Abstract
Introduction
In recent decades, the use of pre-operative needle biopsy for breast cancer diagnosis has shifted. There is also an increased demand for availability of predictive factors. This study aims to quantify these changes.
Material and methods
From the Dutch nationwide pathology database (PALGA), all reports on breast cancer for five periods of 3 months between 1996 and 2016 were retrieved. Reports were categorised using automatic recognition of keywords. Classification was checked manually for the first 200 reports per period. The first 100 resected cases in each period underwent detailed investigation.
Results
For automatic analysis 34,639 reports were retrieved. Accuracy was 98% compared to manual assessment. Fine needle aspiration cytology (FNAC) decreased from 77% (1996) to 58% (2001), 34% (2006), 25% (2011), and 17% (2016). For detailed assessment, 498 cases were analysed. Diagnostic surgical excision decreased from 24% in 1996 to 3% in 2016, cases with only cytology from 65% to 1%, respectively. Cytology and core needle biopsy (CNB) were combined in 21% of cases in 2016. Pre-operative availability of ER status increased from 3% in 1996 to 36% in 2006 and 78% in 2016 (as compared to 47%, 92%, and 97% for post-operative availability, respectively) and for HER2 status from 0% to 13% and 66% (as compared to 1%, 89%, and 96% for post-operative availability, respectively).
Conclusion
Results suggest that nationwide, clinics prioritise reliability and availability of ER and HER2 status, replacing FNAC by CNB. However, for optimal treatment planning for all patients, availability of pre-operative receptor status warrants further improvement.
Introduction
The diagnostic workup of suspicious breast lesions has progressively changed over the last decades. The use of pre-operative histological biopsies has become ubiquitous, as patients and practitioners value maximal pre-operative diagnostic certainty ever more, and pre-operative knowledge of prognostic and predictive factors are increasingly important for an optimal treatment plan [Citation1]. Historically, fine needle aspiration cytology (FNAC) was the first available needle biopsy tool to evaluate mass lesions of the breast [Citation2]. In more recent years, core needle biopsy (CNB) has become commonplace. The tissue sample provided by CNB provides more diagnostic certainty, and prognostic and predictive factors such as oestrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) status are more readily available [Citation3]. While FNAC has a major advantage in results being rapidly available, it is also highly operator dependant [Citation3]. Trends towards more neo-adjuvant systemic therapy amplify the requirement of diagnostic certainty and availability of prognostic and predictive markers [Citation1]. The European Society of Medical Oncology Breast Cancer Guideline reflects this change in practice: its 2009 update includes the statement that a pathological diagnosis in breast cancer should be based on a CNB [Citation4]. In the USA, current NCCN guidelines for breast cancer screening also prefer CNB over FNAC [Citation5]. However, not all guidelines have followed. Amongst others, Dutch breast cancer guidelines consider FNAC an equivalent option to CNB for solid lesions [Citation6]. British NICE guidelines make no recommendation whatsoever on pre-operative biopsy, other than recommending triple diagnosis (imaging, physical examination, and biopsy) be performed in a single visit [Citation7].
We wanted to quantify the changes over time in the use of pre-operative needle biopsy in the Netherlands, including the type of biopsy performed, the availability of ER and HER2 status, and the number of pre-operative biopsies performed. This was done by using the Dutch Nationwide Pathology Database (PALGA), which is a nationwide registry of histopathology and cytopathology in the Netherlands. It contains all pathology reports written in the Netherlands. Anonymous excerpts of these reports can be requested for research purposes [Citation8].
Material and methods
PALGA was used to retrieve excerpts from all pathology reports of primary invasive breast carcinomas. Ductal carcinoma in situ (DCIS) in absence of invasive cancer was not included, because these reports will be harder to classify automatically and are not easily separated from invasive cases once included in the dataset. Furthermore, hormone and HER2 receptor status is not routinely established for DCIS only cases in the Netherlands. As a sample, the period of March through May of the years 1996, 2001, 2006, 2011, and 2016 was selected. These months were chosen as a sample in order to exclude the summer recess of the Dutch breast cancer screening program, as patient mix can be profoundly different during this period. Blocks of three months at five year intervals were chosen to reduce the number of reports to a workable number.
Excerpts included a patient identifier code, date of sample acquisition, type of sample (cytology vs. histology), macroscopic description, and conclusion of the report. Using key words and phrases, these reports were categorised as being: cytology, a histological needle biopsy, a resection specimen, a revision or external consultation on a previous excerpt, or impossible to classify. A list of key words and phrases (in Dutch, with English translation) is included as digital supplement. When key words or phrases from both histological needle biopsy and resection specimen were present, samples were considered to be resection specimens. Revisions/external consultations and excerpts impossible to classify were excluded. To verify accuracy of this procedure, the first 200 reports for each time period were manually categorised in the same categories, and compared for accuracy.
The first 100 patients with a report of a breast cancer resection in each time period were selected for additional detailed investigation. Using the patient identifier code, excerpts from all pathology reports concerning these patients between 1 year previous to the resection, and three months after the resection were retrieved. This period was chosen to include diagnostic biopsies performed before neo-adjuvant systemic therapy and re-excisions after incomplete resection. Individual review of the excerpts was performed to establish the nature of the excerpt: cytological biopsy, histological biopsy, lumpectomy, mastectomy, or re-excision (including mastectomy after incomplete lumpectomy). When excerpts contained sufficient data on multiple malignancies (left and right breast tumours) cases were split. Excerpts concerning only axillary lymph nodes were excluded. Availability and results of ER and HER2 status was extracted.
Data were analysed using IBM SPSS Statistics for Windows (V.25.0.0.1, IBM, Armonk, NY, USA). For the detailed manual assessment, the database was restructured from report based to patient based, by combining reports from the same patient into single ‘cases’. Trends over time in the proportion of patients undergoing cytology, histologic needle biopsy, both types of needle biopsy and surgical biopsy were tested with a Cochran–Armitage test for trend, as well as trends in the proportion of cases with available ER and HER2 status pre- and postoperatively ().
Results
For our automatic analysis, a total of 34,639 pathology reports were included. Of these 34,639 reports, 15,977 (46%) reports described resection, 5046 (15%) FNAC, 9495 (27%) CNB, and 4121 (12%) were impossible to classify using keywords and phrases. Most unclassifiable cases were not primary reports (e.g., only administrative information, or only results of secondary testing (mostly HER2)), a small proportion are valid cases automatic analysis failed to classify. The validity cheques showed 1–3% mistakes in automatic recognition (). Numbers of in- and excluded cases and reports are shown in .
Figure 1. Inclusion and exclusion of cases. aSamples contain all reports filed in March through April of the indicated year. bAutomatic analysis using keywords. cExcluded because report described a revision or external consultation. dManual validity check of the automatic process, performed on the first 200 reports per sample. eExcluded because reports did not contain a recent primary breast cancer (e.g., axillary surgery only, revision of older pathology specimen). fBilateral tumours in a single patient (parenthesis) were included as separate cases. gManual classification of all included reports. hTotal number of reports pertaining to the aforementioned number of cases.
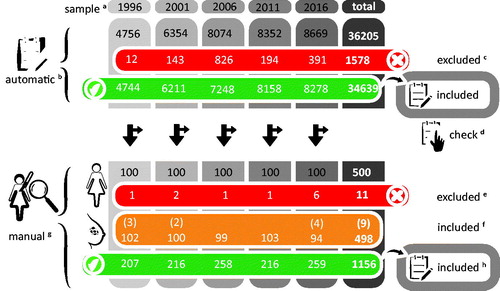
Table 1. Results of automatic keyword recognition of pathology reports.
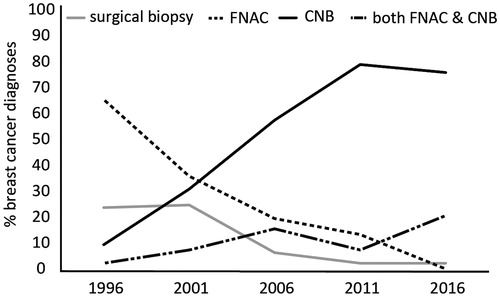
The percentages of FNAC vs. CNB of all needle biopsies gradually decrease in time from 77% vs. 23% in 1996 to 17% vs. 83% in 2016, respectively ().
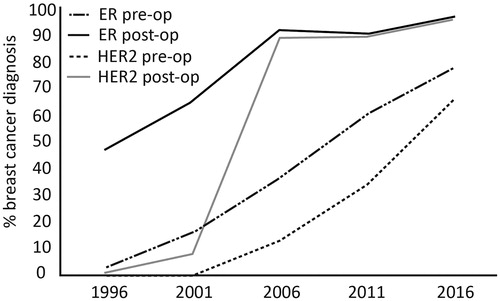
For our detailed manual analysis of five times 100 invasive carcinomas, there was an increase in the percentage of patients that had a pre-operative needle biopsy, from 76% in 1996 to 97% in 2016. At the same time, there was a shift towards more CNB which increased from 1% in 1996 as the first biopsy to 79% in 2016. Conversely, FNAC as the first biopsy dropped from 87% to 21%. The total percentage of patients that underwent CNB pre-operatively was 13% in 1996, and 96% in 2016. The percentage of patients that underwent FNAC preoperatively was 68% in 1996 and 22% in 2016. However, FNAC was increasingly combined with CNB: in 1996 only 3% underwent both FNAC and CNB pre-operatively, in 2016 this was 21%. Therefore, only 1% of patients underwent only FNAC in 2016, as compared to 65% in 1996 (, ). As a consequence of the increase in CNB, there was also a sharp increase in pre-operative availability of ER and HER2 status, from 3% and 0% in 1996 to 78% and 66% in 2016, respectively (, ). This rise should be placed in context to a concurrent increase of post-operative availability of ER and HER2 status, from 47% and 1% in 1996 to 97% and 96% in 2016, respectively. All these trends are statistically significant, p < .001. The percentage of breast conserving procedures as opposed to mastectomies increased over time from 50% in 1996 to 62% in 2016.
Table 2. Results of detailed manual assessment.
In contrast to the increase in needle biopsies, there is a sharp decrease in the use of surgical excision as a diagnostic tool: while this still occurred in 24% of cases in 1996, this was down to 3% in 2016.
Discussion
In the present study, data of more than 34,000 sequential pathology reports were studied from 1996 to 2016. We clearly showed a shift towards more CNB as preferential biopsy modality instead of FNAC for pre-operative invasive breast cancer diagnosis in the Netherlands. In fact, in 2016, 96% of patients underwent a CNB procedure pre-operatively: a dramatic change when compared to a mere 13% seen in 1996. Since the use of accelerated processing procedures for CNB has not been widely adopted yet in the Netherlands, and receptor status is not determined on FNAC in clinical practice, most breast clinics clearly value diagnostic accuracy and predictive and prognostic information over the optimisation of speed [Citation3,Citation9]. However, a sizable minority of clinics combine multiple biopsy techniques with 21% of patients undergoing both cytology and CNB. Another factor that may have influenced the relative increase in CNB use, is the implementation of digital mammography in the national breast cancer screening program between 2008 and 2009: this especially increased referrals for microcalcifications, which are less amenable to FNAC [Citation10].
The corresponding increase in the availability of hormone receptor and HER2 status can largely be explained by three factors. First, the shift from FNAC to CNB makes it much easier to determine hormone receptor and HER2 status pre-operatively. Second, the increasing use of neo-adjuvant systemic therapy requires reliable determination of these prognostic and predictive factors [Citation11]. Third, at the time of the first sample of this study (1996) HER2 status had not yet acquired its current role in breast cancer care, but was still subject to clinical trials [Citation12]. This is witnessed by the dramatic increase of postoperative availability of HER2 status from 8% in 2001 to 89% in 2006 ().
The data show a sharp decrease in diagnostic resections without previous needle biopsy between 2001 and 2006, with an additional decreasing trend after 2006. Two factors are likely to explain a large part of this change. First, the publication of the first national breast cancer screening and diagnosis guideline in 2000, requiring an attempt at pre-operative needle biopsy, and a conclusive preoperative diagnosis in at least 70% of cases [Citation13]. This percentages was increased to 90% of patients in the guideline revision of 2008 [Citation14]. Second, in 2004, the Dutch health care inspectorate started publishing results of quality indicators in Dutch hospitals, including the number of re-operations [Citation15]. Breast surgery was listed as the third most frequent type surgery followed by a re-operation. More use of needle biopsies was one of the strategies to decrease the number of re-excisions.
Our study was performed using a national database that covers 100% of pathology reports in the Netherlands. The database created for this study is therefore representative of the entire Dutch population. Both large scale automatic processing of pathology reports and smaller scale, manual ‘highly detailed’ processing of reports (in effect creating a nationwide consecutive case series) show the same trends in the use of pre-operative diagnostic biopsies: more biopsies per case, with an increase in CNB and a corresponding decrease in FNAC. A Dutch radiologic database study in 2013 found similar trends in a study group consisting of only screen detected lesions, over a shorter time period: a 60% relative drop in FNAC use from 1997–1998 to 2009–2010, an increase of 500% in CNB use, and a 96% decrease of surgical biopsy [Citation16].
The automatic recognition of reports in our study is an imperfect system. Manual accuracy verification showed that when a report is classified, the result is very accurate. However, when a report cannot be classified only 20–60% of reports are dismissed correctly, the other 40–80% of reports represent a ‘missed’ case: in the first three samples (1996, 2001, 2006) this was most often a missed resection, in the last two samples (2011 and 2016) a missed needle biopsy. As such, completeness of recognition in all five data samples is over 90%.
While it would be interesting to track results back to individual breast cancer clinics to see what type of clinic utilises which type of needle biopsy, PALGA does not provide this data to investigators. We would have expected to see FNAC used mostly in high volume breast clinics, where sufficient exposure of all involved personnel assures that the operator dependency of FNAC does not result in poor test characteristics [Citation3]. Furthermore, it would be interesting to correlate radiologic findings and the results of multidisciplinary discussions to the results of needle biopsies, especially concerning the conclusiveness of FNAC. However, as PALGA is strictly a pathology database, these data cannot be retrieved. It is also impossible to reliably determine which biopsies were stereotactic, MRI guided and which were ultrasound guided. Furthermore, while excerpts included the ‘macroscopic evaluation’ and ‘conclusion’ section of all reports, other fields (e.g., ‘microscopy’) were not provided. As a consequence, some information might be missing. However, it stands to reason that all information deemed crucial by the pathologist is included in the ‘conclusion’.
Internationally, very little data are available on current trends in breast biopsy use. Data from a study on the impact of ‘choosing wisely’ recommendation in American breast cancer patients do show a relatively high use of surgical biopsy, that decreases from 13.3% in 2010 to 7.9% in 2014 [Citation17].
Conclusion
This study shows very clear trends in the use of pre-operative biopsies in the Netherlands. Over time CNB increasingly replaced FNAC, and clinics that still use cytology combine it with CNB. This results in improved pre-operative availability of predictive and prognostic tests such as ER and HER2 status. The performance of diagnostic incisional or excisional biopsy without a prior attempt of needle biopsy has been all but eliminated.
Ethical approval
The study was reviewed and approved by the ethical review board of PALGA (the nationwide network and registry of histo- and cytopathology in the Netherlands).
Supplemental Material
Download MS Word (12.6 KB)Acknowledgments
The authors thank Dr. Annette Gijsbers-Bruggink for her assistance in extracting the raw datasets from PALGA, Dr. Marissa van Maaren, and Drs. Thijs van Vegchel for their assistance in recovering and interpreting historical documents and guidelines, Dr. Rogier Donders for his assistance with statistical analysis, and Dr. Marja ter Meer for her aid in image preparation and the design of the graphical abstract.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Duffy MJ, Harbeck N, Nap M, et al. Clinical use of biomarkers in breast cancer: updated guidelines from the European Group on Tumor Markers (EGTM). Eur J Cancer. 2017;75:284–298.
- Kline TS, Joshi LP, Neal HS. Fine-needle aspiration of the breast: diagnoses and pitfalls. A review of 3545 cases. Cancer. 1979;44(4):1458–1464.
- Willems SM, van Deurzen CH, van Diest PJ. Diagnosis of breast lesions: fine-needle aspiration cytology or core needle biopsy? A review. J Clin Pathol. 2012;65(4):287–292.
- Kataja V, Castiglione M, Group EGW. Primary breast cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(Suppl. 4):10–14.
- Gradishar W, Anderson BO, Abraham J, et al. NCCN guidelines: breast cancer, version 2.2019. Huntington, NY: National Comprehensive Cancer Network; 2019.
- Nortier JWR, Rutgers EJT, Sangen van der MJC, et al. Dutch multidisciplinary breast cancer guideline ('Richtlijn Mammacarcinoom'). Amsterdam: NABON. Available from: http://www.oncoline.nl/mammacarcinoom2012
- Quality standard [QS12]: breast cancer: National Institute for Health and Care Excellence; 2016. Available from: https://www.nice.org.uk/guidance/qs12
- Casparie M, Tiebosch AT, Burger G, et al. Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol. 2007;29(1):19–24.
- Bulte JP, Polman L, Schlooz-Vries M, et al. One-day core needle biopsy in a breast clinic: 4 years experience. Breast Cancer Res Treat. 2013;137(2):609–616.
- Nederend J, Duijm LE, Louwman MW, et al. Impact of transition from analog screening mammography to digital screening mammography on screening outcome in The Netherlands: a population-based study. Ann Oncol. 2012;23(12):3098–3103.
- Herold CI, Marcom PK. Primary systemic therapy in breast cancer: past lessons and new approaches. Cancer Invest. 2008;26(10):1052–1059.
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792.
- Mammacarcinoom: screening en diagnostiek. Utrecht: kwaliteitsinstituut voor de gezondheidszorg CBO; 2020.
- Richtlijn mammacarcinoom. Utrecht: Kwaliteitsinstituut voor de gezondheidszorg CBO; 2020.
- Het resultaat telt. Prestatie-indicatoren als onafhankelijke graadmeter voor de kwaliteit van in ziekenhuizen verleende zorg. Health Care Inspectorate (IGZ); 2004; [cited 2019 Jun 4]. Available from: https://www.medischcontact.nl/web/file?uuid=5ed1e4cf-82bd-4f06-8f7d-5d98ff842947&owner=1e836119-cfd1-4e33-a731-da3efbb2a701&contentid=53692
- van Breest Smallenburg V, Nederend J, Voogd AC, et al. Trends in breast biopsies for abnormalities detected at screening mammography: a population-based study in the Netherlands. Br J Cancer. 2013;109(1):242–248.
- Neuner JM, Nattinger AB, Yen T, et al. Temporal trends and regional variation in the utilization of low-value breast cancer care: has the choosing wisely campaign made a difference? Breast Cancer Res Treat. 2019;176(1):205–215.