Abstract
Objective
Radiation therapy (RT) is used for the treatment of sacral chordoma, in combination with surgery or alone for unresected tumours, to improve local control (LC) and potentially overall survival (OS). The purpose of the present study was to evaluate efficacy and toxicity of proton therapy (PT), and/or intensity modulated radiation therapy (IMRT), particularly Tomotherapy, for sacral chordoma treatment.
Material: Between November 2005 and June 2018, 41 consecutive patients who were not included in clinical trials, received sacral chordoma radiation treatment in Institut Curie with Tomotherapy alone in 13 patients, and combined PT and Tomotherapy boost (Proton – Tomo) in 28 patients. RT was delivered as the exclusive local treatment in 11 patients, and as a post-operative complementary treatment in 30 patients.
Results
After a median follow-up of 46 months (range, 0–125 months), eight local relapses were observed, and seven patients developed distant metastasis (particularly bone and lung). The 2- and 5- year local relapse rates were 11.4% CI (0.65–22.2%) and 29% (10.5–47.4%), respectively.
Over the follow-up period, ten patients died (24.4%). The estimated 2- and 5-year OS rates were 91.4% CI (82.5–100%) and 74.5% (59.4–93.5%), respectively. Fibrosis, cauda equina syndrome, and pain were the most common late toxicities. The comparison between Tomotherapy alone and Proton – Tomo revealed that acute and late cystitis were significantly more frequent in the Tomotherapy group: SHR = 0.12 IC95% (0.01–0.90 [p = .04]), as well as late proctitis. A dosimetric comparison confirmed the interest of PT to spare rectum and bladder in this context.
Conclusion
RT remains essential to improve local control in sacral chordoma. The combination of proton and photon seems to improve organ at risk sparing, resulting in a decreased rate of reported late toxicities.
Introduction
Chordoma is the most prevalent primary malignant bone tumour of the spine and frequently affects the skull base. In Europe, incidence rates ranged from 0.18 to 0.52 per million persons per year [Citation1]. The sacrum is the most often affected site of the spine [Citation2]. Surgery with en bloc resection of the tumour is the mainstay of chordoma management. However, en bloc resection is not always possible [Citation3], and inadequate surgical margins remain the most significant prognostic factor for local and distant recurrence [Citation4,Citation5]. Therefore, high-dose radiation is often used in combination with surgery (if marginal resection or positive tumour margins microscopically [R1] or macroscopically [R2]) to improve local control (LC) and potentially overall survival (OS) [Citation6,Citation7], or alone for the unresected lesions, especially S1–S2 lesions [Citation8]. The tolerance dose of organ at risk (OAR) surrounding the spine, initially resulted in limitation of total radiation therapy (RT) dose that can safely be delivered to the tumour using the classical three-dimensional technique [Citation9]. In fact, most earlier studies revealed that the dose of RT did not exceed 60 Gy, and researchers reported poor LC [Citation10]. The use of three-dimensional proton beam therapy in combination with conformal photon irradiation or IMRT was the first step to increase the total delivered dose (>70 Gy relative biological effectiveness [RBE]), increasing LC and OS, as well as sparing surrounding OAR [Citation11–13]. More recently, three-dimensional proton beam therapy or intensity-modulated proton therapy (IMPT) also resulted in higher dose gradient without increasing the toxicities [Citation14,Citation15]. The purpose of the present study was to evaluate the efficacy and toxicity of high dose RT in the treatment of sacral chordoma. A dosimetric and statistical study was also performed to compare the toxicities obtained by the combination of rotational IMRT (Tomotherapy) and conventional PT versus Tomotherapy alone.
Material and methods
Patients
Patients with primary sacral chordoma receiving a definitive treatment using photon and/or PT between November 2005 and June 2018 were included. Irradiation was performed after surgery when complete resection was not obtained or without surgery when lesions appeared to be unresectable with acceptable morbidity. The diagnosis of chordoma has been proven by microscopic analysis of the biopsy or surgical specimen, looking mostly for immunohistochemistry: cytokeratin, EMA, S100 and sometimes brachyury. The initial staging evaluation included clinical examination and magnetic resonance imaging (MRI) of the pelvis. Some patients underwent contrast-CT or positron emission tomography (PET) for systemic assessment. The treatment of each patient was discussed by a multidisciplinary team.
Radiation therapy
All patients underwent simulation for radiation treatment planning purposes, which consisted of fabrication of a custom thermoplastic mask and a thermoformed mattress for immobilisation in the supine position followed by computed tomography imaging without and with contrast enhancement. The target volume and normal tissue structures were delineated on each axial CT slice, supplemented with fused diagnostic MRI. Hi-Art TomoTherapy and Eclipse photon therapy treatment planning system was performed for the photon plans and Isogray or Eclipse proton treatment planning system for proton plans. In cases with unresectable tumours treated with definitive RT, the gross tumour volume (GTV) included the primary tumour visible on MRI. In most cases, two clinical target volumes (CTVs) were delineated. The low-risk clinical target volume (LR-CTV = CTV1) encompassed all volumes at the risk of microscopic disease, including areas of preoperative tumour extension (taking into consideration the post-surgery structures shrinkage). A second volume, named high-risk clinical target volume (HR-CTV = CTV2) receiving a higher boost-dose of radiation. The CTV2 includes any residual macroscopic disease in the tumour bed after surgery or the GTV + 0.5 cm.
In the case of R1 resection, CTV2 includes the area of the positive resection margin as reconstructed by description after surgical and pathological reports. Some patients received helicoidal IMRT (Tomotherapy) only, using simultaneous integrated boost (SIB) with 6 MV X-rays for the totality of the treatment. For patients treated with combined proton-Tomotherapy approach, the CTV1 was treated with 201 MeV proton beams using the double scattering (DS) technique and more recently using pencil beam scattering (PBS) technique, and the boost in the CTV2 was delivered with Tomotherapy. The prescribed doses were 70–73.8 Gy RBE (1.8-2 Gy/fraction) for the HR-PTV and 52.2-54 Gy (1.8-2 Gy/fraction) for the LR-PTV. RBE used for protons = 1.1. Dose constraints to critical organs are summarised in .
Table 1. Dose constraints to organs at risk.
Follow-up
The follow-up was defined as the time from the end of RT to the last news and calculated using the reverse Kaplan Meier method. Each follow-up visit included a clinical physical examination. An MRI of the pelvic region was conducted every six months during the first five years, and every 12 months thereafter or until death. The Common Terminology Criteria for Adverse Events, Version 5.0 (CTCAE v5.0), formerly called the Common Toxicity Criteria (CTC or NCI-CTC), were used to assess the late toxicities of RT at each patient visit. All patients originally noted in an older version of the CTCAE have been reclassified to version 5.0 for this article. For patients with evidence of local-regional recurrence or distant metastasis, additional examination or imaging modalities were performed to confirm or exclude disease progression at the discretion of the treating physician.
Statistical analysis
Baseline characteristics were summarised as numbers and percentages for qualitative data while as mean and standard deviation or median with minimum and maximum for continuous variables. The chi-square or Fischer’s exact test was performed to examine the associations between categorical variables. The t-test was used to compare continuous variables if the common assumptions of the t-test were met, otherwise the Wilcoxon rank-sum test was utilised. Kaplan-Meier method was conducted to estimate OS, and the log-rank test was utilised to compare the two techniques of irradiation. OS was defined as the time between the end date of radiotherapy and the date of death for deceased patients. Patients still alive were censored at the date of their last news. Univariate Cox regression models were utilised to examine the relative influence of prognostic factors using the Cox procedure. The local relapse (LR) free interval was defined as the time between the end of RT and the first local recurrence for local relapse. Each late toxicity free interval, was defined as the time between the end of RT and the first late toxicity for each late toxicity. Surviving patients and patients who did not experience any LR/late toxicity were censored at the date of the last visit. As death is a competing risk for LR/late toxicity, assessment of LR risk/late toxicity based on survival analysis was performed according to methodological recommendations within the framework of competing risks, using cumulative incidence functions and models for sub-distribution hazards ratio (SHR). A Fine-Grey model was implemented to illustrate the regression models of SHRs. All univariate analyses were performed. The cumulative incidence function of LR was displayed by the Fine-Grey method for competing risks. A p-value lower than 0.05 was considered to be statistically significant. Analyses of the study were performed using software R 3.4.2 [Citation16].
Results
Patient characteristics
Forty-one consecutive patients, not included in clinical trials, were analysed in this study. Baseline characteristics are summarised in . The median age of the cohort was 64 years old, ranging from 37 to 86. Median tumour size at presentation was 255 cm3, interquartile range [IQR]: 136–525. Thirty patients (73%) were operated with a macroscopic residue for 15 of them (median residue size: 241 cm3, IQR: 46–643). Pre-surgical volume was significantly higher in the tomotherapy group than in the Proton - Tomo group (Supp Data 1). For all patients, the diagnosis was biopsy proved. The main histological subtype was myxoid, although it was mentioned in less than 50% of cases. The diagnosis of chordoma was most often confirmed by immunohistochemical analysis showing a positive EMA, cytokeratin, and S100. Brachyury was mentioned and positive in 12% of cases. Thirteen (32%) patients treated before 2013 received Tomotherapy alone; whereas after 2013, 28 patients (68%) received a combination of PT to CTV1 followed by Tomotherapy to CTV2 (Proton - Tomo). This combined protocol was developed in Institut Curie only from January 2013 in the context of a new gantry room. Among the 28 patients treated with the proton-photon combination, 24 (86%) were treated before March 2017 with the classical proton therapy technique (double scattering) while 4 (14%) were treated after March 2017 with the modern proton therapy technique (Pencil Beam Scanning). HR – CTV and HR – PTV were significantly higher in the tomotherapy group than in the Proton - Tomo group (Supp Data 1). None of the subjects had osteosynthetic material at the time of RT.
Table 2. Baseline characteristics of the 41 patients with sacral chordoma.
Locoregional control, distant metastases free-survival, and overall survival
The average overall treatment time was 64 days (range, 59-86 days). With a median follow-up of 46 months (range, 0–125 months), a total number of 15 patients (36.5%) relapsed. The median follow-up was: 99.7 months in the Tomotherapy alone group and 34.0 months in the Proton – Tomo group, respectively. In total, eight patients (19.5%) developed locoregional recurrences. For the whole cohort studied, the 2- and 5- year cumulative incidence of locoregional relapse rates were 11.4% CI95% [0.7–22.2%] and 29% [10.5–47.4%], respectively (Supp Data 2). For the Tomotherapy alone group, the 2- and 5- year cumulative incidence of locoregional relapse rates were 15.4% CI95% [0–35.8%] and 32% [4.2–59.9%], respectively. For the Proton - Tomo group, the 2- and 5- year cumulative incidence of locoregional relapse rates were 9.9%CI95% [0–23.4%] and 25.2% [1.4–49%], respectively. In addition, seven patients (17%) have developed distant metastasis: bone metastases in 2 patients and lung metastases in 6 patients. The 2- and 5-year metastatic relapse rates were 11.3% CI95% [0.0–23.8] and 27.4% [7.4–47.3] respectively. In the whole cohort studied, over the follow-up period, ten patients (24.4%) died. The estimated 2- and 5-year OS were 91.4% CI95% [82.5–100%] and 74.5% [59.4–93.5%], respectively (Supp Data 3). For the Tomotherapy alone group, the estimated 2- and 5-year OS were 84.6% CI95% [67.1–100%] and 69.2% [48.2–99.5%], respectively. For the Proton - Tomo group, the estimated 2- and 5-year OS were 95.7% CI95% [87.7–100%] and 80.3% [61.8–100%], respectively. There was no significant difference in overall survival between the 2 groups, HR = 0.61 [0.13–2.79], p = .52. Supp Data 4 summarises the main results of the univariate analysis performed to determine independent risk factors of local control. In the univariate analysis, the factors significantly associated with lower local relapse were post-surgical tumour volume (cc/100) SHR = 1.48 [1.19-1.85] and CTV-HR volume (cc/100) SHR = 1.09 [1.02-1.17]. The 2- and 5- year locoregional relapse rates appeared to be lower in the Proton-Tomo group than in the Tomotherapy alone group. But there was no significative difference in LR free interval between the 2 groups, (SHR = 0.73 [0.18; 2.96], p = .66).
Acute toxicities
Dermatitis, cauda equina syndrome, and pain were the most prevalent acute toxicities observed in our cohort. Three patients (7.3%) experienced grade 3 acute toxicity (dermatitis). The detailed results are shown in . The comparison between patients treated with Tomotherapy alone and patients treated with Proton-Tomo revealed that cystitis was significantly more frequent in tomotherapy group: 38% versus 0% severe toxicity, respectively (p < .001). Proctitis and acute cauda equina syndrome (any stage combined) also seemed to be more frequent in the Tomotherapy group than in the Proton - Tomo group: 38.5% versus 14.3% and 38.5% versus 25%. In contrast, dermatitis (grade > 2) seemed to be more frequent in Proton - Tomo group than in Tomotherapy group: 75% versus 54%, and pain was significantly more frequent in the Proton-Tomo group: grade 2: 3.5% and 0, grade 1: 78.5% versus 46.1%, respectively (p = .043).
Table 3. Incidence of major acute toxicities for the 41 patients.
Late toxicities
Fibrosis, cauda equina syndrome, and pain were the most prevalent late toxicities observed in our cohort. The detailed results are presented in . The comparison between patients treated with tomotherapy alone and patients treated with Proton - Tomo revealed that late cystitis were significantly less frequent in Proton - Tomo group: SHR = 0.12 IC95% [0.01-0.90], (p = .04). Late proctitis, cauda equina syndrome, fibrosis, and pain (any stage combined) were not being significantly different in the groups of tomotherapy alone and the Proton - Tomo. In the Proton - Tomo group, the findings revealed that the chronic pain decreased for two patients, and the chronic pain had completely disappeared for the other five patients. Chronic pain was also reduced in two patients in the Tomotherapy group, but no disappearance was recorded. During the last visit, we also noted in the Proton - Tomo group, a patient for whom cauda equina syndrome was reduced, and four patients who no longer had symptoms of cauda equina syndrome. No disappearance or even reduction of symptoms of cauda equina syndrome was observed in the patients in the Tomotherapy group.
Table 4. Incidence of major late toxicities for the 41 patients.
Dosimetric analysis
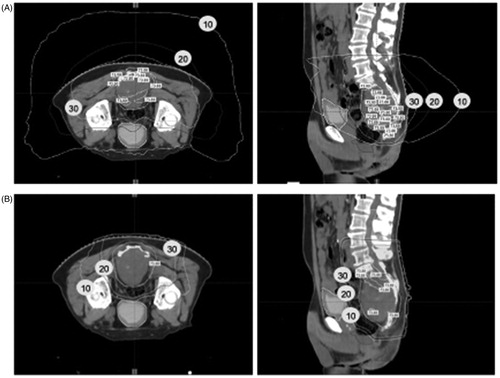
Coverage of the target volume was optimal regardless of the technique used. The mean V95 (target volume receiving at least 95% of the prescribed dose) were for GTV and HR-CTV: 100% (range, 97-100%) and 100 (range, 97-100%), respectively. The mean D95 (the dose received by 95% of the target volume) were for GTV and HR-CTV: 100% (range 96-100%) and 100 (97-101%), respectively. No significant differences were observed between the two groups. Moreover, a dosimetric analysis showed that for the same coverage rate of the provisional target volume PTV (95% of the volume covered by at least 95% of the prescribed dose) the mean dose in the bladder, rectum, and skin was significantly lower in the Proton - Tomo group than in the Tomotherapy group with 4.7 versus 24.8 Gy RBE (p < .001), 27.6 versus 45.6 Gy RBE (p = .008) and 17.5 versus 44.6 Gy RBE (p < .001), respectively (Supp Data 5). shows an example of comparative dosimetry between tomotherapy alone and Proton - Tomo for a same patient. In this case, the minimum, maximum, and mean doses in the bladder in Proton - Tomo versus Tomotherapy alone were 2 versus 8,1 Gy, 15.3 versus 29.2 Gy, 3.8 versus 15.3 Gy, respectively. Since pre-surgical volume, HR – CTV and HR – PTV were significantly higher in the tomotherapy group than in the Proton - Tomo group (Supp Data 1), a comparative Tomo – Proton plans for the 13 patients in the Tomotherapy group was made to better estimate the dose that would have been given to the various OAR. This analysis showed that doses to the rectum, anal canal, bladder and caudal equine would have been much lower if these 13 patients had been treated with the Proton-Tomo combination (Supp Data 6). The dose in OAR for these 13 comparative plans were similar to the doses in OAR given to the 28 patients actually treated with Proton – Tomo (Supp Data 7).
Figure 1. Tomotherapy vs. Proton – Tomo plan. A dosimetric analysis was performed to compare Tomotherapy alone (A) and Proton – Tomo (B) for the same patient. This example shows that the bladder and the skin are particularly well spared in Proton – Tomo plan vs. Tomotherapy alone. The white, black, and grey isodose lines correspond to the isodose 10 Gy, 20 Gy and 30 Gy, respectively.
Discussion
In the present study, we first analyse the outcome and the toxicities observed in patients treated with IMRT and/or PT for sacral chordoma. The diagnosis of chordoma was biopsy proven, and confirmed in the majority of case in immunohistochemical analysis. Brachyury is currently the most specific biomarker of chordoma but it was mentioned for only a few patients of our cohort. Moreover, recent studies evaluated the role of radiomics to precise the diagnosis of sacral chordoma, and differentiate with other pathologies which would require different treatment [Citation17–19]. As previously demonstrated [Citation4], larger post-surgical tumour volume and CTV-HR volume were significantly associated with a higher local relapse rate.
In 2006, Park et al. reported the efficacy and toxicities observed in 27 patients treated with the combination of IMRT and PT [Citation13]. Five-year locoregional control (LRC) was comparable to that found in our study (90%, Supp Data 8). However, 2- and 5-year LRC and OS observed in our study were lower than those observed in two other studies [Citation13,Citation20]. The plausible explanation for this could be that 32% of patients in our study cohort were treated with Tomotherapy alone, and it was already demonstrated that LRC and OS were lower when patients received IMRT only. Acute toxicities in our study cohort were usual, with a majority of dermatitis, cauda equina syndrome, and pain, and did not lead to premature end of RT. Late toxicities were also usual and comparable with other studies for cystitis and proctitis (Supp Data 8).
The main quality of our study was to compare the toxicities obtained with IMRT alone and the combination of PT and IMRT. Indeed, recent studies reported that the use of PT, either in combination with photon or alone, allowed to increase the dose delivered in the HR-CTV as well as sparing surrounding OAR [Citation6,Citation13–15]. The findings of our study postulated that acute cystitis was significantly lower, and acute proctitis and cauda equina syndrome appeared to be lower in the combination group than in IMRT alone group, but we can note a lack of power due to our small sample size. Late cystitis was also significantly lower and late proctitis appeared to be lower in the Proton - Tomo group than in IMRT alone group. These results were correlated to a dosimetric analysis, which confirmed the interest of PT to spare rectum and bladder in this context, as already shown in a previous study [Citation21,Citation22]. The median follow-up was different between both groups, it’s a limit of our study. Analysis was done by using survival analysis, but result after 36 months should be taken carefully (exclusively descriptive).
In our cohort, the choice to use a combination of protons and photons, rather than using protons alone, came from the fact that the only PT technique that could be used in Institut Curie in 2013 was DS and not intensity-modulated proton therapy (IMPT). Comparative dosimetric studies conducted in Institut Curie have shown that the combination of Proton - Tomo provides better coverage of target volumes, sparing all organs at risk (including the skin) than conventional PT alone. The IMPT will surely replace this combination when it becomes easily available in our centre [Citation23].
Since 2016 in Institut Curie, a phase II clinical trial is open and recruits (PROTONCHORDE 01 - ClinicalTrials.gov: NCT02802969), which will allow researchers to verify these results and answer other questions, always with the aim of improving the treatment of this rare tumour type. All the patients included in this clinical trial were excluded from the cohort studied in this article to avoid any statistical bias, as the construction of the clinical target volumes and radiation doses in the protocol are not the same as those used in routine clinical practice and reported in our study.
The strength of our study is the relatively important number of patients receiving sacral chordoma treatment in the same centre. The comparison of the two radiotherapy approaches is also very important to guide the choice of treatment, especially in the case of democratisation of access to PT. Limits are the shorter follow-up of the Proton - Tomo group (the first patient treated in 2013 only) and the high number of lost to follow-up in our IMRT only group.
Conclusion
This study therefore showed that the combination of proton therapy and tomotherapy is a very good option for the treatment of sacral chordoma, as it allows the risk of acute and late toxicities to be reduced compared to the use of tomotherapy alone. These results need to be confirmed in a prospective study with a larger number of patients.
Supplemental Material
Download MS Word (61.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Bakker SH, Jacobs WCH, Pondaag W, et al. Chordoma: a systematic review of the epidemiology and clinical prognostic factors predicting progression-free and overall survival. Eur Spine J. 2018;27(12):3043–3058.
- Whelan J, McTiernan A, Cooper N, et al. Incidence and survival of malignant bone sarcomas in England 1979-2007. Int J Cancer. 2012;131(4):E508–E517.
- Varga PP, Szövérfi Z, Fisher CG, et al. Surgical treatment of sacral chordoma: prognostic variables for local recurrence and overall survival. Eur Spine J. 2015;24(5):1092–1101.
- Kerekes D, Goodwin CR, Ahmed AK, et al. Local and distant recurrence in resected sacral chordomas: a systematic review and pooled cohort analysis. Glob. Spine J. 2019;9(2):191–201.
- Colangeli S, Muratori F, Bettini L, et al. Surgical treatment of sacral chordoma: en bloc resection with negative margins is a determinant of the long-term outcome. Surg Technol Int 2018;33:343–348.
- Rotondo RL, Folkert W, Liebsch NJ, et al. High-dose proton-based radiation therapy in the management of spine chordomas: outcomes and clinicopathological prognostic factors. J. Neurosurg Spine. 2015;23(6):788–797.
- Dial BL, Kerr DL, Lazarides AL, et al. The Role of Radiotherapy for Chordoma Patients Managed with Surgery: analysis of the national cancer database. Spine. 2020;45:E742–E751.
- Stacchiotti S, Sommer J. Chordoma Global Consensus Group. Building a global consensus approach to chordoma: a position paper from the medical and patient community. Lancet Oncol. 2015;16(2):e71–83–e83.
- Pennicooke B, Laufer I, Sahgal A, et al. Safety and local control of radiation therapy for chordoma of the spine and sacrum: a systematic review. Spine. 2016;41:S186–S192.
- Bjornsson J, Wold LE, Ebersold MJ, et al. Chordoma of the mobile spine. A clinicopathologic analysis of 40 patients. Cancer. 1993;71(3):735–740.
- Park L, DeLaney TF, Liebsch NJ, et al. Sacral chordomas: Impact of high-dose proton/photon-beam radiation therapy combined with or without surgery for primary versus recurrent tumor. Int. J. Radiat. Oncol. 2006;65(5):1514–1521.
- DeLaney TF, Liebsch NJ, Pedlow FX, et al. Long-term results of Phase II study of high dose photon/proton radiotherapy in the management of spine chordomas, chondrosarcomas, and other sarcomas. J Surg Oncol. 2014;110(2):115–122.
- Kabolizadeh P, Chen Y-L, Liebsch N, et al. Updated Outcome and Analysis of Tumor Response in Mobile Spine and Sacral Chordoma Treated With Definitive High-Dose Photon/Proton Radiation Therapy. Int J Radiat Oncol. 2017;97(2):254–262.
- Youn SH, Cho KH, Kim J-Y, et al. Clinical outcome of proton therapy for patients with chordomas. Radiat Oncol J. 2018;36(3):182–191.
- Aibe N, Demizu Y, Sulaiman NS, et al. Outcomes of patients with primary sacral chordoma treated with definitive proton beam therapy. Int J Radiat Oncol Biol Phys. 2018;100(4):972–979.
- R Development Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. Available from: http://www.R-project.org
- Yin P, Mao N, Zhao C, et al. Comparison of radiomics machine-learning classifiers and feature selection for differentiation of sacral chordoma and sacral giant cell tumour based on 3D computed tomography features. Eur Radiol. 2019;29(4):1841–1847.
- Yin P, Mao N, Zhao C, et al. A Triple-Classification Radiomics Model for the Differentiation of Primary Chordoma, Giant Cell Tumor, and Metastatic Tumor of Sacrum Based on T2-Weighted and Contrast-Enhanced T1-Weighted MRI. J Magn Reson Imaging. 2019;49(3):752–759.
- Yin P, Mao N, Wang S, et al. Clinical-radiomics nomograms for pre-operative differentiation of sacral chordoma and sacral giant cell tumor based on 3D computed tomography and multiparametric magnetic resonance imaging. BJR. 2019;92(1101):20190155.
- Chen Y-L, Liebsch N, Kobayashi W, et al. Definitive high-dose photon/proton radiotherapy for unresected mobile spine and sacral chordomas. Spine. 2013;38(15):E930–E936.
- Zabel-Du Bois A, Nikoghosyan A, Schwahofer A, et al. Intensity modulated radiotherapy in the management of sacral chordoma in primary versus recurrent disease. Radiother Oncol. 2010;97(3):408–412.
- Bobin M, Zacharatou C, Sargos P, et al. Helical tomotherapy of spinal chordomas: French Multicentric, retrospective study of a cohort of 30 cases. Radiat Oncol. 2017;12(1):32.
- Schneider C, Vyfhuis M, Morse E, et al. Dramatic response of a large sacral chordoma to intensity modulated proton beam therapy. Cureus. 2017;9(9):e1670.
