Abstract
Background
Neoadjuvant chemotherapy (NACT) is offered to an increasing number of breast cancer (BC) patients, and comprehensive monitoring of treatment response is of utmost importance. Several imaging modalities are available to follow tumor response, although likely to provide different clinical information. We aimed to examine the association between early radiological response by three conventional imaging modalities and pathological complete response (pCR). Further, we investigated the agreement between these modalities pre-, during, and post-NACT, and the accuracy of predicting pathological residual tumor burden by these imaging modalities post-NACT.
Material and methods
This prospective Swedish cohort study included 202 BC patients assigned to NACT (2014–2019). Breast imaging with clinically used modalities: mammography, ultrasound, and tomosynthesis was performed pre-, during, and post-NACT. We investigated the agreement of tumor size by the different imaging modalities, and their accuracy of tumor size estimation. Patients with a radiological complete response or radiological partial response (≥30% decrease in tumor diameter) during NACT were classified as radiological early responders.
Results
Patients with an early radiological response by ultrasound had 2.9 times higher chance of pCR than early radiological non-responders; the corresponding relative chance for mammography and tomosynthesis tumor size measures was 1.8 and 2.8, respectively. Post-NACT, each modality, separately, could accurately estimate tumor size (within 5 mm margin compared to pathological evaluation) in 43–46% of all tumors. The diagnostic precision in predicting pCR post-NACT was similar between the three imaging modalities; however, tomosynthesis had slightly higher specificity and positive predictive values.
Conclusion
Breast imaging modalities correctly estimated pathological tumor size in less than half of the tumors. Based on this finding, predicting residual tumor size post-NACT is challenging using conventional imaging. Patients with early radiological non-response might need improved monitoring during NACT and be considered for changed treatment plans.
Background
The indication for neoadjuvant chemotherapy (NACT) in breast cancer (BC) has broadened and become a major trend in BC care. Originally designed to down-stage and consequently shrink large tumors to an operable size [Citation1,Citation2], later, NACT was recommended for patients with locally advanced BC, inflammatory BC, or patients with T2 tumors and other risk factors (e.g., triple-negative or HER2 overexpressing subtype) [Citation3–5]. Currently, the important concept of salvage adjuvant chemotherapy (ACT) according to ypTN-staging has been added to the list of profits of NACT [Citation6–8].
Ideally, oncological and surgical BC treatment should be performed according to a patient’s individual tumor response to NACT through repeated response evaluations; assessed clinically by inspection/palpation of the breast and lymph nodes, by imaging, and ultimately, pathological assessment. Mammography, ultrasound, and/or magnetic resonance imaging (MRI) are the imaging modalities most frequently used, and several studies have investigated the ability of these modalities in measuring tumor response during NACT for predicting residual pathological tumor size post-NACT [Citation9–16]. However, the accuracy of the novel 3D-mammography tomosynthesis relative to other imaging modalities, has been less studied [Citation14]. During the last decade, studies have shown the superiority of MRI in detecting pathological complete response (pCR) [Citation17–19]; however, while the modality is clinically advantageous, shortcomings in terms of costs and availability remain [Citation20]. Also, a recent review questioned the high performance of MRI as presented by individual studies [Citation20].
For BC-patients receiving NACT, it is of great importance to identify prospective responders from non-responders, ideally prior to treatment start, but at least as early as possible during treatment. The image-based evaluation post-NACT is vital when deciding on the optimal surgical treatment [Citation21] or possibly abstaining from surgery. There is an ongoing discussion regarding the safety of omitting breast surgery for patients with radiological complete response (rCR). Preliminary results of the MICRA trial, show lacking accuracy of minimal invasive ultrasound biopsies post-NACT in patients showing rCR (MRI), and should not be used as ground of omission of surgery [Citation22]. The succeeding result from the pathology examination is determining the post-NACT staging (ypTN) [Citation23], providing additive information to the subsequent treatment decisions (including salvage ACT) [Citation24,Citation25]. Different imaging modalities might be useful at different time points during NACT; a study of patients with human epidermal growth factor receptor 2 (HER2) overexpressing BC receiving NACT, showed that ultrasound was better than mammography at predicting pCR after 6 weeks of treatment with HER2-blockade, whereas mammography was more informative than ultrasound after completion of NACT [Citation26]. In this prospective study, we aimed to examine the association between early radiological response by three clinically used conventional imaging modalities (mammography, ultrasound, and tomosynthesis) and pCR. Further, we investigated the agreement between these modalities pre-, during, and post-NACT, and the accuracy of predicting pathological residual tumor burden by these imaging modalities post-NACT.
Material and methods
Cohort
This ‘NeoDense’ study cohort is part of the overarching SCAN-B study (Clinical Trials ID NCT02306096) [Citation27,Citation28], which prospectively included 207 BC-patients receiving NACT provided at Skåne University Hospital, Sweden (2014–2019). The study name ‘NeoDense’, short for neoadjuvant density, originates from the volumetric mammographic density study conducted within this cohort [Citation29]. At diagnosis, BC-patients older than 18 years and accepting NACT were considered for study inclusion and were, after written informed consent, included in the study. Reasons for the exclusion (N = 5) are previously published [Citation29].
The standard NACT included three courses of fluorouracil, epirubicin, and cyclophosphamide (FEC) or epirubicin and cyclophosphamide (EC) followed by three courses of a taxane (docetaxel or paclitaxel), and HER2-blockade in case of HER2-overexpression. A total of 97% (N = 196) of the patients received a chemotherapy regimen of FEC/EC and taxane, whereas 2% (N = 5) of the patients received a taxane-only NACT-regimen, and one patient received EC only. Among the patients with HER2-overexpressing tumors (N = 49), the majority (N = 46, 94%) received double HER2-blockade (trastuzumab and pertuzumab), and the remaining three patients received single HER2-blockade (trastuzumab).
Clinical and pathological data were retrieved from prospectively collected study-specific patient questionnaires (including questions regarding lifestyle factors, previous breast diseases, reproductive factors, and current medications), medical charts, and clinical pathology reports. The focus of this study was the radiological evaluation of the tumor in the breast only, and not axillary lymph nodes, and we performed our analyses with a substitute-variable of pCR, pCRbreast, defined as the absence of any invasive focus (i.e., allowing remaining ductal carcinoma in situ (DCIS)) in the pathological breast specimen. However, since current guidelines define a true pCR as the absence of any residual invasive cancer in the resected breast and axillary lymph nodes [Citation30], sensitivity analyses were performed with this variable definition (pCRtrue, N = 45). This study was designed in accordance with the STROBE guidelines [Citation31]. The study was approved by the Regional Ethics Committee in Lund, Sweden (official record numbers: 2014/13, 2014/521, and 2016/521).
Imaging
Study patients were referred to a set of breast imaging examinations at three time points (pre-NACT (T0), during NACT (after two courses of chemotherapy, T1), and post-NACT (T2)): bilateral mammograms (three views), unilateral ultrasound, and, at one of the two treatment sites, bilateral one-view, wide-angle digital breast tomosynthesis was used (mediolateral oblique view) (N = 156) (Supplementary Material 1). The timing of the imaging examinations followed the clinical routine. Percutaneous ultrasound-guided approach was used to mark the tumors with coils (usually one coil) according to clinical routine, except for the two tumors considered as un-detectable with ultrasound and where stereotactic guidance was used. Through prospectively collected imaging study forms (previously published [Citation29]), assessment of tumor size (largest diameter), tumor focality, and measurability (i.e., whether the radiologist could give a tumor size estimate) were retrieved and noted in real-time at the examination. There was only a very small number of missing examinations () and the protocol-defined time frames were satisfactorily followed as presented in Supplementary Material 1. Mammography images were acquired on the following machines: GE Senographe Pristina (3%), Philips MammoDiagnost DR (17%), Philips MicroDose (2%), and Siemens Mammomat Inspiration (77%). Tomosynthesis was available at one of the two sites, and a Siemens Mammomat Inspiration was used.
Table 1. Patient and tumor characteristics pre-NACT.
Patients presenting with either rCR, representing no visible tumor, or radiological partial response (rPR) indicating ≥30% decrease in the (largest) diameter of the largest foci (adapted after Response Evaluation and Criteria in Solid Tumors (RECIST) criteria, version 1.1 [Citation32]) as measured during NACT compared to pre-NACT, were classified as early radiological responders. Otherwise, they were classified as early radiological non-responders. For the purpose of this study, we used a simplified interpretation of RECIST 1.1, and defined only the single largest tumor pre-NACT as target lesions, rather than the total sum of all target lesions [Citation32,Citation33].
In case of multifocal BC (N = 25 on mammography) or bilateral BC (N = 4), as defined pre-NACT, only the single largest focus by imaging was considered a target lesion and the pathology data was derived from this lesion. Accordingly, the single largest remaining invasive focus was used as a pathological outcome since this variable defines the ypT-stage (additional to pCR). Only one patient presented clinically with inflammatory BC and for this tumor, the radiologist could still give a size estimation with all three modalities.
Statistical analyses
We summarized characteristics of the cohort pre-NACT, including tumor size, as assessed by mammography, tomosynthesis, and ultrasound. When the tumor was not visible (i.e., undetectable pre-NACT for a modality), the patient was omitted from the statistics for all time points for that particular modality since there was nothing to have a response relative to. We also investigated the agreement between imaging modalities pre-, during, and post-NACT using the Bland–Altman plots (i.e., for each person and each pair of imaging modalities, we plotted differences against the mean of estimated tumor size as assessed by each modality). Patients for whom one or both imaging modalities were either not performed or the tumor size was non-measurable, were excluded. We compared imaging modalities post-NACT to the pathological specimen, and categorized each imaging modality as accurate or over- or underestimating the size, depending on whether the imaging modality estimated a tumor size within 5 mm of the pathology-based tumor size. In a sensitivity analysis, we used a limit of 2 mm. We also assessed the accuracy of the different imaging modalities by the Bland–Altman plots comparing imaging modalities to the pathological specimen. Next, we estimated sensitivity, specificity, positive, and negative predictive value (PPV and NPV), as well as Cohen’s kappa, for rCR post-NACT as an indicator for pCR. To enable easier comparison between the modalities, only patients with a visible tumor in all three modalities were included in these test characteristics analyses (i.e., exclusion of patients either lacking examination or having a non-detectable tumor in one or more modalities). Patients with non-measurable tumors post-NACT were defined as non-rCR. We did this for each imaging modality separately, and by combining different modalities (pairwise or all three). When we combined modalities, we would require either just one of the two/three modalities to show rCR, or require both/all three to show rCR. We visualized the association between rCR-assessment by the three modalities separately in a Venn diagram. Finally, we estimated the probability of achieving pCR among those who showed early response for each imaging modality, i.e., if the tumor size had decreased by at least 30% or gone from being unmeasurable (although visible) to size 0 during NACT, compared to pre-NACT.
Results
The tumor- and patient characteristics at the time of pre-NACT are outlined in . According to the different imaging modalities, the median tumor size was 30 mm (IQR 20–40), 28 mm (IQR 21–36), and 28 mm (IQR 19–35), as assessed with mammography, tomosynthesis, and ultrasound, respectively. Lymph node metastases to the axilla were verified at diagnosis for 71% of the patients (ultrasound). A total of 60% of the tumors were estrogen receptor-positive, 51% were progesterone receptor-positive, 24% had HER2 overexpressing BC, and 79% presented with high proliferative tumors as measured by Ki67. The largest proportion of non-measurability (i.e., the radiologist could not give a size estimate of the tumor), as well as un-detectability, were seen for mammography (5.0% and 5.4% vs. 3.0% and 3.0% for tomosynthesis and 2.5% and 1.0% for ultrasound, respectively), although the differences were small. Post-NACT, few patients showed progressive disease defined as ≥20% increase in tumor diameter in comparison to baseline (N = 8, N = 8, and N = 5 as assessed with mammography, tomosynthesis, and ultrasound, respectively).
Agreement between breast imaging modalities
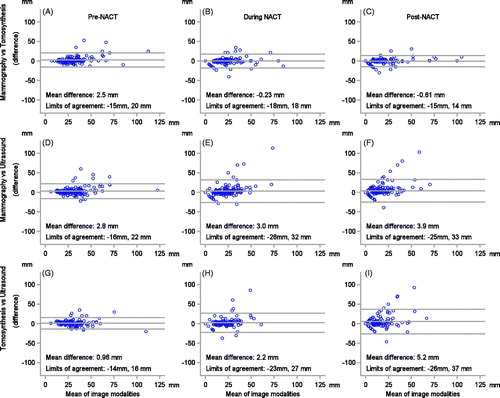
The agreement between the imaging modalities at each time point is visualized in the Bland–Altman plots in . Pre-NACT, the agreement, in terms of limits of agreement, between the modalities, was similar, although tomosynthesis vs. ultrasound had the smallest mean difference. At the later time points, mammography and tomosynthesis showed the best agreement with one another. During and post-NACT, ultrasound seemed to underestimate tumor size in comparison to both mammography and tomosynthesis, especially for larger tumors, and the agreement for ultrasound vs. mammography, and ultrasound vs. tomosynthesis, was similar. The funnel shape (i.e., increasing variation in the difference with increasing mean values) seen in the panels indicate that there is not a constant level of agreement across the range of measurement.
Figure 1. Agreement between mammography and tomosynthesis (A–C), mammography and ultrasound (D–F), and tomosynthesis and ultrasound (G–I), at baseline, after two courses of chemotherapy, and after completed chemotherapy (six courses), visualized in a Bland–Altman plot. The mean of the difference and limits of agreement (thin dashed lines) are illustrated in each graph.
Post-NACT evaluation
A total of 52 (26%) patients accomplished pCRbreast and 45 patients accomplished pCRtrue (baseline characteristics in Supplementary Material 2). Post-NACT, the accuracy of tumor size estimation by imaging (each modality individually) in relation to pathology (within 2 and 5 mm margins), was highest for ultrasound (31% and 46%, respectively) and mammography (30% and 46%, respectively), and slightly lower for tomosynthesis (27% and 43%, respectively) () – only small differences between the modalities considered of minor clinical relevance.
Table 2. Accuracy of radiological modalities post-NACT (in relation to the invasive focus on pathology).
Post-NACT, in the evaluation of rCR as an indicator for pCR according to the three imaging modalities individually, the sensitivity was highest for mammography (65%), and the specificity was highest for tomosynthesis (91%), although there were overlapping 95% confidence interval (CI) for all three modalities (). For all three modalities individually, the NPV was between 85% and 88%. If all three modalities were individually interpreted as rCR, then the combined sensitivity was only 35% (95%CI 21–52%); the (combined) specificity was, on the other hand, the highest of our models, with 94% (95%CI 88–97%). The (combined) PPV (probability of pCRbreast) when all three modalities were interpreted as rCR was 63% (95%CI 41–82%); the same PPV was seen for tomosynthesis alone (PPVtomosynthesis) 63% (95%CI 44–79%) and for any combination of two modalities. If one (or more) of the three modalities was interpreted as rCR, the sensitivity, specificity, and PPV were 79% (95%CI 64–90%), 71% (95%CI 62–79%), and 47% (95%CI 34–59%), respectively. The agreement between rCR and pCRbreast, quantified using kappa-statistics [Citation34], showed similar agreement for the three modalities; slightly higher kappa point estimates for mammography and tomosynthesis in comparison to ultrasound (κ = 0.43 (95%CI 0.25–0.60) and κ=0.44 (95%CI 0.25–0.63) vs. κ=0.39 (95%CI 0.21–0.57)). The corresponding analyses with the variable pCRtrue increased the level of agreement for tomosynthesis (κ=0.50 (95%CI 0.31–0.68)) and this trend was reversed for mammography (κ=0.37 (95%CI 0.19–0.56)) (Supplementary Material 3). In , 41% (N = 58) of the patients were assessed as rCR by any modality, of whom 33% (N = 19) of the patients were assessed as rCR by all three modalities (Supplementary Material 4). Importantly, all patients categorized as rCR by tomosynthesis were also assessed as rCR by mammography. Stratified by inclusion in , baseline patient and tumor characteristics were similar (Supplementary Material 2). A total of 77% (N = 46) were excluded merely due to tomosynthesis not being available.
Table 3. Post-NACT: test characteristics of mammography, ultrasound, and tomosynthesis in predicting pCRa following NACT (95%CI).
According to the Bland–Altman analysis, for all three imaging modalities, it appears that there was a better accuracy post-NACT between imaging-based tumor size and pathology-based tumor size for up-front smaller tumor sizes (up to a tumor size of approximately 20 mm), compared to larger tumors (Supplementary Material 5). Again, the panels indicate that there is not a constant level of agreement across the range of measurement, causing a funnel-like appearance. Ultrasound had the smallest mean difference (–0.79 mm) between tumor size assessed with imaging and pathology post-NACT, and the narrowest limits of agreement. Thus, ultrasound seems to underestimate the tumor size, which is again most pronounced in large tumors, whereas the other methods vary more, as can be seen by their wider limits of agreement.
Early radiological response
For patients categorized as early radiological responders by mammography, 35% accomplished pCRbreast post-NACT, whereas only 19% of the patients categorized as early radiological non-responders accomplished pCRbreast post-NACT (). Correspondingly, the proportions of pCRbreast among radiological early responders for tomosynthesis and ultrasound were slightly higher; 44% and 42%, respectively. The chance of pCRbreast was significantly higher for early radiological responders compared to early radiological non-responders for all three modalities. For ultrasound, the relative chance of pCRbreast was 2.9 (95%CI 1.6–5.2) when comparing patients with early radiological response with early radiological non-responders; the corresponding relative chance for mammography and tomosynthesis were 1.8 (95%CI 1.1–3.0) and 2.8 (95%CI 1.5–5.2), respectively. Evaluating the numbers from another perspective, for patients classified as early radiological non-responder with ultrasound, only 14% accomplished pCRbreast. Similar numbers were seen for pCRtrue (Supplementary Material 6). A total of 118, 89, and 112 patients were categorized as radiological early non-responders with mammography, tomosynthesis, and ultrasound, respectively ( and Supplementary Material 6), of whom N = 21 (18%), N = 12 (13%), and N = 14 (13%) for mammography, tomosynthesis, and ultrasound, respectively, still accomplished pCRtrue (Supplementary Material 6).
Table 4. pCRa/non-pCR-rate by radiological early response.
Discussion
The primary purpose of breast imaging during NACT is to provide an early and accurate indicator of treatment effect translated into an accurate prediction of pCR. In this study, we found an association between early radiological response by breast imaging and pCR for three conventional imaging modalities (mammography, ultrasound, and tomosynthesis), as previously shown for mammography and ultrasound [Citation26,Citation35], although the association between early radiological response and pCR has not previously been evaluated for tomosynthesis. Similarly, the I-SPY trial showed that early radiological response by MRI was a strong predictor of pCR [Citation19]. Being categorized as an early radiological responder was associated with a 2–3-times higher chance of accomplishing pCR. Regardless of the routine change in chemotherapy drugs from the anthracyclines-based regimen to taxane-regimen mid-way through NACT, only a small proportion of the radiological early non-responders accomplished pCR post-NACT. Guidelines recommend delivering all pre-planned chemotherapy without additional treatment disruptions [Citation5]; only in case of (radiological) progression (estimated to only 3% of all patients receiving NACT in one meta-analysis [Citation36]) should the treatment strategy be reevaluated [Citation5,Citation37]. It has repeatedly been shown that the survival rates are similar whether chemotherapy is given as NACT or in the adjuvant setting, and solemnly differs in terms of an increased rate of breast-conserving surgery for patients receiving NACT [Citation38,Citation39]. Regardless of the obvious advantages of salvage ACT in NACT-treated patients, still, such ‘non-inferiority’ survival rates in relation to adjuvant treatment raise the question of whether the full potential of NACT in terms of personalized treatment is truly utilized. For patients not accomplishing pCR, the combined duration of both NACT and ACT is extensive, and early identification of non-responders could help escalate treatment. The concept of response-guided NACT (i.e., an adaptive treatment plan according to the on-treatment response), as introduced in the GeparTrio-study [Citation40] is currently lacking convincing evidence of its benefits. However, clinical trials testing this hypothesis for neoadjuvant endocrine therapy, report supportive results of such tailored approach [Citation41] and the possibilities of de-escalation for early-responders have been investigated [Citation42]. Besides evident progression and intolerable side-effects, reasons for discontinuing NACT are not clearly stated in clinical guidelines, although it could save patients from exposure to toxic but insufficiently active therapy [Citation43]. In order to enable enrollment in many ‘early switch’ trials, and thus facilitate access to the latest innovations, early identification of patients not likely to reach pCR is important [Citation44]. Early radiological non-responders, still accomplishing pCR is troublesome, since these patients are at risk of (unfavorable) treatment modification. In the present study, examination by ultrasound had the smallest proportion of patients accomplishing pCRtrue among radiological non-responders (13% of the radiological non-responders corresponding to 7% of the entire cohort). In many studies, the early non-responders accomplishing pCR are underreported, e.g., in the ACRIN 6698 trial (sub-study to the I-SPY-2-trial) [Citation45] and the WSG-ADAPT HER2+/HR − phase II trial [Citation42], these patients were not addressed. In the NeoAlto trial (HER2 overexpressing cohort) [Citation46], 20% of the non-responders by FDG-PET/CT accomplished pCR, possibly explained by the activity of paclitaxel in tumors independent of HER2. We consider clinical awareness of early radiological non-responders important, including radiological early non-responders still accomplishing pCR, and prospectively conducted trials designed to evaluate the clinical efficacy of changing or adding treatment are warranted.
Breast imaging evaluation post-NACT influences surgical decision-making, and in this study, the accuracy of the imaging modalities of predicting post-NACT pathological tumor size, assessed with kappa-statistics, showed point estimates of 0.39–0.44 (corresponding to ‘fair’ to ‘moderate’ – agreement), which is a level of the agreement considered as rather unsatisfactory in many clinical settings. In comparison to a previous study, our kappa-values (point estimates) were similar for mammography but slightly lower for ultrasound (κ = 0.45 (95%CI 0.24–0.66)) [Citation47]. However, our CI substantially overlapped with theirs. Using a different statistical approach to address this question, we found that in less than half of the tumors, conventional breast imaging modalities, separately, could correctly estimate pathological tumor size within a 5 mm margin. Based on the appearance of the Bland–Altman plot, ultrasound was considered to be the most accurate of the three modalities.
NACT is associated with higher rates of local recurrence compared to ACT, which might be explained by the higher rates of breast conserving surgery among NACT-treated patients compared to patients undergoing primary breast surgery [Citation48]. Several studies have shown difficulties in achieving tumor-free margins when performing primary breast conserving surgery, and a considerable number of re-operations are performed to obtain radical tumor excision [Citation49]. Contributing factors might be difficulties associated with evaluating tumor size with imaging post-NACT [Citation50,Citation51]. Other imaging modalities, e.g., MRI, correlating most accurately with pathological size [Citation18], or possibly 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT), might provide better aid in the surgical decision [Citation5,Citation52]. In addition, early changes according to both MRI and FDG-PET/CT parameters are associated with treatment response during NACT [Citation19,Citation45,Citation53–55].
In order to safely omit breast surgery altogether, rCR post-NACT by imaging (possibly in combination with minimal invasive procedures) must accurately mirror pCR, hence prediction of pCR is important. Previous studies [Citation9,Citation12,Citation15] have shown a wide range of sensitivity and specificity for mammography (28–89% and 48–87%, respectively) and ultrasound (36–94% and 33–96%, respectively). Few studies have evaluated the test performance measures of tomosynthesis. However, a previous study, though only based on 50 patients, showed a sensitivity of 45%, and specificity of 98% for tomosynthesis [Citation14]. Our results are thus in line with the previous literature, although we found slightly reduced specificity for tomosynthesis. However, for a clinician, the predictive values of each modality and their combinations are considered of greater interest [Citation56] when making specific treatment decisions. A previous study [Citation47] had shown a probability of pCR (PPV) of 80% when both mammography and ultrasound demonstrated rCR; in our studies, the corresponding probability was slightly more than 60%. Our results indicate that tomosynthesis and ultrasound makes an advantageous combination; the high sensitivity of ultrasound is balanced with the high specificity of tomosynthesis. The addition of mammography did not substantially increase diagnostic precision. All patients showing rCR by tomosynthesis also showed rCR by mammography, explaining the identical test characteristics of tomosynthesis alone and ‘mammography and tomosynthesis’. Two-view tomosynthesis, instead of one-view (as used in this study), might increase the test accuracy of tomosynthesis. Larger studies are warranted to establish the potential benefits of tomosynthesis in tumor response evaluation for patients receiving NACT.
We, therefore, conclude that all three tested conventional imaging modalities have similar diagnostic precision and that they all fail to provide an accurate indicator of response to NACT in the pre-surgical setting. Thus, other modalities, such as MRI or potentially FDG-PET/CT, could be considered, as suggested as imaging alternatives by international guidelines/expert panels [Citation24,Citation52,Citation57]. However, access to these modalities is still limited in many parts of the world. Conventional breast imaging methods, as well as contrast-enhanced MRI, are all reliable on change in tumor size to evaluate treatment response (i.e., anatomical imaging). However, both advanced MRI-techniques and FDG-PET are considered functional imaging modalities, detecting changes in tumor vascularity and glucose metabolism, respectively. Both FDG-PET and MRI can acceptably identify patients with an increased likelihood of accomplishing pCR [Citation19,Citation46,Citation58–62]. A meta-analysis showed sensitivity and specificity of 63–88% and 55–91% for MRI (both anatomical and functional) and 80–85% and 66–79% for FDG-PET/CT [Citation52]. Thus, MRI and FDG-PET/CT have a similar diagnostic performance in response assessment following completion of NACT, whereas FDG-PET/CT was superior to MRI after three courses of NACT [Citation63]. Our conventional breast imaging results, and their combinations, show similar diagnostic accuracy as MRI, but slightly lower sensitivity than FDG-PET/CT. However, the vast majority of the patients in the included studies in the referenced meta-analysis received NACT before 2013, and the indication for NACT might have changed over time; thus, making a comparison with our results difficult.
For solid tumor imaging evaluation RECIST-guidelines have become an international standard in tumor response evaluation [Citation32]. The RECIST-guidelines advocate breast-MRI as the preferred modality for lesion evaluation during NACT for BC, and only a few studies have used RECIST criteria for mammographic evaluation [Citation26,Citation64].
Our study has many strength, including the prospective nature of the study with considerable patient and tumor information, our predefined protocol, and the pre-specified questions originating from an oncological clinical problem.
Some study limitations must be acknowledged. The accuracy of imaging might depend on BC subtype [Citation33]; however, we were unable to make subgroup analyses based on the St Gallen BC subtype due to the limited number of patients enrolled in the study. On the other hand, it is worth noting that clinical guidelines do not stipulate differentiated NACT monitoring for different BC subtypes, nor different chemotherapy regimens, with the exception of adding HER2-blockade in HER2-overexpressing BC [Citation24,Citation37]. Since we aimed at mirroring Swedish clinical practice, our study protocol did not include the more expensive and more complicated imaging methods of MRI and FDG-PET/CT. Due to the limited field-of-view with a standard ultrasound probe and the operator-dependency of ultrasound [Citation65], the limitations with standardization of tumor size measurements, especially for large tumors [Citation66], must be acknowledged. However, measurements followed protocol and our results did not indicate a larger range of size estimates for ultrasound for large lesions in comparison to the other modalities. In this context, the funnel-shape of the panels in the Bland–Altman plots ( and Supplementary Material 5) deserves a short reflection; there is apparently not a constant level of agreement across the range of measurements, and this condition is suboptimal in a Bland–Altman plot, justifying caution when interpreting the mean differences and limits of agreement. However, we still believe that the Bland–Altman is a valuable tool to visualize the results in our study and, purposely, we abstained from a log-transformation, as this would add difficulties in interpreting the results. In the pCR definition, remaining DCIS alone in the pathological specimen was allowed, however since not possible to safely distinguish from invasive cancer on mammography, post-NACT, the concept of rCR might not be in alignment with the pCR-concept. A considerable minority of the patients presented with multifocal/bilateral disease; however, only the largest focus was considered as target lesion. Considering the sum of the largest three foci (data not shown), did not change the progressive disease-status, limiting the source of potential bias. The ‘unmeasurable’ tumors by a modality as well as the ‘undetectable’ tumors, excluded from most of the analyses, are a potential source of potential systematic error; however, baseline characteristics in this subgroup were similar to the entire cohort (data not shown). Lastly, the concerns with a binary endpoint such as pCR (whether defined as ypT0 or ypT0N0) needs to be addressed. A more nuanced and individualized prognosis can be provided by one of many post-NACT pathological assessment scores, reflecting also partial response. The importance is evident in the age of salvage ACT, e.g., residual cancer burden score I (‘near-pCR’), show as good outcome as patients accomplishing pCR [Citation67]. However, without doubt, pCR is still the universally accepted primary endpoint in NACT-studies.
In conclusion, our results show that predicting residual tumor size after NACT is challenging using conventional imaging approaches (mammography, ultrasound, and tomosynthesis). In less than half of the tumors could our imaging modalities, separately, correctly estimate pathological tumor size. Early radiological non-response is worrisome, and prospectively conducted trials designed to evaluate the clinical efficacy of changing or adding treatment are warranted.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Regional Ethics Committee in Lund, Sweden (Official records number: 2014/13, 2014/521 and 2016/521).
Informed consent
Informed written consent was obtained from all participants included in the study.
Author contributions
IS contributed to the study design, made the study protocols, organized the enrollment of patients and the data/image collection, wrote the statistical plan, interpreted the data, solely drafted the manuscript (except for “Statistical analyses”), and organized the joint revision of the manuscript. DF delivered technical aid during image gathering, interpreted the data, and revised the manuscript. UH gave important input on the statistical plan, executed the statistical analyses, co-interpreted the data, co-wrote the “Statistical analyses” part of the manuscript, and substantially revised the manuscript. LR participated in data interpretation, and intellectually revised the manuscript. SZ partook in the over-all design of the study, interpreted the data, and contributed to the revision of the manuscript. SB was the foremost contributor to the initial design of the study, interpreted the data, and revised the manuscript. All authors have read and approved the final manuscript.
| Abbreviations | ||
| NACT | = | neoadjuvant chemotherapy |
| BC | = | breast cancer |
| ACT | = | adjuvant chemotherapy |
| MRI | = | magnetic resonance imaging |
| pCR | = | pathological complete response |
| HER2 | = | human epidermal growth factor receptor 2 |
| FEC | = | fluorouracil, epirubicin, and cyclophosphamide |
| EC | = | epirubicin and cyclophosphamide |
| DCIS | = | ductal carcinoma in situ |
| rCR | = | radiological complete response |
| rPR | = | radiological partial response |
| RECIST | = | Response Evaluation and Criteria in Solid Tumors |
| PPV | = | positive predictive value |
| NPV | = | negative predictive value |
| CI | = | confidence interval |
| FDG-PET/CT | = | 18F-fluorodeoxyglucose positron emission tomography/computed tomography |
Supplemental Material
Download MS Word (29.7 KB)Supplemental Material
Download Rich Text Format File (75 KB)Supplemental Material
Download PDF (109.9 KB)Supplemental Material
Download PDF (320.8 KB)Supplemental Material
Download Rich Text Format File (56.8 KB)Supplemental Material
Download Rich Text Format File (164.7 KB)Supplemental Material
Download PDF (429.6 KB)Acknowledgements
The authors thank all the study participants. The authors acknowledge the outstanding work by research nurse Lina Zander. The authors thank the technicians, administrators, and radiologists at Unilabs Malmö and Helsingborg for their cooperation.
Disclosure statement
SZ: Speakers’ fees and travel support from Siemens Healthcare AG, and consultancy fees from Collective Minds Radiology AB. SB: Speakers’ fees from Pfizer, study-specific advisor Pfizer, and travel support from Roche. The other authors declare that they have no competing interests.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Mieog JS, van der Hage JA, van de Velde CJ. Neoadjuvant chemotherapy for operable breast cancer. Br J Surg. 2007;94(10):1189–1200.
- Kaufmann M, von Minckwitz G, Smith R, et al. International expert panel on the use of primary (preoperative) systemic treatment of operable breast cancer: review and recommendations. J Clin Oncol. 2003;21(13):2600–2608.
- Masood S. Neoadjuvant chemotherapy in breast cancers. Womens Health (Lond). 2016;12(5):480–491.
- Burstein HJ, Curigliano G, Loibl S, et al. Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann Oncol. 2019;30(10):1541–1557.
- Cain H, Macpherson IR, Beresford M, et al. Neoadjuvant therapy in early breast cancer: treatment considerations and common debates in practice. Clin Oncol (R Coll Radiol). 2017;29(10):642–652.
- Denduluri N, Chavez-MacGregor M, Telli ML, et al. Selection of optimal adjuvant chemotherapy and targeted therapy for early breast cancer: ASCO Clinical Practice Guideline Focused Update. J Clin Oncol. 2018;36(23):2433–2443.
- Masuda N, Lee SJ, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147–2159.
- von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–628.
- Berg WA, Gutierrez L, NessAiver MS, et al. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology. 2004;233(3):830–849.
- Yeh E, Slanetz P, Kopans DB, et al. Prospective comparison of mammography, sonography, and MRI in patients undergoing neoadjuvant chemotherapy for palpable breast cancer. AJR Am J Roentgenol. 2005;184(3):868–877.
- Chagpar AB, Middleton LP, Sahin AA, et al. Accuracy of physical examination, ultrasonography, and mammography in predicting residual pathologic tumor size in patients treated with neoadjuvant chemotherapy. Ann Surg. 2006;243(2):257–264.
- Schulz-Wendtland R. Neoadjuvant chemotherapy-monitoring: clinical examination, ultrasound, mammography, MRI, elastography: only one, only few or all? Eur J Radiol. 2012;81(Suppl. 1):S147–S148.
- Schaefgen B, Mati M, Sinn HP, et al. Can routine imaging after neoadjuvant chemotherapy in breast cancer predict pathologic complete response? Ann Surg Oncol. 2016;23(3):789–795.
- Park J, Chae EY, Cha JH, et al. Comparison of mammography, digital breast tomosynthesis, automated breast ultrasound, magnetic resonance imaging in evaluation of residual tumor after neoadjuvant chemotherapy. Eur J Radiol. 2018;108:261–268.
- Atkins JJ, Appleton CM, Fisher CS, et al. Which imaging modality is superior for prediction of response to neoadjuvant chemotherapy in patients with triple negative breast cancer? J Oncol. 2013;2013:1–7.
- Peintinger F, Kuerer HM, Anderson K, et al. Accuracy of the combination of mammography and sonography in predicting tumor response in breast cancer patients after neoadjuvant chemotherapy. Ann Surg Oncol. 2006;13(11):1443–1449.
- Fatayer H, Sharma N, Manuel D, et al. Serial MRI scans help in assessing early response to neoadjuvant chemotherapy and tailoring breast cancer treatment. Eur J Surg Oncol. 2016;42(7):965–972.
- Dialani V, Chadashvili T, Slanetz PJ. Role of imaging in neoadjuvant therapy for breast cancer. Ann Surg Oncol. 2015;22(5):1416–1424.
- Hylton NM, Blume JD, Bernreuter WK, et al. Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy-results from ACRIN 6657/I-SPY TRIAL. Radiology. 2012;263(3):663–672.
- Yu N, Leung VWY, Meterissian S. MRI performance in detecting pCR after neoadjuvant chemotherapy by molecular subtype of breast cancer. World J Surg. 2019;43(9):2254–2261.
- Bhattacharyya M, Ryan D, Carpenter R, et al. Using MRI to plan breast-conserving surgery following neoadjuvant chemotherapy for early breast cancer. Br J Cancer. 2008;98(2):289–293.
- van der Noordaa MEM, van Duijnhoven FH, Loo CE, et al. Identifying pathologic complete response of the breast after neoadjuvant systemic therapy with ultrasound guided biopsy to eventually omit surgery: study design and feasibility of the MICRA trial (Minimally Invasive Complete Response Assessment). Breast. 2018;40:76–81.
- Giuliano AE, Connolly JL, Edge SB, et al. Breast cancer—major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(4):290–303.
- Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(10):1674.
- Regionalt Cancercentrum. Bröstcancer Nationellt vårdprogram; 2020; [cited 2020 Sep 22]. Available from: https://kunskapsbanken.cancercentrum.se/globalassets/cancerdiagnoser/brost/vardprogram/nationellt-vardprogram-brostcancer.pdf
- Di Cosimo S, Campbell C, Azim HA Jr., et al. The use of breast imaging for predicting response to neoadjuvant lapatinib, trastuzumab and their combination in HER2-positive breast cancer: results from Neo-ALTTO. Eur J Cancer. 2018;89:42–48.
- Saal LH, Vallon-Christersson J, Hakkinen J, et al. The Sweden Cancerome Analysis Network – Breast (SCAN-B) initiative: a large-scale multicenter infrastructure towards implementation of breast cancer genomic analyses in the clinical routine. Genome Med. 2015;7(1):20.
- Ryden L, Loman N, Larsson C, et al. Minimizing inequality in access to precision medicine in breast cancer by real-time population-based molecular analysis in the SCAN-B initiative. Br J Surg. 2018;105(2):e158–e168.
- Skarping I, Fornvik D, Heide JU, et al. Mammographic density changes during neoadjuvant breast cancer treatment: NeoDense, a prospective study in Sweden. Breast. 2020;53:33–41.
- Bossuyt V, Provenzano E, Symmans WF, et al. Recommendations for standardized pathological characterization of residual disease for neoadjuvant clinical trials of breast cancer by the BIG-NABCG collaboration. Ann Oncol. 2015;26(7):1280–1291.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247.
- Semiglazov V. RECIST for response (clinical and imaging) in neoadjuvant clinical trials in operable breast cancer. J Natl Cancer Inst Monogr. 2015;2015(51):21–23.
- McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 2012;22(3):276–282.
- Huober J, von Minckwitz G, Denkert C, et al. Effect of neoadjuvant anthracycline-taxane-based chemotherapy in different biological breast cancer phenotypes: overall results from the GeparTrio study. Breast Cancer Res Treat. 2010;124(1):133–140.
- Caudle AS, Gonzalez-Angulo AM, Hunt KK, et al. Impact of progression during neoadjuvant chemotherapy on surgical management of breast cancer. Ann Surg Oncol. 2011;18(4):932–938.
- Regionalt cancercentrum. Nationellt vårdprogram Bröstcancer; 2019; [cited 2020 Sep 22]. Available from: http://www.swebcg.se/wp-content/uploads/2016/09/nationellt-vardprogram-brostcancer.pdf
- Mieog JS, van der Hage JA, van de Velde CJ. Preoperative chemotherapy for women with operable breast cancer. Cochrane Database Syst Rev. 2007;18(2):CD005002.
- Mohiuddin JJ, Deal AM, Lund JL, et al. Evaluating the effectiveness of neoadjuvant chemotherapy in reducing mastectomy for women with breast cancer. JNCI Cancer Spectr. 2017;1(1):pkx004.
- von Minckwitz G, Blohmer JU, Costa SD, et al. Response-guided neoadjuvant chemotherapy for breast cancer. J Clin Oncol. 2013;31(29):3623–3630.
- Sato N, Masuda N, Morimoto T, et al. Neoadjuvant endocrine therapy with exemestane followed by response-guided combination therapy with low-dose cyclophosphamide in postmenopausal patients with estrogen receptor-positive breast cancer: a multicenter, open-label, phase II study. Cancer Med. 2018;7(7):3044–3056.
- Nitz UA, Gluz O, Christgen M, et al. De-escalation strategies in HER2-positive early breast cancer (EBC): final analysis of the WSG-ADAPT HER2+/HR– phase II trial: efficacy, safety, and predictive markers for 12 weeks of neoadjuvant dual blockade with trastuzumab and pertuzumab +/– weekly paclitaxel. Ann Oncol. 2017;28(11):2768–2772.
- Thompson AM, Moulder-Thompson SL. Neoadjuvant treatment of breast cancer. Ann Oncol. 2012;23(Suppl. 10):x231–x236.
- Reyal F, Hamy AS, Piccart MJ. Neoadjuvant treatment: the future of patients with breast cancer. ESMO Open. 2018;3(4):e000371.
- Partridge SC, Zhang Z, Newitt DC, et al. Diffusion-weighted MRI findings predict pathologic response in neoadjuvant treatment of breast cancer: the ACRIN 6698 Multicenter Trial. Radiology. 2018;289(3):618–627.
- Gebhart G, Gamez C, Holmes E, et al. 18F-FDG PET/CT for early prediction of response to neoadjuvant lapatinib, trastuzumab, and their combination in HER2-positive breast cancer: results from Neo-ALTTO. J Nucl Med. 2013;54(11):1862–1868.
- Keune JD, Jeffe DB, Schootman M, et al. Accuracy of ultrasonography and mammography in predicting pathologic response after neoadjuvant chemotherapy for breast cancer. Am J Surg. 2010;199(4):477–484.
- Early Breast Cancer Trialists' Collaborative G. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19(1):27–39.
- Volders JH, Negenborn VL, Spronk PE, et al. Breast-conserving surgery following neoadjuvant therapy—a systematic review on surgical outcomes. Breast Cancer Res Treat. 2018;168(1):1–12.
- O’Halloran N, Lowery A, Curran C, et al. A review of the impact of neoadjuvant chemotherapy on breast surgery practice and outcomes. Clin Breast Cancer. 2019;19(5):377–382.
- King TA, Morrow M. Surgical issues in patients with breast cancer receiving neoadjuvant chemotherapy. Nat Rev Clin Oncol. 2015;12(6):335–343.
- Fowler AM, Mankoff DA, Joe BN. Imaging neoadjuvant therapy response in breast cancer. Radiology. 2017;285(2):358–375.
- Pickles MD, Lowry M, Manton DJ, et al. Prognostic value of DCE-MRI in breast cancer patients undergoing neoadjuvant chemotherapy: a comparison with traditional survival indicators. Eur Radiol. 2015;25(4):1097–1106.
- Li W, Newitt DC, Yun B, et al. Tumor sphericity predicts response in neoadjuvant chemotherapy for invasive breast cancer. Tomography. 2020;6(2):216–222.
- Humbert O, Cochet A, Coudert B, et al. Role of positron emission tomography for the monitoring of response to therapy in breast cancer. Oncologist. 2015;20(2):94–104.
- Guggenmoos-Holzmann I, van Houwelingen HC. The (in)validity of sensitivity and specificity. Stat Med. 2000;19(13):1783–1792.
- Slanetz PJ, Moy L, Baron P, et al. ACR appropriateness criteria® monitoring response to neoadjuvant systemic therapy for breast cancer. J Am Coll Radiol. 2017;14(11S):S462–S475.
- Mghanga FP, Lan X, Bakari KH, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography-computed tomography in monitoring the response of breast cancer to neoadjuvant chemotherapy: a meta-analysis. Clin Breast Cancer. 2013;13(4):271–279.
- McDermott GM, Welch A, Staff RT, et al. Monitoring primary breast cancer throughout chemotherapy using FDG-PET. Breast Cancer Res Treat. 2007;102(1):75–84.
- Wang Y, Zhang C, Liu J, et al. Is 18F-FDG PET accurate to predict neoadjuvant therapy response in breast cancer? A meta-analysis. Breast Cancer Res Treat. 2012;131(2):357–369.
- Kim HJ, Im YH, Han BK, et al. Accuracy of MRI for estimating residual tumor size after neoadjuvant chemotherapy in locally advanced breast cancer: relation to response patterns on MRI. Acta Oncol. 2007;46(7):996–1003.
- Marinovich ML, Sardanelli F, Ciatto S, et al. Early prediction of pathologic response to neoadjuvant therapy in breast cancer: systematic review of the accuracy of MRI. Breast. 2012;21(5):669–677.
- Chen L, Yang Q, Bao J, et al. Direct comparison of PET/CT and MRI to predict the pathological response to neoadjuvant chemotherapy in breast cancer: a meta-analysis. Sci Rep. 2017;7(1):8479.
- Shin HJ, Kim HH, Ahn JH, et al. Comparison of mammography, sonography, MRI and clinical examination in patients with locally advanced or inflammatory breast cancer who underwent neoadjuvant chemotherapy. Br J Radiol. 2011;84(1003):612–620.
- Hashimoto BE, Morgan GN, Kramer DJ, et al. Systematic approach to difficult problems in breast sonography. Ultrasound Q. 2008;24(1):31–38.
- Hooley RJ, Scoutt LM, Philpotts LE. Breast ultrasonography: state of the art. Radiology. 2013;268(3):642–659.
- Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25(28):4414–4422.
