Abstract
Background
Comorbidities have shown to highly influence the outcome and risk of death of head and neck cancer patients. The purpose of this study was to examine the comorbidities among oral cavity squamous cell carcinoma (OSCC) patients, and to investigate the impact of comorbidities on overall survival (OS) and recurrence free survival (RFS).
Methods
Patients diagnosed with OSCC in Eastern Denmark in the period 2000–2014 and treated with curative intend were included. Patients data were linked to the Danish National Patients Register to identify comorbidities based on the Charlson Comorbidity Index (CCI) at the time of diagnosis and five years after diagnosis. Each patient was age-and sex-matched in a 1:10 ratio with an age and sex matched reference group.
Results
A total of 1,183 patients and 11,830 controls were included. Overall this study found comorbidities to be more common among OSCC compared to the reference group both at the time of diagnosis and five years after. The 5-year OS among patients with a CCI score of zero, one, two, and three or above was 60%, 44%, 41%, and 40%, respectively. Similarly, the multivariate cox-regression analysis showed that patients with increasing CCI score also had an increasing risk of death compared to patients with no comorbidities.
Conclusion
OSCC patients had significantly higher comorbidity burden at diagnosis and risk of developing additional comorbidities after diagnosis compared to the reference population. Survival outcomes decreased significantly with higher CCI.
Introduction
Oral cavity squamous cell carcinomas (OSCC) make up a noteworthy proportion of head and neck cancers with a global incidence of approximately 300,000 cases annually [Citation1–3]. In Denmark, OSCC accounts for more than 20% of the head and neck cancer cases and the incidence is increasing [Citation4,Citation5]. From 1980 to 2014 the incidence rate increased from 1.9 to 3.5 per 100.000 per year in the Danish population [Citation6]. However, despite the clinical improvements in diagnosis, surgical techniques, and treatment opportunities the 5-years survival rate has remained at approximately 50% [Citation1,Citation2,Citation6]. Tobacco and alcohol consumption are major risk factors for developing OSCCs [Citation1,Citation7,Citation8]. Further, it is shown that tobacco and alcohol consumption have a synergistic effect on carcinoma development [Citation8,Citation9]. Moreover, both tobacco and alcohol consumption are associated to several other diseases such as: pulmonary, cardiovascular, and hepatic [Citation10–12].
Comorbidities in head and neck cancer patients have a high impact on mortality with death from nonmalignant comorbidities reaching 35% at 5 years [Citation13–15] and have been shown to influence treatment decision in these patients [Citation16]. One of the most widely used indexes to predict survival among cancer patients is the Charlson Comorbidity Index (CCI) [Citation17–19]. CCI uses medical records to categorize comorbidities in weighted and summed score [Citation20]. The Danish health care system provides free access to diagnostic and treatment from general practitioners to public university hospitals for every citizen, financed by general taxes. All OSCC patients are treated in a close collaboration with oncologist accordingly to national guidelines by the Danish Head and Neck Cancer Group (DAHANCA) [Citation21,Citation22]. This provides the ideal basis for investigation the impact of comorbidities on patient with OSCC.
The purpose of this study was to report on the comorbidities based on the CCI score for OSCC patients at diagnosis and five years after cancer treatment in reference to an age- and gender matched population. Additionally, the impact of comorbidities on the overall survival (OS) and recurrence free survival (RFS) is reported.
Materials and methods
All patients from the Copenhagen Oral Cavity Squamous Cell Carcinoma (COrCa) database treated with curative intend were enrolled. The COrCa database contains information on 1,399 OSCC patients diagnosed or treated in Eastern Denmark (2.4 million residents; 46% of the Danish population) in the period 2000 to 2014. All patients registered with OSCC in the Danish Pathology Registry (DPR) and/or treated at the University Hospital of Copenhagen between 2000 to 2014 is included in the COrCa database [Citation23]. DPR is a national database which contains information on pathology performed since 1970 in Denmark [Citation24]. Using DPR numerous patient data could be extracted to the COrCa database [Citation23].. The precise content of the COrCa-database has been described previously [Citation23].
Data on comorbidities were derived from the Danish National Patient Register (DNPR). The DNPR contains information on all inpatient consultations after 1978 according to the ICD-classification in Denmark and implemented ambulatory hospital consultations after 1995 (Citation25,Citation26]. Data from the DNPR were combined with data from the COrCa database using the unique personal identification numbers of the patients [Citation26].
Each OSCC patient in the study was matched on age, sex, and calendar time with ten subjects from the general population (henceforth the ‘reference population’) identified through the DNPR. No subject from the reference population was diagnosed with an OSCC at the time of matching.
The medical comorbid disease burden was extracted by means of the Charlson Comorbidity Index (CCI) at time of diagnosis and five years after diagnosis or the last date of follow-up (the April 14th 2020). CCI consists of 19 medical conditions weighted from one to six based on its potential to influence mortality with a maximal score possible of 33 (see Supplementary Table 1) [Citation20]. The use of CCI in the DNPR has been reported to have an overall positive predictive value of 98%. The high overall positive predictive value indicates that the CCI ICD-classification diagnostic codes are coded in the DNPR very accurately [Citation27]. Records on patient comorbidities were extracted from the DNPR as mentioned above and stratified on CCI score (Supplementary Table 1). Records of any OSCC diagnosis were excluded from calculations of the CCI score. Additionally, age was not included in the CCI calculations.
For the survival analysis, patients were grouped according to their weighted CCI score at time of diagnosis comprising CCI score of zero, one, two, and three or above. Patients with a CCI score equal or above one were considered comorbid.
OS was defined as time from diagnosis to death by any cause. RFS was defined as time from diagnosis to recurrence or death by any cause. Patients who were alive at the last follow-up date were censored at this date. (See Supplementary Table 3, for STROBE [Citation28] check list)
Statistical analysis
Statistical analysis was performed in the statistical environment R statistics version 3.6.1 (Stanford University, Stanford, CA, USA) [Citation29]. A correlation of Union for International Cancer Control (UICC)-stage (8th edition) and CCI was conducted. Uni- and multivariate cox regression analyses were conducted for the following variables: age, sex, anatomical sublocation, CCI score, UICC, T-stage, N-stage, smoking, and treatment. An interaction analysis of CCI and UICC was also included. Kaplan–Meier analyses were used to asses 5-year OS and RFS based on CCI score. The calculations were performed with the ‘survival’ package and/or ‘survminer’ package [Citation30,Citation31]. Additionally, the odds ratio (OR) calculations were performed with the ‘fmsb’ package [Citation32]. We considered p-values less than .05 to indicate statistical significance.
Results
A total of 1,183 OSCC patients and 11,830 age- and sex matched controls were enrolled in this study. The majority were males (62.5%, n = 740) and median age at time of diagnosis was 62 years (range: 23–99 years). A detailed characterization of the patient cohort has been published previously [Citation23]. The mean CCI score of the patients at the time of diagnosis was 0.76 (95%CI 0.70–0.82) compared to a mean score of 0.27 (95%CI 0.26–0.28) in the reference population. Five years after diagnosis the mean CCI score had increased to 1.00 (95%CI 0.96–1.08) among the patients and 0.38 (95%CI 0.37–0.39) in the reference population. No correlation between UICC-stage and CCI score (p = .94) was identified ().
Table 1. Average CCI score at the time of diagnosis and five years after.
Comorbidities
At the time of diagnosis, the three most common comorbidities among the OSCC patients were non-metastatic malignancies (i.e. other than OSCC) (14%, n = 167), chronic pulmonary disease (6.6%, n = 78), and peripheral vascular disease (6.3%, n = 75) (). While in the reference population the most common comorbidities were cerebrovascular disease (4.1%, n = 325), chronic pulmonary disease (3.9%, n = 464), and myocardial infarction (3.1%, n = 370).
Table 2 . The odds ratio of specific comorbidities at the time of diagnosis was calculated for patients with OSCC compared to an age-and sex-matched reference group.
Patients with OSCC had a significantly higher risk of having a secondary malignancy both metastatic (OR = 90.7, 95%CI 11.5–716, p < .01) and non-metastatic malignancies (OR = 88.2, 95%CI 56.3–138, p < .01) compared to the reference population. Further, the OSCC patients had significantly increased risk of several other comorbidities including moderate-severe liver disease (OR= 10.1, 95%CI 4.93:20.7, p = .03), and chronic pulmonary diseases (OR = 18.0, 95%CI 13.3–24.5, p < .01), (). No comorbidities were significantly increased in risk among the reference population compared to OSCC patients at the time of diagnosis.
The most common comorbidities acquired after the OSCC treatment and up to five years after continued to be non-metastatic malignancies (24%, n = 284). Further, metastatic malignancies rose to be the second most common comorbidity (11%, n = 131) among the OSCC patients followed by chronic pulmonary disease (4.8%, n = 57) and peripheral vascular disease (4.7%, n = 56) (). Malignancies after treatment of OSCC continued to be of significant higher risk for the OSCC patients compared to the reference population with an OR for non-metastatic of 3.17 (95%CI 2.74–3.66, p < .01) and an OR for metastatic malignancy of 11.9 (95%CI 9.20 − 15.3, p < .01). Overall, the same comorbidities continued to have increased OR among OSCC patient compared to the reference population. Congestive heart failure did not have increased OR among the patients five years after OSCC treatment. Additionally, OSCC patients had significantly lower risk of developing connective tissue disease compared the reference population (OR= 0.48, 95%CI 0.24–0.98, p = .04). Connective tissue disease was the only comorbidity with lower risk of development among the OSCC patients compared to the reference population ( and Citation3).
Table 3. The odds ratio of specific comorbidities acquired from the date of the OSCC diagnosis up to five years after the diagnosis date was calculated for patients with OSCC compared to an age-and sex-matched reference group.
Survival
The 5-year OS was 60% among patients with a CCI score of zero. Patients with CCI scores of one, two, or three or above had a 5-year OS of 44%, 41%, and 40%, respectively (), and the 5-year rates for RFS were 51%, 37%, 33%, and 36%, respectively.
Figure 1. Kaplan–Meier survival curves stratified CCI score. (A) 5-year overall survival and (B) Recurrence free survival. *This analysis was not age-adjusted. CCI: Charlson Comorbidity Index.
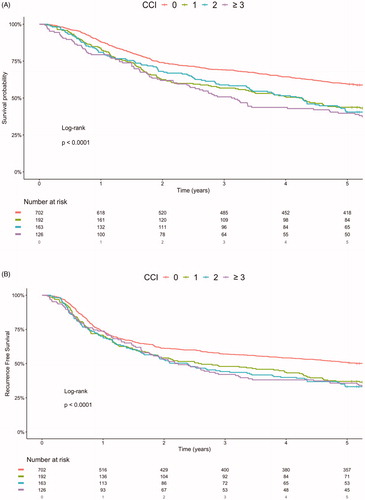
Multivariate cox-regression analysis adjusting for age, sex, anatomical sublocation, UICC-stage, smoking, and treatment showed significantly increased hazard ratios (HR) for patients with CCI score one, two, and three or above, compared to patients with a CCI score of zero for both OS and RFS (See and Supplementary Table 2). The effect of a CCI score of one had a HR for OS of 1.47 (95%CI 1.20–1.79), a CCI score of two had a HR for OS of 1.57 (95%CI 1.27–1.94) and a CCI score of three or above had a HR for OS of 1.98 (95%CI 1.58–2.48) compared to CCI score zero. While the HR of CCI score one for RFS was 1.33 (95%CI 1.10–1.62), of CCI score two for RFS was 1.48 (95%CI 1.21–1.80) and of CCI score three or above for RFS was 1.55 (95%CI 1.25–1.94) compared to CCI score zero (). UICC and CCI score had no significant interaction effect (p = .09) on the survival among the OSCC patients, while the interaction of CCI and treatment modality significantly impacted survival (p = .05).
Table 4. Univariate and multivariate analysis of factors affecting overall survival and recurrence free survival -->a.
Discussion
This retrospective population-based study found that OSCC patients had a significant higher comorbidity burden at diagnosis and higher risk of developing additional comorbidities after diagnosis compared to the general population. Additional, patients with a high CCI score had worse 5-year outcome compared to patients with no comorbidities. This was also evident in the multivariate cox regression which showed significantly increased HR for patients with CCI scores of one, two, and three or above compared to zero.
Overall, the comorbidities were predominantly associated with tobacco smoking and high alcohol intake beside additional malignancy, cardiovascular, pulmonary, hepatic, and ulcer diseases. This was seen both at the time of diagnosis and five years after diagnosis. Two large population-based studies on comorbidities in head and neck cancer patients reported similar findings [Citation33,Citation34]. Boje et al. [Citation33] (n = 12623) reported chronic pulmonary disease (7.6%), diabetes (7.2%), and cerebrovascular disease (6.9%) as the three most common comorbidities. Stordeur et al. [Citation34] (n = 8821) reported chronic pulmonary disease (19.4%), diabetes (8.0%), and peripheral vascular disease (5.6%) as the three most common comorbidities. In a recent study from our research group with oropharyngeal cancer patients [Citation35] the smoking-and high alcohol intake-associated comorbidities were most common among the HPV-negative patients compared to HPV-positive patients. In line with the latter findings a Taiwanese study by Yang et al. [Citation36] found malignancy (metastatic (40.1%) and non-metastatic (11.3%)) to be the most common comorbidity among EBV-positive nasopharyngeal cancer patients. This is corresponding to our findings where OSCC patients had a significantly increased OR of non-metastatic malignancy at the time of diagnosis with 14% of the study population having an additional malignancy. Additional malignancy increases the CCI score with two points, and an increased CCI score is correlated with worse OS among the OSCC patients. Clinicians need to be aware that OSCC patients have a great risk of additional malignancies when diagnosed with OSCC.
Timely treatment of the OSCC is essential to achieve optimal outcome for the OSCC patients. However, comorbidities have been implicated to affect time to treatment and thus survival. The study by Stordeur et al. [Citation37] found an significant association between CCI score and time to treatment in head and neck cancer patients and multiple studies report comorbidities as being a major risk factor for mortality and complications in head and neck surgery [Citation38,Citation39]. This demonstrate the need for further studies focusing on the correlation of comorbidities and time to treatment for OSCC patients.
We found the OS to be decreasing with increasing CCI among OSCC patients. Similar survival outcomes were reported in studies from Boje et al. and Storedeur et al. on head and neck cancer patients accessing comorbidities using CCI [Citation33,Citation34]. An interesting finding in our study was that CCI score had a greater impact on the HR for OS compared to the RFS. An explanation for this could be that comorbidities greatly increase the risk of death, while not necessary increasing the risk of recurrence. This is supported by the fact that rate of recurrence was similar among the different CCI groups: 31% of patients with a CCI score of zero had a recurrence, 30% of patients with CCI score of one had a recurrence, 36% of patients with a CCI score of two had a recurrence, and 25% of patients with a CCI score of three or above had a recurrence. An additional explanation could be that patients with a high CCI score dies before a potential recurrence can occur. This is supported by that the lowest recurrence rate was seen among patients with a CCI score of three or above.
The Danish health care system is universal and tax-financed. Patients are provided treatment independently of social status, income or insurance at public university hospitals and patient data is uniformly registered in nationwide registers. This diminishes selection bias. An additional strength of this study is the elaborated information included in the COrCa-database. The high quality of data was collected thorough examination of medical charts of each individual patient [Citation23]. A limitation of this study is the lower certainty factor in the beginning of the study period. The increase in certainty factor is based on the implementation of various image modalities like CT, MRI, and PET-CT as well as the sentinel node biopsy procedure leading to a possible stage migration [Citation40]. The technique evolution may also influence the comorbidities.
In this study the CCI score of the patients was calculated based on data from the DNPR. There are limitations to this since the data is based on the accuracy of input by clinicians and nurses and that only comorbidities treated in hospitals are registered, meaning that comorbidities treated in primary care facilities or general practitioners is not encoded. This will cause a small percentage of misclassifications and coding errors to be present. However, as previously mentioned the use of CCI in the DNPR has been reported to have an overall positive predictive value of 98% [Citation27].
In conclusion, OSCC patients have a significantly higher comorbidity burden at diagnosis and risk of developing additional comorbidities after diagnosis compared to age- and sex-matched reference population. The most frequent comorbidities among OSCC patients were all somewhat associated with tobacco smoking and high alcohol intake. Further, survival outcomes decreased significantly with higher CCI, most evident for OS compared to RFS.
Supplemental Material
Download MS Word (29.5 KB)Disclosure statement
The authors declare on conflicts of interest.
References
- Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol [Internet]. 2009;45(4-5):309–316.
- Chi AC, Day TA, Neville BW. Oral cavity and oropharyngeal squamous cell carcinoma-an update. CA Cancer J Clin [Internet]. 2015;65(5):401–421.
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86.
- Blomberg M, Nielsen A, Munk C, et al. Trends in head and neck cancer incidence in Denmark, 1978-2007: focus on human papillomavirus associated sites. Int J Cancer. 2011;129(3):733–741.
- Jakobsen KK, Grønhøj C, Jensen DH, et al. Increasing incidence and survival of head and neck cancers in Denmark: a nation-wide study from 1980 to 2014. Acta Oncol. 2018;57(9):1143–1151.
- Karnov KKS, Grønhøj C, Jensen DH, et al. Increasing incidence and survival in oral cancer: a nationwide Danish study from 1980 to 2014. Acta Oncol (Madr). 2017;56(9):1204–1209.
- Bundgaard T, Bentzen SM, Wildt J. The prognostic effect of tobacco and alcohol consumption in intra-oral squamous cell carcinoma. Eur J Cancer Part B Oral Oncol. 1994;30(5):323–328.
- Morse DE, Psoter WJ, Cleveland D, et al. Smoking and drinking in relation to oral cancer and oral epithelial dysplasia. Cancer Causes Control. 2007;18(9):919–929.
- Mello FW, Melo G, Pasetto JJ, et al. The synergistic effect of tobacco and alcohol consumption on oral squamous cell carcinoma: a systematic review and meta-analysis. Clin Oral Investig. 2019;23(7):2849–2859.
- Rocco A, Compare D, Angrisani D, et al. Alcoholic disease: liver and beyond [Internet]. World J Gastroenterol. 2014;20(40):14652–14659.
- Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43(10):1731–1737.
- Salvi S. Tobacco smoking and environmental risk factors for chronic obstructive pulmonary disease. Clin Chest Med. 2014;35(1):17–27.
- Rose BS, Jeong J-H, Nath SK, et al. Population-based study of competing mortality in head and neck cancer. J Clin Oncol. 2011;29(26):3503–3509.
- Mell LK, Dignam JJ, Salama JK, et al. Predictors of competing mortality in advanced head and neck cancer. J Clin Oncol. 2010;28(1):15–20.
- Argiris A, Brockstein BE, Haraf DJ, et al. Competing causes of death and second primary tumors in patients with locoregionally advanced head and neck cancer treated with chemoradiotherapy. Clin Cancer Res [Internet]. 2004;10(6):1956–1962.
- Bøje CR. Impact of comorbidity on treatment outcome in head and neck squamous cell carcinoma – a systematic review. Radiother Oncol. 2014;110(1):81–90.
- Bannay A, Chaignot C, Blotiere PO, et al. The best use of the charlson comorbidity index with electronic health care database to predict mortality. Med Care. 2016;54:188–194.
- Lee MS, Hsu CC, Wahlqvist ML, et al. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer. 2011;11:20.
- Extermann M. Measuring comorbidity in older cancer patients. Eur J Cancer. 2000;36(4):453–471.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Bilde A, Von Buchwald C, Johansen J, Danish Society for Head and Neck Oncology, et al. The Danish national guidelines for treatment of oral squamous cell carcinoma. Acta Oncol (Madr). 2006;45(3):294–299.
- Dansk Selskab for Hoved- og Hals Onkologi; DSHHO. Behandling af planocellulaert karcinom i mundhulen Nationale retningslinjer [National Guidelines for Treatment of Oral Cavity Sqaumous Cell Carcinoma]. [Internet]. [cited 2020 Apr 12]. Available from: https://www.dahanca.oncology.dk/assets/files/GUID_Mundhulekraeft.
- Jensen JS, Jakobsen KK, Mirian C, et al. The Copenhagen Oral Cavity Squamous Cell Carcinoma database: protocol and report on establishing a comprehensive oral cavity cancer database. Clin Epidemiol. 2019;11:733–741.
- Bjerregaard B, Larsen OB. The Danish pathology register. Scand J Public Health. 2011;39(7 Suppl):72–74.
- Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(7 Suppl):30–33.
- Thygesen LC, Daasnes C, Thaulow I, et al. Introduction to Danish (nationwide) registers on health and social issues: structure, access, legislation, and archiving. Scand J Public Health. 2011;39(7 Suppl):12–16.
- Thygesen SK, Christiansen CF, Christensen S, et al. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol. 2011;11(1):83.
- Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. BMJ. 2009;2(2):806–808.
- R: The R Project for Statistical Computing [Internet]. [cited 2019 Aug 8]. Available from: https://www.r-project.org/.
- Terry M, Therneau M. Package ‘survival’ title survival analysis. 2019.
- Package “survminer” Type Package Title Drawing Survival Curves using “ggplot2” [Internet]. 2019. [cited 2019 Sep 6]. Available from: https://cran.r-project.org/web/packages/survminer/survminer.pdf.
- Nakazawa M. fmsb: functions for medical statistics book with some demographic data. Cran. 2015.
- Boje CR, Dalton SO, Gronborg TK, et al. The impact of comorbidity on outcome in 12 623 Danish Head and Neck Cancer Patients: a population based study from the DAHANCA database. Acta Oncologica. 2013;52(2):285–293.
- Stordeur S, Schillemans V, Savoye I, et al. Comorbidity in head and neck cancer: is it associated with therapeutic delay, post-treatment mortality and survival in a population-based study? Oral Oncol. 2020;102:104561.
- Grønhøj C, Kronberg Jakobsen K, Kjaer E, et al. Comorbidity in HPV + and HPV- oropharyngeal cancer patients: a population-based, case-control study. Oral Oncol. 2019;96:1–6.
- Yang CC, Chen PC, Hsu CW, Chang SL, et al. Validity of the age-adjusted charlson comorbidity index on clinical outcomes for patients with nasopharyngeal cancer post radiation treatment: a 5-year nationwide cohort study. PLoS One. 2015;10(1):e0117323.
- Stordeur S, Schillemans V, Savoye I, et al. Comorbidity in head and neck cancer: is it associated with therapeutic delay, post-treatment mortality and survival in a population-based study? Oral Oncol. 2020;102: 104561.
- Shepherd SJ, Creber N, Mansour K, et al. Relationship between age, comorbidities and complications in head and neck cancer patients undergoing curative surgery. ANZ J Surg. 2019;90(5):851–855.
- Ferrier MB, Spuesens EB, Le Cessie S, et al. Comorbidity as a major risk factor for mortality and complications in head and neck surgery. Arch Otolaryngol – Head Neck Surg. 2005;131(1):27–32.
- Bilde A, Von Buchwald C, Therkildsen MH, et al. Need for intensive histopathologic analysis to determine lymph node metastases when using sentinel node biopsy in oral cancer. Laryngoscope. 2008;118(3):408–414.
