Abstract
Background
Metastatic patterns have been linked with prognosis in colorectal cancer. We aim to determine the distribution of metastases, their dynamics during disease and their prognostic impact for specific clinical treatment scenarios (resection of metastasis and/or systemic treatment, best supportive care).
Material and methods
978 patients diagnosed with metastatic colorectal adenocarcinoma treated at three oncological centers from 2006 to 2018 were included. Overall survival was assessed depending on tumor load, distribution of metastases and treatment of the patients.
Results
Most patients had single site metastasis (n = 684; 69.9%): 398 patients had liver (n = 398; 40.7%) and 103 patients had lung only metastasis (10.6%). The number of organs involved in metastases at diagnosis was highly prognostic (HR 0.77; CI 0.65, 0.90), whereas the additional gain of metastases during progression of the disease was not. The majority of patients (62.9–74.2%) with initial lung, liver or both metastases retained their initial metastatic status. In the overall population, lung only metastases were associated with the most favorable outcome (HR 0.64; CI 0.50, 0.81). This was also observed in patients receiving best supportive care (HR 0.45; CI 0.27, 0.75). Resection of lung only metastases resulted in longer median survival (102.2 months). A relevant survival difference in patients treated by systemic therapy alone was not observed. Lung only metastasis was associated with rectal cancer (p < .001) and RAS-mutation (p = .01); both, lung and liver metastasis were associated with time from diagnosis to first metastasis (p < .001).
Conclusion
The number of organs involved in metastasis at diagnosis but not the total cumulative number of involved organs is of prognostic relevance in colorectal adenocarcinoma. This prognostic relevant initial metastasis distribution remains unchanged in the majority of patients during the disease. However, the prognostic impact of the metastatic pattern is potentially altered by treatment modality.
Introduction
Colorectal cancer (CRC) has become an emerging health issue over the last centuries, accounting for approximately 10% of cancers and cancer related deaths globally [Citation1]. About 20% of patients show metastases at diagnosis and another 25% develop these later, implying a relevant worsening of overall survival (OS) [Citation2]. Estimation of OS, however, is difficult but essential for clinical practice. Numerous factors influencing survival in metastatic CRC (mCRC) have been described over the last years. Some of these factors, like RAS/BRAF mutation status or anatomic location of the primary tumor, gained high clinical relevance and are considered in clinical decision making for treatment options [Citation3]. Yet, there are other factors not routinely considered in clinical practice, which also influencing prognosis.
Two of these are metastatic pattern and tumor load as discussed recently [Citation4–6]. Especially tumor load, represented by the number of organs afflicted by metastases, showed potential for predicting survival. However, results of studies addressing this issue are conflicting, as some showed evidence for an association [Citation5] whereas others did not [Citation6]. In addition, it seems to be important which organ or organs are involved in metastatic spread. Prasanna et al. addressed this question and analyzed data of a large number of patients from two independent registries [Citation4]. Their results underline the association between clinical outcome and anatomic site of metastasis, but differ heavily between the two registries included. Additionally, only patients receiving treatment, without differentiation of treatment modality applied were included. Other studies showed worse prognosis for patients suffering from metastasis outside of typical organs, which are the liver and the lung [Citation5,Citation7–9]. Finally, other researchers looked at survival in a relatively large cohort but only included patients from a time before modern treatment options [Citation4,Citation10].
Modern treatments for mCRC are highly effective and potentially overrule the prognostic impact of other factors, including metastatic pattern. To our knowledge, metastatic pattern as a prognostic factor has only yet been investigated independent of treatment settings. We therefore investigated the impact of metastatic pattern on overall survival (OS) in patients suffering from mCRC within a well-documented, real-life patient population. Availability of treatment information allowed us to describe a potential difference of the prognostic impact of metastatic pattern in patient groups treated by modern treatments (systemic treatment with or without resection of metastasis or best supportive care only). Additionally, detailed knowledge of the change in metastatic pattern during the course of the disease enabled us to study the dynamic change of the sites of metastasis and its influence on prognosis. The metastatic patterns were correlated with other known prognostic factors like sidedness of the primary tumor, RAS mutation status and time from diagnosis to detection of the first metastasis. In doing so, we aim to contribute to the understanding of the prognostic value of metastatic patterns in patients treated with modern oncological treatments.
Material and methods
Patients
We retrospectively identified 2915 patients diagnosed with colorectal adenocarcinoma treated at three different oncological centers from January 2006 to March 2020. Clinical data were obtained either from monitored cancer registries (two centers) or by chart review (one center). Patients were eligible for analysis if they suffered from metastasized colorectal adenocarcinoma, if their first metastasis was diagnosed before Dec 31st 2018 and if complete clinical information regarding metastasis location at diagnosis and at the end of the disease was available. End of disease was defined by either death or last follow-up of the patient. The last follow up was March 31st 2020.
Documentation of metastases
Medical documentaries documented sites of metastases by reviewing radiologic records of each case. In centers 1 (Klinikum Wels-Grieskirchen) and 2 (Ordensklinikum Linz), documentation was done in a continuous manner and at least once a year until death of the patient. For patients in center 3 (Wilhelminenspital Wien), this was done in a retrospective manner for each patient in the cohort.
Statistical analysis
OS was defined as the time from the first histological proof of metastatic disease to death. OS of each patient cohort (e.g. lung metastases only) was compared to survival of the rest of the population (ROP), which was defined as all patients without metastasis in this particular organ or having more than one site of metastasis involved. Second comparison was against patients with metastases in one other single organ (SOM), and liver only was compared to lung only and reverse if applicable. Time-to-event data were analyzed by Kaplan–Meier product limit estimation. The reverse Kaplan–Meier method was used for calculation of median follow-up time. Chi-square test was used for comparing independent samples of categorical data. A two-sided level of significance of p < .05 was applied. For all analyses we used R version 3.6.3 (R core Team, 2020) with the packages survival (survival analyses, Kaplan–Meier-analyses, cox-regression, diagnostics), survminer (survival-curves, diagnostics), reporttools (pairwise fisher’s exact test) and /pander (creation of tables). Statistical procedures are documented in R-markdown and can be provided upon request.
Table 1. Patient and tumor characteristics (n = 978).
Ethics
This retrospective analysis was approved by the ethics committee of the Land Oberoesterreich (Upper Austria) (Ethics Committee Land Oberoesterreich, 1002/2020, February 12th 2020).
Results
Description of the patient cohort
Totally, 2915 patients with either localized or metastatic CRC were identified from three Austrian oncologic referral hospitals (1456, 1309,153 patients respectively). To obtain sufficient follow up, the cohort was truncated at Dec 31st 2018 (latest time point for diagnosis of the first metastasis). After exclusion of cases with local or locoregional CRC and data cleaning (inclusion of cases with complete information on metastases location and follow up), a total of 978 patients were included in the subsequent analysis (Supplementary Figure). A proportion of 18.9% (n = 185) of the patients were treated from 2005 to 2009, the remainder from 2010 to 2018. Median patient age was 69 years (IQR 60.00 − 76.00) and the majority were male (62.4%). Most patients suffered from a synchronous disease. A total of 68.5% patients received at least one line of systemic antineoplastic treatment. From these, 65.7% received at least one second and 37.3% at least one third line of treatment. The rate of metastasis resection was 20.8% in total, whereas resection without systemic therapy was performed in 7.0% of the patients. Roughly one quarter of patients (24.5%) received no treatment at all, either due to the patient’s wish or medical suggestion (best supportive care, BSC). Primary tumor locations were rather equally distributed (rectal 35.5%, left colon 33.7%, right colon 30.8%). Most common organ affected by metastasis at diagnosis was the liver (67.1%), followed by the lung (27.2%), and the peritoneum (17.3%). This sequence of affected organs remained during the course of the disease, which is shown by a cumulative rate of 74% of liver, 43.9% of lung and 22.7% of peritoneal metastasis acquired during the course of the disease. RAS mutational status was available in 78.4% of patients (). The median follow up time of the cohort was 69.19 months (CI 42.05, 99.31).
Metastatic pattern and dynamics of metastasis
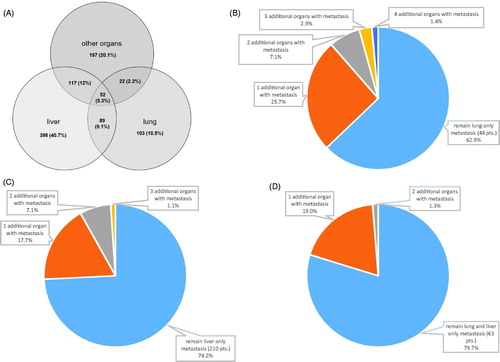
At diagnosis the majority of patients presented with single site metastasis (n = 684; 69.9%). The liver was the most common single site for metastasis (n = 398; 40.7%), followed by other locations with 20.1% (n = 197; group of patients with metastasis in lymph nodes, peritoneum, bones, brain and other rare sites) and the lung (n = 103; 10.6%). shows the numbers, frequencies and combinations of metastatic sites. The majority of patients retained their individual metastatic pattern during the course of the disease. From 70 patients with initial lung only metastases, 44 (62.7%) retained this metastasis status and 26 (37.1%) gained metastases in additional organs. From 283 patients with initial liver only metastases, 210 (74.2%) retained this metastatic status and 73 (25.8%) gained additional organ metastases (). Similar results are found in patients with initial metastases in both, lung and liver: from 79 patients, 63 (79.7%) kept this metastatic pattern and 16 (20.3%) developed additional organs with metastases (). To prevent potential skewing of these results by resection of metastasis, patients receiving resection were excluded from this analysis. In detail, from 103 patients with lung only metastasis, 33 received resection (32%), from 398 patients with liver only metastases, 115 received resection (28.9%), and from 89 patients with metastases in lung and liver, 10 (11.2%) received resection of metastases. In the whole cohort, the average number of metastases per patients was 1.4 at diagnosis and increased to a maximum of 2.2 during the course of the disease.
Clinical outcome according to tumor load
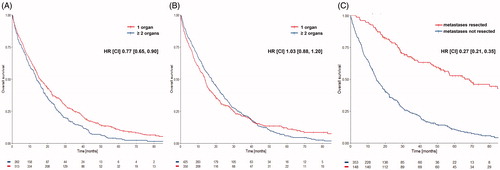
Patients with single organ metastasis (SOM) at diagnosis showed a longer median OS compared to patients with more than one organ with metastases (HR 0.77, CI 0.65, 0.90; ). This difference was not present when patients were compared by the maximal number of metastatic sites reached during the course of the disease (HR 1.03, CI 0.88, 1.20; ). Patients with metastasis resection again were excluded from this analysis, as resection influences prognosis of metastasized patients most and was more often done in patients with SOM (84.2%) than in patients with more than one organ affected by metastases (15.8%). In these patients with SOM, resection further increased median OS (HR 0.27, CI 0.21, 0.35). shows the comparison of patients with either lung only or liver only metastases with and without resection.
Prognostic implications of single organ metastasis
In the overall population, lung only metastasis displayed the longest median survival, when compared to the remainder of the population (ROP; HR 0.64; CI 0.50, 0.81), to other patients with SOM (HR 0.71, CI 0.55, 0.91), or to patients with liver metastasis only (HR 0.73, CI 0.57, 0.95). A similar comparison for liver metastasis alone showed no relevant differences (). The prognostic relevance of lung only metastasis remained significant in patients receiving no treatment. In this BSC cohort, patients with lung only metastases showed a median OS of 14.6 months, resulting in a HR of 0.45 (CI 0.27, 0.75) when compared to the ROP, a HR of 0.53 (CI 0.31, 0.89) when compared to patients with other SOM, and a HR of 0.53 (CI 0.31, 0.91) when compared to liver only metastasis. As for the whole population, liver only metastasis showed no relevant differences. Treatment by resection of metastasis (with or without systemic treatment) showed the longest median OS for lung only metastasis with 102.2 months, resulting in an HR of 0.71 (CI 0.40, 1.27) when compared to the ROP, a HR 0.70 (CI 0.39, 1.27) when compared to other SOM, and a HR of 0.74 (CI 0.41, 1.36) when compared to liver only metastasis. Much smaller differences were found for liver only metastasis () in a similar comparison. For patients treated by systemic therapy alone, lung only metastasis resulted in a survival of 27.1 months when compared to the ROP (HR 0.80, CI 0.58, 1.10), other SOM (HR 0.88, CI 0.64, 1.23), or liver only (HR 0.82, CI 0.58, 1.16). Differences in other organ locations were not as pronounced ().
Table 2. Median OS according to metastatic pattern in different treatment groups.
Metastasic pattern and selected clinicopathological features
Later occurrence of metastasis was associated with more single organ metastasis, lung metastasis and lung only metastasis. Short intervals from diagnosis to first metastasis was associated with liver metastasis in general. The rate of liver only metastasis declined over time. The numbers for time to first metastasis separated by 0 months (simultaneous occurrence of diagnosis and metastasis) are provided in . Rectal cancer was associated with lung metastasis in general and lung only metastasis in particular. Liver metastasis and other metastatic patterns showed no clear association with either location of the primary tumor or RAS mutation status (). Again, lung metastasis in general and lung only metastasis was more common in patients with RAS mutated tumors. Metastasis in other organs than liver and lungs were more frequent in the RAS wild-type population ().
Table 3. Metastatic pattern and clinicopathological features.
Discussion
To date only little is known about the incidence of metastasis to different organs and the dynamics of metastatic pattern during the course of colorectal cancer. In particular, assessment of the impact on prognosis of tumor load and metastatic patterns is difficult and sparely done. In our work, we present data of the distribution of colorectal cancer metastases including a dynamic aspect and describe their prognostic value on clinical outcome in a real-life patient group treated by modern oncological treatments.
Most patients presented with single organ metastasis at diagnosis (69.9%). The liver was most commonly affected as single organ (40.7%), followed by the lung (10.5%). These numbers are in the magnitude of the numbers observed by other authors, showing 47.5% for liver only and 6.0% for lung only metastasis, and 7.8% to both organs [Citation11]. The average numbers of metastatic sites per patient at diagnosis (1.4) and during the course of the disease (2.1) are also comparable to the results of these authors (1.4 vs 2.6/2.2) [Citation11]. The slightly less average number of metastatic sites at the end of the disease in our cohort might be due to different resection rates. Our results on organ specific metastasis incidence and its dynamics during the course of the disease confirm the data by Holch et al. with our patient population being twice as large. The similarity of the results underline the reliability of the results and the quality of data used for the analysis.
A more detailed view on the dynamics of metastasis during the course of the disease revealed that the majority of patients retain their initial metastatic pattern (). We show this for patients with lung only, liver only metastases or metastases in both, liver and lung. The numbers of additional metastatic sites is highest in the lung only metastases group, and lowest in the group with both, lung and liver metastases at diagnosis. This can be explained by the longer survival time of patients with lung only metastasis, leading to a higher risk for metastatic spread. To our knowledge, this has not been reported in the scientific literature in this detail.
Tumor load at diagnosis, measured by numbers of organs with metastases, was of prognostic relevance. In contrast, cumulative incidence of organs with metastases was not (). As shown in various reports, resection of metastases significantly reduces tumor load leading to improved prognosis even in unfavorable patient groups with BRAF mutations [Citation12–14]. To avoid an overestimation of the effect on OS by resection of metastases, patients receiving surgery for metastases were excluded for this analysis. This exclusion underlines the prognostic value of metastatic pattern at diagnosis ( and ). A separate analysis of SOM patients with and without resection showed the striking impact of resection on OS ().
Almost one third of the patients received BSC. Information on this clinical setting is sparsely available in the literature, as these patients are commonly excluded from such analysis. Second line treatment was given to 65.7% of the patients and 37.3% of patients received a third line therapy. These frequencies are well comparable to an analysis of subsequent treatments in the FIRE-3 trial population, where 69.9% and 43.2% received second and third line treatment, respectively [Citation15]. The slightly lower rates in our analysis can be explained by obvious differences between our real world cohort and a study population, including selected patients only.
Lung only metastasis was associated with a longer median OS compared to the ROP, to patients with other SOM or to patients with liver only metastasis. In general, the OS values in our analysis are well comparable to others with similar patient groups having a larger patient population [Citation4]. Slight differences might be explained by the fact that Prasanna et al. excluded patients not treated, which we did not. In contrast, the BSC population is of particular interest as it allows a closer view to the natural course of the disease. We show that the prognostic information of sole lung metastasis observed in the overall population is seen in this cohort as well with a survival of 14.6 months, which is meaningful longer than in patients with other distribution of metastases (). Similar results are obtained in patients receiving resection of metastases with or without systemic treatment. Numerically, the median OS in the lung only metastasis group is the longest observed in our population (102.2 months). This numerical difference is smaller in patients receiving systemic treatment only. However, the numbers of patients with lung only metastasis in these groups are small, leading to a wide range of the CI yet. Other metastatic patterns did not show such a correlation with prognosis ().
Summarizing our findings we assume that the prognostic impact of metastasis is altered by specific antitumor treatment and appears to be lowest in patients treated by systemic treatment alone. This implies a relative prognostic nature of metastatic spread. To our knowledge, this issue has not been considered from this perspective yet. In our analysis, lung metastases, especially lung only metastases, are associated with rectal cancer and metachronous disease (). Both, metachronous disease and rectal cancer, as part of ‘left sided colorectal cancer’, are known features for a good prognosis [Citation4,Citation16–18]. The cumulation of these factors may contribute to the long OS data obtained in this group. This is in contrast to liver and liver only metastasis, which is more common in synchronous than in metachronous disease, possibly causing the less beneficial impact of this metastatic pattern to the course of the disease. Further, RAS mutations are also associated with more lung only metastatic patterns, but not with liver metastasis (). The reason for this is not clear but can be found in the literature, mainly for KRAS [Citation4,Citation19,Citation20].
In conclusion, we found that within a real-life population the initial sites of metastatic spread is retained in the majority of the patients over the course of the disease in mCRC. Moreover, only the initial but not the cumulative metastatic pattern is of prognostic relevance, which in turn is assumed to be of different impact depending on the specific treatment setting. Lung as a single organ with metastases represents the group of patients with the longest median survival, even if patients receive BSC. The results of our study contribute to understanding of the prognostic value of metastatic patterns in colorectal cancer.
Limitations of the study
Several potential limitations should be considered when interpreting the results of our study. Foremost, it is an explorative study, which implies the risk of selection and information bias. The latter is enhanced by possibly different methods for data collection in the three participating centers, which would mainly influence disease dynamics like progression of disease. By looking at OS as an endpoint, we reduced biases based on these differences. Additionally, metastasis in the peritoneum and lymph nodes are difficult to detect clinically. We therefore focused on liver and lung metastases, which are reliably detected by clinical imaging. The information on BRAF mutational status was low and due to this missing information we cannot assess the impact of this relevant patient group on our data. The same holds true for the mismatch repair status. This is mainly due to the fact that these biomarkers have become clinically relevant for treatment decisions only recently and will therefore be able to be studied from now on.
Author contributions
All authors contributed to the conception, design and interpretation of the data.
DN, JT and HR provided the data.
PK and HW partly drafted and proofread the manuscript.
HR did the statistical analysis and wrote the manuscript.
The final version was approved by all authors.
Supplemental Material
Download MS Word (11.2 KB)Supplemental Material
Download PDF (186.4 KB)Acknowledgments
The authors thank D. Zauner, M. Pötscher and F. Knotz for their support with data management.
Disclosure statement
The authors report no conflicts of interest.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon request.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J Clin. 2018;68(6):394–424.
- Vatandoust S, Price TJ, Karapetis CS. Colorectal cancer: metastases to a single organ. WJG. 2015;21(41):11767–11776.
- Yoshino T, Arnold D, Taniguchi H, et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol. 2018;29(1):44–70.
- Prasanna T, Karapetis CS, Roder D, et al. The survival outcome of patients with metastatic colorectal cancer based on the site of metastases and the impact of molecular markers and site of primary cancer on metastatic pattern. Acta Oncol (Madr). 2018;57(11):1438–1444.
- Elias D, Sideris L, Pocard M, et al. Incidence of unsuspected and treatable metastatic disease associated with operable colorectal liver metastases discovered only at laparotomy (and not treated when performing percutaneous radiofrequency ablation). Ann Surg Oncol. 2005;12(4):298–302.
- Carpizo DR, D’Angelica M. Liver resection for metastatic colorectal cancer in the presence of extrahepatic disease. Ann Surg Oncol. 2009;16(9):2411–2421.
- Damiens K, Ayoub JPM, Lemieux B, et al. Clinical features and course of brain metastases in colorectal cancer: an experience from a single institution. Curr Oncol. 2012;19:254–258.
- Koppe MJ, Boerman OC, Oyen WJG, et al. Peritoneal carcinomatosis of colorectal origin: Incidence and current treatment strategies. Ann Surg. 2006;243(2):212–222.
- Nozue M, Oshiro Y, Kurata M, et al. Treatment and prognosis in colorectal cancer patients with bone metastasis. Oncol Rep. 2002;9:109–112.
- Riihimaki M, Hemminki A, Sundquist J, et al. Patterns of metastasis in colon and rectal cancer. Sci Rep. 2016;6(1):29765.
- Holch JW, Demmer M, Lamersdorf C, et al. Pattern and dynamics of distant metastases in metastatic colorectal cancer. Visc Med. 2017;33(1):70–75.
- Prasanna T, Wong R, Price TJ, et al. Metastasectomy and BRAF mutation: an analysis of survival outcome in metastatic colorectal cancer. J Clin Oncol. 2019;37(15_suppl):3531.
- Rajakannu M, Magdeleinat P, Vibert E, et al. Is cure possible after sequential resection of hepatic and pulmonary metastases from colorectal cancer? Clin Colorectal Cancer. 2018;17(1):41–49.
- Shah SA, Haddad R, Al-Sukhni W, et al. Surgical resection of hepatic and pulmonary metastases from colorectal carcinoma. J Am Coll Surg. 2006;202(3):468–475.
- Modest DP, Stintzing S, Von Weikersthal LF, et al. Impact of subsequent therapies on outcome of the FIRE-3/AIO KRK0306 trial: First-line therapy with FOLFIRI plus cetuximab or bevacizumab in patients with KRAS wild-type tumors in metastatic colorectal cancer. J Clin Oncol. 2015;33(32):3718–3726.
- Goey KKH, Sørbye H, Glimelius B, et al. Consensus statement on essential patient characteristics in systemic treatment trials for metastatic colorectal cancer: supported by the ARCAD Group. Eur J Cancer. 2018;100:35–45.
- Kumar R, Price TJ, Beeke C, et al. Colorectal cancer survival: an analysis of patients with metastatic disease synchronous and metachronous with the primary tumor. Clin Colorectal Cancer. 2014;13(2):87–93.
- Venook AP, Ou F-S, Lenz H-J, et al. Primary (1°) tumor location as an independent prognostic marker from molecular features for overall survival (OS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB/SWOG 80405 (Alliance). J Cin Oncol. 2017;35(15_suppl):3503.
- Tie J, Lipton L, Desai J, et al. KRAS mutation is associated with lung metastasis in patients with curatively resected colorectal cancer. Clin Cancer Res. 2011;17(5):1122–1130.
- Yaeger R, Cowell E, Chou JF, et al. RAS mutations affect pattern of metastatic spread and increase propensity for brain metastasis in colorectal cancer. Cancer. 2015;121(8):1195–1203.