Introduction
High-risk stage II colon cancer is defined in European and American guidelines as the presence of at least one of the following high-risk factors: T4 stage, tumor differentiation grade 3, bowel obstruction or perforation, presence of lymphovascular invasion (LVI), or <12 investigated lymph nodes [Citation1–3]. Perineural invasion (PNI), high tumor budding and compromised surgical margin are also strong predictors of disease recurrence [Citation3–6]. Circulating tumor DNA is a promising novel biomarker in colorectal cancer (CRC) and could be the most influential prognostic marker after radical surgery, but its predictive value has not yet been established [Citation7–9].
A disease-free survival benefit from fluoropyrimidine-based adjuvant chemotherapy in stage II colon cancer has been demonstrated in the Quasar trial [Citation10] and in systematic reviews, with an OS benefit in some [Citation10,Citation11] but not statistically significant in all [Citation12–14]. However, the inclusion and definition of high-risk stage II patients varies between studies and thus also the efficacy. Patients with stage II CRC harboring high microsatellite instability (MSI) show a good prognosis compared to patients with proficient mismatch repair and do not benefit from fluoropyrimidine-based adjuvant chemotherapy [Citation12,Citation15].
Several studies on stage II colon cancer have shown that each of these high-risk factors has a distinct impact on survival, and the relapse risk rises with the number of high-risk factors [Citation16–19]. The relative benefit of adjuvant chemotherapy also varies depending on the high-risk factor [Citation18–20].
Our aim was to assess the significance of each high-risk factor in stage II colon cancer and to develop an easily adaptable risk score, which could predict cancer-specific survival (CSS), with or without adjuvant chemotherapy.
Material and methods
Ethics
Auria Biobank and Clinical Informatics collects tissue samples and clinical data from patients treated at the Turku University Hospital and Satakunta Central Hospital districts in Finland. An independent validation cohort was obtained from the data registry at the department of oncology at Tampere University Hospital. Both Auria and Tampere obtain clinical data directly from operational electronic health record systems. The current study was approved by the Scientific Steering Committee of Auria Biobank, and research permission was granted by the Institutional Review Boards of Turku, Satakunta and Tampere hospitals.
Study population
All patients diagnosed with CRC during 2004–2017 in Turku University Hospital and during 2004–2012 in Satakunta Central Hospital were identified as described earlier [Citation21]. The validation cohort from Tampere was obtained differently, including all patients diagnosed with CRC who were referred to the department of Oncology during 2010–2018. pStage II colon cancers were identified according to the TNM 2010 system, excluding 65 patients with known MSI or OS <1 month. MSI immunohistochemistry was widely implemented in Turku region starting from 2015. R1 resection was defined as proximal, lateral or distal margin of ≤1mm. Right-sided colon cancer included tumors from the cecum to the transversal colon and left-sided from the splenic flexure to the sigmoid colon. Patients were classified having 0, 1 or 2+ comorbidities according to Charlson comorbidity classification [Citation22], using hospital ICD-10 codes.
Adjuvant chemotherapy data were available for Turku and Tampere cohorts. By our definition adjuvant therapy was considered given if at least three months of fluoropyrimidine- and/or oxaliplatin-based chemotherapy was completed.
Developing the risk score
An experimental risk score (RS) was created by the authors for stage II colon cancer. First, univariable Cox regression analysis for CSS was performed for each of the high-risk factors present: T4 stage, obstruction/perforation, <12 lymph nodes investigated, grade 3 tumor, R1 resection and presence of LVI and/or PNI. The electronic system reports LVI/PNI as either positive or negative and cannot distinguish between them. Second, since it has been shown that each high-risk factor affects survival in different magnitude [Citation17–20], each high-risk factor was weighted based on Cox hazard ratio (HR), modified from the method used by Charlson [Citation22]: Non-significant factors yield 0 points. Factors with HR 1.5–2.49 yield 1 point, factors with HR 2.5–3.49 yield 2 points, and those with HR 3.5 or more 4 points. These points are added together to calculate the RS. Third, RS was compared to the traditional practice where each high-risk factor yields 1 point [Citation16].
Statistics
The primary endpoint was CSS, defined as the period from diagnosis by a pathologist to date of death due to CRC (ICD10 codes C18–C20) or censored at the end of the follow-up in December 2018 or at time of non-CRC death. Date and cause of death was verified from Statistics Finland, an independent national statistical registry.
The HRs were analyzed with Cox regression using the enter method with 95% confidence interval (CI). High-risk factors with a p-value of <.05 were included in the RS model. The variances were analyzed either with Tukey’s ANOVA or Pearson’s chi-square test. Area under the receiver-operator curve (AUC or AUROC) was calculated assuming a positive correlation with high RS and short CSS. Survival estimates were calculated with the Kaplan-Meier log-rank method. All statistical analyses were performed with SPSS Statistics version 26 (IBM, Chicago, IL) software.
Results
Study population
Patients with pStage II colon cancer from Turku (N = 485) and Satakunta (N = 219) cohorts were used for model training. A separate Tampere cohort with 183 pStage II colon cancer patients was used for model validation (). The median follow-up time was 5.9 years in the training cohort and 3.5 years in the validation cohort. Patients in the validation cohort were younger (p < .001) and had received more adjuvant chemotherapy (p = .006). LVI/PNI was not routinely assessed in Satakunta cohort ().
Table 1. Demographics of the stage II colon cancer study populations.
Risk score predicts cancer-specific survival in stage II Colon cancer
In the training cohort, a T4 tumor, presence of LVI/PNI, tumor obstruction or perforation, <12 lymph nodes investigated and R1 resection were negatively associated with CSS. Specifically, R1 resection yielded 4 points (HR 9.8 (3.9–24.9), p < .001), T4 tumor 2 points (HR 2.6 (1.6–4.2), p < .001), obstruction/perforation 2 points (HR 3.0 (2.0–4.7), p < .001), inadequate lymph node sampling 1 point (HR 1.8 (1.2–2.7), p = .006) and LVI/PNI 1 point (HR 2.0 (1.0–3.9), p = .04). Points were added together to form the RS. Tumor grade was not associated with CSS, HR 0.8 (0.6–1.3), and neither was mucinous histology as compared to non-mucinous histology.
A high RS predicted shorter CSS in the training cohort (, AUC 0.72) and in the validation cohort (, AUC 0.69). Median RS in both training and validation cohorts was 1 (range 0–6). For comparison, simply adding the number of high-risk factors together from the six aforementioned high-risk factors, resulted in an AUC 0.68 in the training cohort, and no CSS difference was observed between patients with 2 or 3+ high-risk factors (). Comparing RS 0–1 and 2–6 AUC was 0.68.
Figure 1. Ten-year cancer-specific survival according to the risk score (RS). (A) Training cohort of 704 patients. (B) Validation cohort of 183 patients. (C) Prediction accuracy of the training and validation cohorts. (D) For comparison, traditional practice using the training cohort, where the number of high-risk factors are added together.
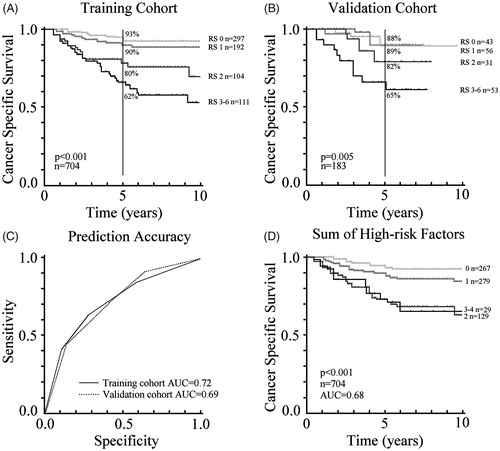
To minimize the effect of missing data () multivariable analysis was performed (Supplementary Table 1). A T4 tumor, presence of LVI/PNI, lymph node sampling <12 and R1 resection remained as independent prognostic factors, while tumor obstruction/perforation did not. Additionally, gender, presence of comorbidities and tumor location in left or right colon did not affect CSS, but age ≥75 years was independently associated with short CSS (HR 2.5 (1.5–4.3)).
Adjuvant chemotherapy
Patients who had received at least 3 months of chemotherapy and were aged <80 years were pooled from Turku and Tampere cohorts for this analysis (N = 510). Overall, in stage II colon cancer CSS did not differ in those who had received adjuvant treatment compared to those who had not (Supplementary Figure 1A). Only in patients with RS 2 or more, a longer CSS was observed in patients who had received adjuvant chemotherapy (HR 0.4 (0.2–0.9), p = .02, Supplementary Figure 1B). Those who had received adjuvant chemotherapy were 6 years younger and had less comorbidities.
Discussion
Numerous studies have been conducted to identify which patients with stage II colon cancer are at high risk of recurrence and would benefit from adjuvant chemotherapy, but the definition of high-risk stage II CRC is variable [Citation10–14,Citation17–20]. Our study is in line with previous findings [Citation16–19] showing that a higher number of high-risk factors in stage II colon cancer increases the risk of death from CRC, and we show that this is independent of tumor grade, gender or comorbidities. Here we introduce a RS which can be easily calculated and incorporated into clinical trial design to assess the risk of death from colon cancer.
Disease recurrence nomograms for colon cancer have been developed, but the nomograms include stage II–III patients and rely on complex calculation procedures with AUC values of 0.64–0.77 [Citation23–25] or biomarkers KRAS and BRAF with AUC 0.66–0.74 [Citation26]. Our RS is easy to calculate and has comparable predictive accuracy (0.72) to these models, even though AUC <0.80 is generally considered as limited diagnostic accuracy. CSS was chosen as the primary endpoint, since it could be verified from an independent source and is more precise than OS. The RS performed equally well in both training and validation cohorts, even though the validation cohort was not formally powered and the inclusion criteria for the cohorts were different; the training cohort was biobank-based from consecutively operated CRC patients and the validation cohort included selected patients referred for oncologist for possible adjuvant chemotherapy. This may explain the younger age and higher frequency of T4 and LVI positive tumors in the validation cohort.
Regular multidisciplinary meetings began during 2004 in Turku region and during 2007 in Tampere region, thus before the current study. In Satakunta the meetings were irregular. At that time in Turku and Satakunta, adjuvant chemotherapy was recommended if at least one of any high-risk factor was present in stage II colon cancer [Citation1], while in Tampere at least two high-risk factors were preferred for adjuvant therapy.
Our study did not support tumor differentiation grade as a prognostic factor for CSS. Similar findings have been reported previously for stage II CRC [Citation7,Citation18,Citation24] while in larger analyses with stage II–III CRCs, tumor grade 3 has remained as a significant prognostic factor [Citation16]. Grading of CRC may be somewhat inconsistent based on known interobserver variation bias, which could affect especially older tumor samples prior to the WHO 2010 classification [Citation27]. This issue has been addressed in the latest 2019 classification where CRC is graded as low or high. The results concerning R1 resection should be interpreted with caution, since some T3R1 tumors may be confused with T4aR0 tumors [Citation6].
It was of great interest to observe how the relative benefit of adjuvant chemotherapy improved as RS increased. CSS benefit from adjuvant therapy was observed only in RS 2–6 patients. Our retrospective study was designed to predict survival with standard adjuvant treatments used in the hospitals of the study, therefore causing selection bias since treatment allocation was not randomized. In the future, new emerging biomarkers including circulating tumor DNA [Citation7–9], could alongside our RS model further improve the tailoring of adjuvant chemotherapy in stage II colon cancer.
In conclusion, our validated RS model predicted survival comparably to other models and nomograms reported previously, without the need for additional biomarkers or complex mathematics. However, due to limited number of patients in the current study, these results should be reproduced in a larger adequately powered prospective study setting. The RS could easily be adapted to clinical trial design by using a structured pathology report.
Supplemental Material
Download MS Word (12 KB)Supplemental Material
Download MS Word (12.9 KB)Supplemental Material
Download MS Word (29.1 KB)Supplemental Material
Download TIFF Image (141.9 KB)Acknowledgments
The authors wish to thank all personnel at Auria Clinical Informatics and Clinical Informatics Unit at the Tampere University Hospital for data curating, gathering and analysis, especially Timo Lohiranta and Satu Järvinen. We also thank Adelaide Lönnberg for revising the English language and Antti Sykkö from Statistics Finland.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Labianca R, Nordlinger B, Beretta GD, et al. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi64–72.
- Costas-Chavarri A, Nandakumar G, Temin S, et al. Treatment of patients with early-stage colorectal cancer: ASCO Resource-Stratified Guideline. J Glob Oncol. 2019;5:1–19.
- Chan GHJ, Chee CE. Making sense of adjuvant chemotherapy in colorectal cancer. J Gastrointest Oncol. 2019;10(6):1183–1192.
- Knijn N, Mogk SC, Teerenstra S, et al. Perineural invasion is a strong prognostic factor in colorectal cancer: a systematic review. Am J Surg Pathol. 2016;40(1):103–112.
- van Wyk HC, Park JH, Edwards J, et al. The relationship between tumour budding, the tumour microenvironment and survival in patients with primary operable colorectal cancer. Br J Cancer. 2016;115(2):156–163.
- Wittekind C, Compton C, Quirke P, et al. A uniform residual tumor (R) classification: integration of the R classification and the circumferential margin status. Cancer. 2009;115(15):3483–3488.
- Tie J, Wang Y, Tomasetti C, et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med. 2016;8(346):346ra92–346ra92.
- Reece M, Saluja H, Hollington P, et al. The use of circulating tumor DNA to monitor and predict response to treatment in colorectal cancer. Front Genet. 2019;10:1118.
- Reinert T, Henriksen TV, Christensen E, et al. Analysis of plasma cell-free DNA by ultradeep sequencing in patients with stages I to III colorectal cancer. JAMA Oncol. 2019;5(8):1124.
- Gray R, Barnwell J, McConkey C, Quasar Collaborative Group, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370(9604):2020–2029.
- Sargent D, Sobrero A, Grothey A, et al. Evidence for cure by adjuvant therapy in colon cancer: observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2009;27:872–877.
- Meyers BM, Cosby R, Quereshy F, et al. Adjuvant chemotherapy for stage II and III colon cancer following complete resection: a cancer care ontario systematic review. Clin Oncol (R Coll Radiol). 2017;29(7):459–465.
- Figueredo A, Coombes ME, Mukherjee S. Adjuvant therapy for completely resected stage II colon cancer. Cochrane Database Syst Rev. 2008;(3):CD005390.
- O'Connor ES, Greenblatt DY, LoConte NK, et al. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J Clin Oncol. 2011;29:3381–3388.
- Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28:3219–3226.
- Osterman E, Glimelius B. Recurrence risk after up-to-date colon cancer staging, surgery, and pathology: analysis of the entire swedish population. Dis. Colon Rectum. 2018;61(9):1016–1025.
- Quah HM, Chou JF, Gonen M, et al. Identification of patients with high-risk stage II colon cancer for adjuvant therapy. Dis Colon Rectum. 2008;51:503–507.
- Babcock BD, Aljehani MA, Jabo B, et al. High-risk stage II colon cancer: not all risks are created equal. Ann Surg Oncol. 2018;25(7):1980–1985.
- Kumar A, Kennecke HF, Renouf DJ, et al. Adjuvant chemotherapy use and outcomes of patients with high-risk versus low-risk stage II colon cancer. Cancer. 2015;121(4):527–534.
- Verhoeff SR, van Erning FN, Lemmens VE, et al. Adjuvant chemotherapy is not associated with improved survival for all high-risk factors in stage II colon cancer. Int J Cancer. 2016;139(1):187–193.
- Heervä E, Carpelan A, Kurki S, et al. Trends in presentation, treatment and survival of 1777 patients with colorectal cancer over a decade: a Biobank study. Acta Oncol. 2018;57(6):735–742.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383.
- Weiser MR, Landmann RG, Kattan MW, et al. Individualized prediction of colon cancer recurrence using a nomogram. J Clin Oncol. 2008;26:380–385.
- Hoshino N, Hasegawa S, Hida K, et al. Nomogram for predicting recurrence in stage II colorectal cancer. Acta Oncol. 2016;55:1414–1417.
- Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol. 2004;22:1797–1806.
- Dienstmann R, Mason MJ, Sinicrope FA, et al. Prediction of overall survival in stage II and III colon cancer beyond TNM system: a retrospective, pooled biomarker study. Ann Oncol. 2017;28:1023–1031.
- Chandler I, Houlston RS. Interobserver agreement in grading of colorectal cancers-findings from a nationwide web-based survey of histopathologists. Histopathology. 2008;52(4):494–499.
