Introduction
As long as 25 years after treatment of primary uveal melanoma (UM), metastases are the leading cause of death and, eventually, over 50% of patients die of metastatic disease [Citation1,Citation2]. The liver is the first site of metastases in 90% of patients and remains the only site in more than 50% of them [Citation2,Citation3]. Thereafter, the median overall survival (OS) has been 13 months with little difference between non-surgical treatments [Citation4,Citation5]. Neither advances in managing the primary tumour nor novel therapies for metastatic cutaneous melanoma have translated to survival benefit in metastatic UM [Citation6,Citation7]. In fact, no consensus exists on which first-line treatments for metastatic UM, if any, provide OS benefit.
There is no published survival data on consecutive patients managed with best supportive care (BSC) to allow historical comparison with those actively treated, although such data would be valuable for planning and analysing trials of metastatic UM, most of which continue to be non-randomised and non-comparative [Citation8,Citation9]. We report population-based OS according to previously validated prognostic stages [Citation10] for patients with metastatic UM managed only with BSC.
Methods
Study design
Eligible to our retrospective observational cohort study were patients previously treated for primary UM in the Ocular Oncology Service, Department of Ophthalmology, Helsinki University Hospital, Finland, who developed metastases between January 1999 and December 2016, and received only BSC, although palliative radiotherapy to control pain was allowed for five patients [Citation11,Citation12]. The BSC decision was made by an oncologist in 96% of the patients and by a general practitioner in 4%. Our service is a national referral centre that manages over 95% of Finnish patients with UM. The institutional review board and the National Institute for Health and Welfare approved our study.
Data collection
We obtained patient charts from all hospitals managing metastatic UM. Because the Finnish law permits destroying patient records 12 years after death, data were incomplete for 21 patients. Of 338 patients with metastatic UM, 111 received no active treatment but two of them were diagnosed only at autopsy and excluded (Supplementary Figure S1).
We adapted definitions of the Collaborative Ocular Melanoma Study (COMS) [Citation1,Citation13] to ascertain whether metastatic UM was present, and obtained histopathologic specimens for review (Supplementary Text). Based on that, one patient was excluded, resulting in 108 enrolled patients.
We recorded the gender, age, date of diagnosis of the primary and metastases, Tumour, Node, Metastasis (TNM) stage [Citation14,Citation15], date of treatment decision (i.e. BSC), liver function tests (LFTs), Eastern Cooperative Oncology Group performance status (PS) [Citation16], sites of metastases, the largest diameter of the largest metastasis (LDLM), symptoms, participation in regular follow-up to detect metastases [Citation17], and the date and registered cause of death. The regular follow-up included annual LFTs and upper abdominal ultrasonography (US), followed by magnetic resonance imaging or computed tomography when metastases were suspected. Follow-up ended on December 31, 2018.
Outcomes
Our primary endpoint is OS from the date of treatment decision to death as most common in clinical trials [Citation4,Citation18]. A secondary endpoint is OS from the date of diagnosis of metastases to death that is less frequently reported in the literature [Citation4].
In TNM staging, metastatic UM is currently divided in three categories (M1a to M1c) based on LDLM [Citation14]. The PS and serum or plasma alkaline phosphatase (AP) level are additional independent predictors of OS [Citation3,Citation10]. The Helsinki University Hospital Working Formulation (WF) staging uses all three variables and has been validated by the European Ophthalmic Oncology Group (Supplementary Table S1) [Citation10]. We assigned patients to the WF stages IVa, IVb, and IVc [Citation10] by calculating their individual predicted median OS (online calculator available at http://www.prognomics.org/huhwf.aspx). As originally described, the WF stages correspond to median predicted OS of ≥12, <12–6, and <6 months, respectively, based on data at the time of diagnosis of metastases. For primary outcome assessment, we used the same data as available at the time of treatment decision.
The LDLM, PS, or AP level were missing for 16 patients, preventing calculation, but we could assign the WF stage for 13 of them using a published summary table [Citation3].
Statistical analysis
Analysis was performed with Stata (version 15, Stata Corp., College Station, TX). All p-values are two-tailed, and p<.05 was considered significant. We report median with range and interquartile range (IQR) for continuous variables and compare gender distribution using binomial test. We estimated OS using Kaplan-Meier product-limit method, report the median OS with 95% confidence interval (CI), and compare unordered and ordered categories with the log-rank test and test for trend, respectively.
We used Cox proportional hazards regression to check whether gender, age at the time of treatment decision (categorized as <80 and ≥80, based on the age criterion for referral to a geriatric oncologist), relapse-free interval (RFI) (from the primary tumour to the diagnosis of metastases), symptoms from metastases, LDLM (TNM categories M1a, <30 mm; M1b, 31–80 mm; M1c, >80 mm) [Citation14], AP level (categories <1.0 x, 1.0–2.0 x, >2.0 x upper normal limit [UNL]), and PS (categories 0–1, 2, 3–4 according to the WF) retained residual predictive power, given the WF stage, and might thus help predict OS. We allowed independent variables in models if p<.10, tested the assumption of proportional hazards using scaled adjustment of Schoenfeld residuals [Citation19], and compared models using the deviance test.
Results
Patient characteristics
The median age of the 108 patients with metastatic UM managed only with BSC was 78 (range, 48–95) at the time of treatment decision (). The median RFI was 32 months (range, 0–194; IQR 2–150; Supplementary Figure S2). The characteristics of the primary UM are summarised in Supplementary Table S2.
Table 1. Baseline characteristics of 108 patients with metastatic uveal melanoma managed with best supportive care, and stratification by the Helsinki University Hospital Working Formulation stage.Table Footnotea
Ninety-four percent of patients attended regular follow-up, 41% were asymptomatic, and 94% had liver metastases with or without other sites of metastases (). The median LDLM was 33 mm (range, 2–270). The AP exceeded UNL in 50% of 94 patients with available data. The PS was 0–2 and 3–4 for 44% and 52% of patients, respectively.
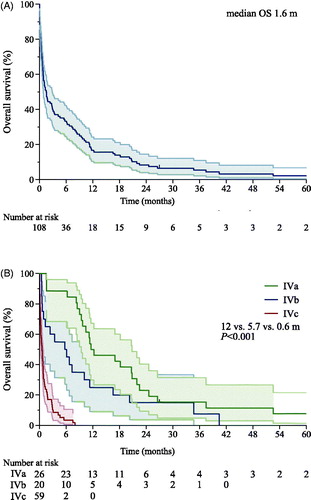
The median interval from diagnosis of metastases to treatment decision (i.e. BSC) was 29 days (range, 0–758; IQR, 7.5–63). The median OS after treatment decision was 1.6 months (range, 0–83; ). One patient was alive with progressive metastases at the time of analysis. The audited cause of death was metastatic UM for others.
Figure 1. Kaplan-Meier graph of overall survival (OS) from decision to treat with best supportive care for metastatic uveal melanoma. (A) Entire cohort. (B) According to the Helsinki University Hospital Working Formulation. Three patients of 108 patients could not be staged and are omitted. Shaded areas show the 95% confidence intervals.
Overall survival by stage
Of the 105 patients who could be staged according to the WF, 24% represented stage IVa, 19% IVb, and 55% IVc. The median OS from treatment decision shortened with increasing stage. It was 12 (range, 1.6–83), 5.7 (range, 0.5–40), and 0.6 (range, 0–8.0) months for stage IVa, IVb, and IVc, respectively (p<.001, log-rank test for trend, ). By univariable Cox regression, WF predicted shorter OS: stage IVb versus IVa (p=.038, HR 1.9), IVc versus IVb (p<.001, HR 4.2), and IVc versus IVa (p<.001, HR 11.9; Supplementary Table S3).
In stage IVa, 50% of patients survived with BSC for ≥12 months (Supplementary Figure S3). In stage IVb, 50% and 25% of patients survived ≥6 and ≥12 months, respectively. In stage IVc, 97% of them died within 6 months.
The weighted kappa for agreement between observed and predicted OS category was 0.614 and 0.615 (agreement 84% versus 59% expected, p<.001 and 83% versus 57% expected, p<.001, Supplementary Table S4), calculated from the treatment decision and diagnosis of metastases, respectively [Citation10].
The historical benchmarks for OS from treatment decision, stratified by WF stage, and an Excel file with the corresponding data to calculate the historical survival curve are provided (Appendix C, D, and https://doi.org/10.5281/zenodo.3369090).
Verification of and search for further prognosticators
Regarding the components of the WF, the median OS from treatment decision was 9.7 months (range, 0.5–83) for PS 0–1, 6.1 months (range, 0.2–40) for PS 2, and 0.6 months (range, 0–27) for PS 3–4 (p<.001, log-rank test for trend). A higher AP level and a larger LDLM (by TNM M1 category) also associated with shorter OS (p<.001), verifying their validity as predictors when analysing OS with BSC.
The median OS was 1.1, 1.0, and 1.9 months for RFI <2.0, 2.0–3.5, and >3.5 years, respectively (p=.033, log-rank test for trend; Supplementary Figure S4 for different variables); and 8.3 months for absence of symptoms from metastases versus 0.6 months for presence of symptoms (p<.001, log-rank test).
In bivariable Cox regression models with WF stage, presence of symptoms independently predicted survival (p<.001), and this model fitted better with the data (–2 log likelihood = 332.59 versus 348.27, p<.001, df = 1; Supplementary Table S3).
Stratified by WF stage, none of the other variables was significant in all three strata of WF stage (Supplementary Table S5).
Discussion
Our nation-wide study with BSC for metastatic UM shows that the WF staging, previously validated by the OOG for mainly actively treated patients [Citation10], differentiates also patients receiving BSC by OS. The agreement between predicted and observed OS, evaluated by weighted kappa, was even stronger in our dataset than in the OOG validation study (0.388), irrespective of whether we based staging on data at the time of treatment decision or diagnosis of metastases (0.614 and 0.615, respectively) [Citation10].
Also, to the best of our knowledge, our cohort is the second largest one of patients receiving BSC for metastatic UM, and we are the first to stage them. We are aware of seven previous reports that included 11 to 191 patients with BSC [Citation9,Citation20–25]. One of these studies also analysed prognostic factors and found, in line with us, that patients who received BSC had worse PS than actively treated [Citation23]. Correspondingly, the PS, but also the AP level and LDLM, the two other components of the WF, were independent predictors of OS in our dataset.
The median OS of 1.6 months in our BSC cohort was substantially shorter than 13 months in our recent meta-analysis of 2,494 actively treated patients and 10 months in another meta-analysis of 921 patients [Citation4,Citation5], but the stage distribution in the latter studies probably was very different [Citation23]. The WF stage was available in 3 of 78 studies [Citation26–28] included in the first meta-analysis, but none of them reported on BSC.
To best match our results with clinical research practice, we chose as our primary outcome the OS from treatment decision, as required of trials by the European Medicines Agency and the U.S. Food and Drug Administration [Citation29,Citation30]. The median interval between diagnosis of metastases and BSC decision was only 29 days in our cohort, and the agreement with observed OS was equivalent for both of these endpoints.
The median OS in the OOG validation study was 11 months, expectedly much longer than 1.6 months with BSC [Citation10]. However, the median OS for stage IVa and, especially, stage IVb was more similar — 17 versus 14 months and 10 versus 8.6 months, respectively. The vast majority of patients with PS 0–1 in our study fell in these two stages. These observations are in line with a previous study that compared OS with systemic chemotherapy and BSC, and found no difference by multivariate analysis [Citation23]. The OS for stage IVc in the OOG cohort was 4 times as long, 4.6 months versus 1.1 months, as with BSC [Citation10]. Of our patients with stage IVc, 85% had a poor PS and they were thus probably excluded from active systemic treatment unlike in the OOG study.
Our overall and WF stage-specific benchmarks remain provisional until verified and refined with independent datasets. Limitations of our study include, in addition to the retrospective data collection, lack of genetic prognosticators and lack of a universal definition of BSC [Citation12]. To improve understanding of the natural course of metastatic UM, we encourage collaboration to enrol patients who receive BSC in order to collect their WF stage and additional prognostic factors, especially genetic ones [Citation31–33]. We strongly advocate using a validated system, such as the WF stage, for evidence-based, stage-specific reporting of outcomes.
Supplemental Material
Download MS Excel (16.1 KB)Supplemental Material
Download PDF (40.2 KB)Supplemental Material
Download PDF (322.4 KB)Supplemental Material
Download MS Word (88 KB)Acknowledgments
The data and specimens were obtained with permission from Helsinki Biobank, Biobank Borealis, Biobank of Eastern Finland, Fimlab Laboratories, Kuopio University Hospital, Tampere University Hospital, Turku University Hospital, Oulu University Hospital, Central Hospitals at Hämeenlinna, Joensuu, Jyväskylä, Kajaani, Kemi, Kokkola, Kotka, Lahti, Lappeenranta, Mariehamn, Mikkeli, Pori, Rovaniemi, Savonlinna, Seinäjoki, and Vaasa, private health care providers Terveystalo, Pihlajalinna Koskiklinikka, and Docrates Cancer Center, several district hospitals, and many health care centers. We gratefully acknowledge the technical assistance of Mrs. Seija Lehtonen.
Disclosure statement
ESR reports personal fees from Théa Nordic, MMH personal fees from BMS, MSD, Novartis, Roche, Sanofi, and Varian, and TTK personal fees from Santen Finland; all outside the submitted work. ML and JL have no conflicts of interests.
Additional information
Funding
References
- Kujala E, Mäkitie T, Kivelä T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2003;44(11):4651–4659.
- Diener-West M, Reynolds SM, Agugliaro DJ, et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch Ophthalmol. 2005;123(12):1639–1643.
- Eskelin S, Pyrhönen S, Hahka-Kemppinen M, et al. A prognostic model and staging for metastatic uveal melanoma. Cancer. 2003;97(2):465–475.
- Rantala ES, Hernberg M, Kivelä TT. Overall survival after treatment for metastatic uveal melanoma: a systematic review and meta-analysis. Melanoma Res. 2019;29(6):561–568.
- Khoja L, Atenafu EG, Suciu S, et al. Meta-analysis in metastatic uveal melanoma to determine progression free and overall survival benchmarks: an international rare cancers initiative (IRCI) ocular melanoma study. Ann Oncol. 2019;30(8):1370–1380.
- Carvajal RD, Piperno-Neumann S, Kapiteijn E, et al. Selumetinib in combination with dacarbazine in patients with metastatic uveal melanoma: a phase III, multicenter, randomized trial (SUMIT). J Clin Oncol. 2018;36(12):1232–1239.
- Zimmer L, Vaubel J, Mohr P, et al. Phase II DeCOG-study of ipilimumab in pretreated and treatment-naïve patients with metastatic uveal melanoma . PLoS One. 2015;10(3):e0118564.
- Augsburger JJ, Corrêa ZM, Shaikh AH. Effectiveness of treatments for metastatic uveal melanoma. Am J Ophthalmol. 2009;148(1):119–127.
- Jochems A, van der Kooij MK, Fiocco M, et al. Metastatic uveal melanoma: treatment strategies and survival-results from the Dutch melanoma treatment registry. Cancers (Basel). 2019;11(7):1007.
- Kivelä TT, Piperno-Neumann S, Desjardins L, et al. Validation of a prognostic staging for metastatic uveal melanoma: a collaborative study of the European Ophthalmic Oncology Group. Am J Ophthalmol. 2016;168:217–226.
- Cherny NI, Catane R, Kosmidis P, ESMO Taskforce on Supportive and Palliative Care. ESMO takes a stand on supportive and palliative care. Ann Oncol. 2003;14(9):1335–1337.
- Lee RT, Ramchandran K, Sanft T, et al. Implementation of supportive care and best supportive care interventions in clinical trials enrolling patients with cancer†. Ann Oncol. 2015;26(9):1838–1845.
- Moy CS, Albert DM, Diener-West M, et al.; Collaborative Ocular Melanoma Study Group, prepared by COMS Mortality Coding Committee. Cause-specific mortality coding: methods in the Collaborative Ocular Melanoma Study. COMS report. Control Clin Trials. 2001;22(3):248–262. no.
- Kivelä TT, Simpson ER, Grossniklaus HE, et al. Uveal melanoma. In: Amin MB, Edge S, Greene F, et al. editors. AJCC cancer staging manual. 8th ed. Chicago, USA: Springer International Publishing; 2017. p. 813–826.
- Kujala E, Damato B, Coupland SE, et al. Staging of ciliary body and choroidal melanomas based on anatomic extent. J Cin Oncol. 2013;31(22):2825–2831.
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655.
- Eskelin S, PyrhöNen S, Summanen P, et al. Screening for metastatic malignant melanoma of the uvea revisited. Cancer. 1999;85(5):1151–1159.
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216.
- Grambsch PM, Therneau TM, Fleming TR. Diagnostic plots to reveal functional form for covariates in multiplicative intensity models. Biometrics. 1995;51(4):1469–1482.
- Lane AM, Kim IK, Gragoudas ES. Survival rates in patients after treatment for metastasis from uveal melanoma. JAMA Ophthalmol. 2018;136(9):981–986.
- Gomez D, Wetherill C, Cheong J, et al. The Liverpool uveal melanoma liver metastases pathway: outcome following liver resection. J Surg Oncol. 2014;109(6):542–547.
- Gragoudas ES, Egan KM, Seddon JM, et al. Survival of patients with metastases from uveal melanoma. Ophthalmology. 1991;98(3):383–389.
- Pons F, Plana M, Caminal JM, et al. Metastatic uveal melanoma: Is there a role for conventional chemotherapy? - A single center study based on 58 patients. Melanoma Res. 2011;21(3):217–222.
- Nicholas MN, Khoja L, Atenafu EG, et al. Prognostic factors for first-line therapy and overall survival of metastatic uveal melanoma: The Princess Margaret Cancer Centre experience. Melanoma Res. 2018;28(6):571–577.
- Xu LT, Funchain PF, Bena JF, et al. Uveal melanoma metastatic to the liver: treatment trends and outcomes. Ocul Oncol Pathol. 2019;5(5):323–332.
- Kivelä T, Suciu S, Hansson J, et al. Bleomycin, vincristine, lomustine and dacarbazine (BOLD) in combination with recombinant interferon alpha-2b for metastatic uveal melanoma. Eur J Cancer. 2003;39(8):1115–1120.
- Pyrhönen S, Hahka-Kemppinen M, Muhonen T, et al. Chemoimmunotherapy with bleomycin, vincristine, lomustine, dacarbazine (BOLD), and human leukocyte interferon for metastatic uveal melanoma. Cancer. 2002;95(11):2366–2372.
- Vihinen PP, Hernberg M, Vuoristo MS, et al. A phase II trial of bevacizumab with dacarbazine and daily low-dose interferon-alpha2a as first line treatment in metastatic melanoma. Melanoma Res. 2010;20(4):318–325.
- US Food and Drug Administration. Clinical trial endpoints for the approval of cancer drugs and biologics - guidance for industry 2018. [cited 2020 July 6]. Available from: https://www.fda.gov/media/71195/download
- European Medicines Agency. Guideline on the evaluation of anticancer medicinal products in man 2005. [cited 2020 July 6]. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-evaluation-anticancer-medicinal-products-man-revision-3_en.pdf
- Rodrigues M, Mobuchon L, Houy A, et al. Outlier response to anti-PD1 in uveal melanoma reveals germline MBD4 mutations in hypermutated tumors. Nat Commun. 2018;9(1):1866.
- Rodrigues M, Mobuchon L, Houy A, et al. Evolutionary routes in metastatic uveal melanomas depend on MBD4 alterations. Clin Cancer Res. 2019;25(18):5513–5524.
- Repo P, Jäntti J, Järvinen R-S, et al. Germline loss-of-function variants in MBD4 are rare in Finnish patients with uveal melanoma. Pigment Cell Melanoma Res. 2020;33(5):756–762.
