Abstract
Background
Overall survival has improved significantly in patients with human epidermal growth receptor 2 (HER2)-positive breast cancer due to the use of the monoclonal antibody trastuzumab blocking HER2. However, patients may develop trastuzumab-induced cardiotoxicity (TIC) leading to congestive heart failure. Here we assessed whether analysing NT-proBNP and assessment of electrocardiography (ECG) could detect TIC during trastuzumab therapy.
Methods
One hundred thirty-six patients undergoing adjuvant, neoadjuvant or palliative chemotherapy and HER2 blockade for HER2-positive breast cancer were prospectively assessed with echocardiography, ECG and N-terminal – pro hormone B-type natriuretic peptide (NT-proBNP) testing at baseline and at 6 and 12 months of trastuzumab therapy. TIC was defined as a left ventricular ejection fraction (LVEF) of less than 50% and a decline from baseline of ≥10 units.
Results
Six patients developed TIC under 12 months of trastuzumab therapy (incidence 4.4%). NT-proBNP increased from 198.8 ± 64.0 pg/ml to 678.7 ± 132.4 pg/ml (p < .05) in TIC patients. With a cut-off point of 276.5 pg/ml for NTproBNP and increase in NT-proBNP by 75.8 pg/ml from baseline the sensitivity was 100% and the specificity 95% to detect TIC. Compared with controls, TIC patients were older (68.3 ± 1.1 years and 56.2 ± 1.4 years, respectively; p < .01), had more often diabetes mellitus (OR = 63.5, 95% CI: 5.63–915, p < .01) and atrial fibrillation (OR = 12.3; 95% CI: 1.89–74.62; p < .05) and had lower baseline LVEF (57.1 ± 1.4% and 61.4 ± 0.3%, respectively; p < .001). Abnormal ECGs were common in patients developing TIC.
Conclusions
Measuring changes in NTproBNP may be used to monitor patients for TIC under trastuzumab therapy. Patients with a cardiovascular risk profile are more at risk of developing TIC.
Keywords:
Introduction
Human epidermal growth factor receptor 2 (HER2) overexpressing breast cancer account for around 15% of all breast cancer [Citation1,Citation2]. While historically HER2 positivity was a prognostically negative factor for survival, it was shown that combining trastuzumab, a recombinant monoclonal antibody against HER2, with chemotherapy led to a significant decrease in mortality in women with metastatic breast cancer [Citation3,Citation4]. This was followed by studies showing that combining chemotherapy with trastuzumab in the adjuvant setting also led to a significant better overall survival in HER2-positive breast cancer [Citation5]. The most important side effect of trastuzumab treatment is cardiotoxicity resulting in congestive heart failure (CHF) [Citation6]. The underlying mechanisms behind trastuzumab-induced cardiotoxicity (TIC) are not fully understood but it is suggested that trastuzumab may interfere with DNA repair, cardiac and mitochondrial functions and autophagy leading to oxidative stress in myocytes [Citation7,Citation8]. In the early days, trastuzumab was administered in conjunction with anthracyclines, which resulted in 27% of patients developing TIC [Citation3]. The risk was substantially lower when trastuzumab was given alone or together with paclitaxel and not given concomitantly with anthracyclines [Citation3]. Today, trastuzumab is not combined with anthracyclines, however, 3–7% of patients still develop TIC [Citation6]. While trastuzumab infusion does not seem to induce acute atrial and ventricular depolarisation and repolarisation disturbances [Citation9,Citation10], previous studies have suggested increased risk of arrhytmogenic side effects from trastuzumab including sick sinus syndrome, non-sustained ventricular tachycardia and ventricular bigeminal rhythm [Citation11,Citation12]. Also, studies suggest that trastuzumab may induce prolongation of the time from electrical activation to myocardial contraction [Citation13].
Studies show that the amino-terminal fragment of brain natriuretic peptide (NT-proBNP) may be used to screen people for CHF [Citation14]. A cut-off value for NT-proBNP at 150 pg/ml has a sensitivity of 100%, a specificity of 79.5%, a positive predictive value (PPV) of 10.1% and a negative predictive value (NPV) of 100% to detect CHF in the general population [Citation14]. In patients with signs of CHF, the cut-off value of 150 pg/ml has a sensitivity of 94%, a specificity of 40%, a PPV of 48% and an NPV of 92% [Citation15]. In Sweden, echocardiography is performed regularly before and during trastuzumab therapy in order to detect TIC. TIC is often reversible after trastuzumab treatment has been discontinued and after CHF medication has been initiated, however, studies suggest that the long-term risk of CHF may be enhanced [Citation16]. Monitoring TIC with echocardiography is time consuming for patients and a burden for the health care system in view of the low risk to develop TIC. Previous studies have assessed whether biomarkers, including troponin I (TNI), troponin T (TNT) and NT-proBNP, instead of echocardiography, may be used to monitor heart function during trastuzumab therapy [Citation17,Citation18]. Ponde et. al. showed that TNT and NT-proBNP could not be used to predict the development of TIC in anthracycline-naïve patients with HER2-positive breast cancer treated with trastuzumab and paclitaxel in the neoadjuvant setting [Citation17]. In the adjuvant setting, patients experiencing TIC developed a non-significant increase in NT-proBNP [Citation19]. To note, NT-proBNP may increase in patients exposed to radiotherapy for left-sided breast cancer and NT-proBNP may increase in patients exposed to other cardiotoxic drugs than trastuzumab such as anthracyclines [Citation20,Citation21].
The present study was undertaken to assess whether changes in NT-proBNP levels and in cardiac repolarisation and depolarisation assessed by electrocardiography (ECG) may detect TIC in breast cancer patients undergoing trastuzumab treatment.
Methods
Recruitment, cohort and therapy
All patients with HER2-positive breast cancer where the physician judged the patient to be eligible to trastuzumab treatment were offered to participate in the study. After informed consent, 137 patients diagnosed with HER2-positive breast cancer were recruited at the Department of Oncology, Sahlgrenska University Hospital, Gothenburg, Sweden, between 2013 to 2018. One patient accepted to participate in the study but did not undergo trastuzumab therapy due to personal reasons. For the statistical analyses we therefore have included 136 patients. The majority of patients were treated with trastuzumab (Herceptin®) subcutaneously (600 mg) or intravenously (6 mg/kg, start dose 8 mg/kg) in the adjuvant setting (85%), while a small number of patients were treated in the neoadjuvant or the palliative settings. The majority (113 patients) were treated with the subcutaneous form of Herceptin®, five patients with the intravenous form of Herceptin® and 17 patients shifted from the intravenous to the subcutaneous form of Herceptin® during the 12 months of adjuvant HER2 blockade due to changes in treatment recommendations at the clinic.
Assessment of the cohort at baseline, 6 months and 12 months
Patients underwent echocardiography at baseline (before trastuzumab), at 6 months and at 12 months (end of trastuzumab therapy) according to clinical practice. Within a two-week period after echocardiography, the patients underwent a 12-lead ECG and underwent blood sample testing for NT-proBNP. NT-proBNP immunoassay was performed on the Cobas e602 (measuring range 5–35,000 ng/L; coefficient of variance 9–10%; Roche Diagnostics, Mannheim, Germany) at the Department of Clinical Chemistry, the Sahlgrenska University Hospital, Gothenburg, Sweden.
Baseline characteristics including age, weight, height, cardiovascular risk factors (hyperlipidemia, diabetes mellitus, hypertension, smoking, atrial fibrillation) were retrieved from medical records. Tumour characteristics were retrieved from the pathology report after surgery. In the neoadjuvant setting, tumour characteristics were defined from the core biopsy and tumour size was according to the size defined by ultrasound and alternatively mammography when ultrasound measurements were not available. During 2013–2015 visual estimates of left ventricular ejection fraction (LVEF) were only given and from 2016 LVEF values were given from both visual estimates and by Simpsons biplanar method. To be able to compare declines in LVEF and to be consistent we therefore chose to use visual estimates in the present study. TIC was defined as an LVEF visually assessed by echocardiography of less than 50% and a decline from baseline of ≥10 units. ECG parameters were predefined and included: P duration, PQ duration, QRS duration, QTc duration and heart rate. The TIC group was defined as patients developing TIC at either 6 or 12 months and NT-proBNP values and ECG parameters given in the TIC group were values registered at the point in time for TIC. The control group was defined as patients not developing TIC at neither 6 nor 12 months. For the TIC group, δNTproBNP was defined as the NTproBNP value at the point in time for TIC minus the NTproBNP value at baseline. For the control group, δNTproBNP was defined as the average change in NTproBNP from baseline to 6 months and from baseline to 12 months, i.e. [(NTpro BNP6months-NTproBNPbaseline) + (NTproBNP12months-NT proBNPbaseline)]/2.
Statistics
Patients were dichotomously categorised as either developing TIC or not developing TIC (control group). The Wilcoxon test was used to compare groups for continuous variables and the Fisher’s exact test was used to compare groups for categorical data. Odds ratios (OR) with 95% CI (Baptista-Pike) for potential risk factors associated with the development of TIC were calculated. To assess the diagnostic accuracy to detect TIC with NT-proBNP and δNTproBNP, receiver operating characteristic (ROC) curves were constructed and the area under the curve (AUC) with a 95% confidence interval (CI) were calculated. Data is presented in the form mean ± standard error of the mean (SEM) if not stated otherwise. p Values of less than .05 were considered statistically significant. IBM SPSS Statistics 25.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism program 7.0 (GraphPad Software, Inc., San Diego, USA) were used for statistical analyses. GraphPad Prism program 7.0 was used to create graphs.
Results
Demographics
The mean age of patients was 56.8 ± 1 years (range: 26–82; n = 136; ) and 95.6% of the patients were given therapy with curative intention. Patients were operated with mastectomy and lumpectomy in 55.9% and 43.4% of cases, respectively. Lymph node dissection was performed in 36.8% of the patients and the majority of patients underwent radiotherapy for the breast and/or loco-regionally (72.8% of the cohort; ).
Table 1. Demographics of cohort (TIC and control groups), tumour characteristics and treatments.
Tumour characteristics
The characteristics of the tumours are displayed in . There was a slight overweight of left-sided tumours (51.5%) and the majority of tumours were invasive carcinoma of no special type (invasive ductal carcinoma; 98.5%), grade 3 (59.3%) and with an average size of 26.2 ± 1.3 mm (n = 135). The majority of tumours were oestrogen receptor positive (68.1%), progesterone receptor negative (54.5%) and with high Ki67 index (>20%; 76.5% of the cohort) and with node-negativity (66.2%).
Treatment protocols
In the cohort, 118 patients were treated with trastuzumab in the adjuvant setting, 12 patients in the neoadjuvant setting and 6 patients in the palliative setting. The majority of patients (85%) were administered fluorouracil (5FU)-epirubicin-cyclophosphamide (FEC; 5-FU = 500–600 mg/m2; epirubicin = 60–90 mg/m2; cyclophosphamide 600 mg/m2) or epirubicin-cyclophosphamide (epirubicin = 90 mg/m2; cyclophosphamide 600 mg/m2) prior to trastuzumab therapy (). At the start of trastuzumab therapy, trastuzumab was combined with nine weekly doses of paclitaxel (70–90 mg/m2) in 117 patients (86%) or with three cycles of docetaxel (every third week; 70–100 mg/m2) in 17 patients (12.5%; ). Trastuzumab was in 12 patients combined with pertuzumab in the neoadjuvant setting (8 patients), in the adjuvant setting (2 patients) and in the palliative setting (2 patients). The majority of patients (92%) were administered all 17 trastuzumab treatments during 12 months, while 11 patients (8%) discontinued treatment prematurely (obtaining only 5–13 doses). Premature termination of treatments was either due to side effects including TIC or the own decision of the patient to terminate.
Cardiotoxicity
At baseline, all 136 patients had normal LVEF (range LVEF: 50–71%). Five patients developed TIC after six months and one patient after twelve months of trastuzumab treatment giving an incidence of 4.4% of TIC in the cohort (n = 136; Supplemental Table 1). Of patients developing TIC, three patients were exposed to epirubicin (90 mg/m2) and cyclophosphamide (600 mg/m2) before start of trastuzumab therapy. One patient was not exposed to anthracyclines directly prior to trastuzumab therapy but had previously been exposed to doxorubicin and cyclophosphamide in the 90´s due to breast cancer. The remaining two patients had not been exposed to chemotherapy. All patients with TIC developed symptoms including dyspnoea and/or clinical signs of CHF such as inspiratory crackles. Patients developing TIC immediately terminated trastuzumab therapy and were administered CHF drug therapy (beta-blockers and ACE-inhibitors). All TIC patients ameliorated their LVEF three months after termination of trastuzumab treatment (3 completely and 3 partially). Baseline LVEF was lower for TIC patients than control patients, i.e. 57.08 ± 1.36% vs. 61.42 ± 0.26%, respectively (n = 6 and n = 125, respectively; p < .05; ). Also control patients had a significant but small decrease in LVEF at 6 months and 12 months compared with baseline ().
Table 2. Left ventricular ejection fraction (LVEF) in the trastuzumab-induced cardiotoxicity (TIC) group and the control group.
Patients developing TIC, had an increase in NT-proBNP from 198.8 ± 64.0 pg/ml to 678.7 ± 132.4 pg/ml (p < .05; n = 6; ). In contrast, control patients developed a decrease in NT-proBNP at 6 months, i.e. from 131.2 ± 20.9 pg/ml to 86.7 ± 8.8 pg/ml (p < .05; n = 119; ). In control patients, during 12-month trastuzumab therapy, the P-wave duration, the PQ interval, the QRS duration increased and the heart frequency decreased ().
Table 3. NT-proBNP and ECG parameters in the trastuzumab-induced cardiotoxicity (TIC) group and the control group.
ECG abnormalities including left bundle branch blocks and T wave changes were common at baseline and at the time point of TIC in TIC patients (Supplemental Table 2).
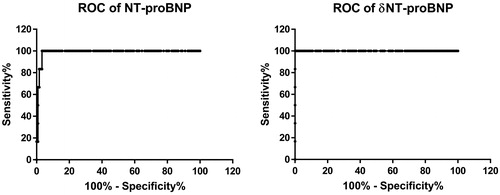
The area under the ROC curve for NT-proBNP was 0.99 (95% CI: 0.97–1.00) and for δNTproBNP was 1.00 (95% CI: 1.00–1.00). For a value equal or above of the cut-off point of 276.5 pg/ml for NT-proBNP, the sensitivity was 100% and the specificity 95% to detect TIC. For a value equal or above of the cut-off point of 75.8 pg/ml for δNTproBNP the sensitivity was 100% and the specificity 95% to detect TIC ().
Risk factors
TIC patients were older than control patients (68.3 ± 1.4 years and 56.3 ± 1.0 years, respectively; p < .01). TIC was associated with present diabetes mellitus (OR = 64.5, 95% CI: 5.72–929.3; p < .01) and atrial fibrillation (OR = 12.5; 95% CI: 1.92–75.81; p < .05; ).
Table 4. Univariate analyses of risk factors associated with the development of trastuzumab-induced cardiotoxicity (TIC).
Discussion
The incidence of TIC in our cohort was 4.4%, which corresponds well to previous incidence figures from larger cohort studies [Citation6,Citation22]. Women developing TIC were older and had more commonly diabetes and arrhythmia in their medical history. Since TIC patients often had more than one risk factor for cardiovascular disease and the number of TIC patients were only six we cannot define which of the identified risk factors that were correlated with development of TIC, since the power to do a multinominal logistic regression analysis was too low. However, our findings are in line with previous studies showing that TIC more often develops in older women with cardiovascular risk factors [Citation6,Citation23]. Furthermore, women in the TIC group had a lower LVEF than women in the control group at baseline indicating that women with lower LVEF may be at risk for developing TIC. The association of lower baseline LVEF and development of TIC was also shown in the NSABP-31 trial [Citation24]. The association between cardiovascular disease and TIC is further supported by our finding that women developing TIC often had an abnormal ECG both at baseline and at the time point for TIC. Trastuzumab treatment also led to a significant but small decrease in LVEF in the control group.
Our study showed that in the control group, an increase in P, PQ and QRS durations occurred at 6 and 12 months. For the TIC group, QRS tended to be increased from baseline, however, significance was not attained. Previous studies have suggested that a prolongation of the time from QRS onset on ECG to the beginning and peak of the transaortic flow on echocardiography (i.e. electromechanical delay) may be an early predictor for TIC [Citation13]. Increase in PQ and QRS durations may also reflect the decrease in heart rate that we observed in control patients [Citation25]. Hence, trastuzumab therapy seems to induce subtle changes in atrial and ventricular depolarisations, however, our study could not detect any change that discriminated TIC patients from control patients.
Women eligible for trastuzumab therapy are normally healthy in terms of cardiovascular risk and few women undergo trastuzumab therapy if CHF is present. In our cohort, all recruited women presented with a normal LVEF at baseline and the predominant part of women had normal baseline NT-proBNP values. All women who developed TIC also developed increases in NT-proBNP. To screen asymptomatic individuals with risk factors for the development of CHF with NT-proBNP has been assessed in many studies. For example, screening with NT-proBNP to detect asymptomatic CHF has been shown to be effective in patients with high cardiovascular risk in the primary care and in asymptomatic individuals with risk profile for CHF [Citation26–28]. Our data suggest that analysing changes in NT-proBNP during 12-month trastuzumab therapy could potentially replace monitoring TIC with echocardiography. This would spare time for the patient and spare both time and money for the health care system considering that the costs for NT-proBNP in Sweden is less than one tenth of the costs for echocardiography. Our data suggest that a cut-off value of 75.8 pg/ml for δNTproBNP could be used to decide on which patients to refer to echocardiography. Since baseline values vary a lot between healthy individuals, we suggest that δNTproBNP is better to use than absolute NTproBNP values to detect TIC. While NT-proBNP increased in TIC patients, NT-proBNP decreased during the 12-month trastuzumab therapy in patients not developing TIC in accordance with previous studies [Citation19]. The majority of patients in our study were exposed to anthracyclines prior to analysing NT-proBNP at baseline, which could have increased baseline NT-proBNP values [Citation21].
Conclusions
Our study indicates that NT-proBNP may be used to monitor women for TIC under trastuzumab therapy. Future studies validating if NT-proBNP can be safely used to detect TIC are warranted. Women with cardiovascular risk profiles and lower LVEF are more at risk of developing TIC.
Ethical approval
The study was performed in accordance with the Declaration of Helsinki (version October 2008) and approved by the Regional Ethical Review Board in Gothenburg (reference number 683-13). Written informed consent was obtained from all participating patients in the study.
Supplemental Material
Download MS Word (12.1 KB)Supplemental Material
Download MS Word (15.4 KB)Acknowledgments
We would like to give our sincere appreciation to the Clinical Trial Unit at the Department of Oncology, Sahlgrenska University Hospital, for help with the study and to physicians at the clinic recruiting patients to the study.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Varga Z, Noske A, Ramach C, et al. Assessment of HER2 status in breast cancer: overall positivity rate and accuracy by fluorescence in situ hybridization and immunohistochemistry in a single institution over 12 years: a quality control study. BMC Cancer. 2013;13:615.
- Stenehjem DD, Yoo M, Unni SK, et al. Assessment of HER2 testing patterns, HER2+ disease, and the utilization of HER2-directed therapy in early breast cancer. Breast Cancer (Dove Med Press)). 2014;6:169–177.
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792.
- Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17(9):2639–2648.
- Moja L, Tagliabue L, Balduzzi S, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev. 2012;18(4):CD006243.
- Lidbrink E, Chmielowska E, Otremba B, et al. A real-world study of cardiac events in > 3700 patients with HER2-positive early breast cancer treated with trastuzumab: final analysis of the OHERA study. Breast Cancer Res Treat. 2019;174(1):187–196.
- Mohan N, Shen Y, Endo Y, et al. Trastuzumab, but not pertuzumab, dysregulates her2 signaling to mediate inhibition of autophagy and increase in reactive oxygen species production in human cardiomyocytes. Mol Cancer Ther. 2016;15(6):1321–1331.
- ElZarrad MK, Mukhopadhyay P, Mohan N, et al. Trastuzumab alters the expression of genes essential for cardiac function and induces ultrastructural changes of cardiomyocytes in mice. PLoS One. 2013;8(11):e79543.
- Yavas O, Yazici M, Eren O, et al. The acute effect of trastuzumab infusion on ECG parameters in metastatic breast cancer patients. Swiss Med Wkly. 2007;137(39-40):556–558.
- Garg A, Li J, Clark E, et al. Exposure-response analysis of pertuzumab in HER2-positive metastatic breast cancer: absence of effect on QTc prolongation and other ECG parameters. Cancer Chemother Pharmacol. 2013;72(5):1133–1141.
- Karaca M, Kocoglu H, Bilgetekin I, et al. Ventricular bigeminal rhythm associated with trastuzumab: a potential cardiac side effect. J Cancer Res Ther. 2018;14(Supplement):S536–S537.
- Piotrowski G, Gawor R, Stasiak A, et al. Cardiac complications associated with trastuzumab in the setting of adjuvant chemotherapy for breast cancer overexpressing human epidermal growth factor receptor type 2 – a prospective study. Arch Med Sci. 2012;2(2):227–235.
- Choe JC, Choi JH, Choi JH, et al. Prolonged electromechanical delay as an early predictor of trastuzumab-induced cardiotoxicity in patients undergoing treatment for breast cancer. Clin Cardiol. 2018;41(10):1308–1314.
- Taylor CJ, Roalfe AK, Iles R, et al. The potential role of NT-proBNP in screening for and predicting prognosis in heart failure: a survival analysis. BMJ Open. 2014;4(4):e004675.
- Fuat A, Murphy JJ, Hungin AP, et al. The diagnostic accuracy and utility of a B-type natriuretic peptide test in a community population of patients with suspected heart failure. Br J Gen Pract. 2006;56(526):327–333.
- Banke A, Fosbol EL, Ewertz M, et al. Long-term risk of heart failure in breast cancer patients after adjuvant chemotherapy with or without trastuzumab. JACC Heart Fail. 2019;7(3):217–224.
- Ponde N, Bradbury I, Lambertini M, et al. Cardiac biomarkers for early detection and prediction of trastuzumab and/or lapatinib-induced cardiotoxicity in patients with HER2-positive early-stage breast cancer: a NeoALTTO sub-study (BIG 1-06). Breast Cancer Res Treat. 2018;168(3):631–638.
- Cardinale D, Colombo A, Torrisi R, et al. Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Cin Oncol. 2010;28(25):3910–3916.
- Demissei BG, Finkelman BS, Hubbard RA, et al. Detailed phenotyping reveals distinct trajectories of cardiovascular function and symptoms with exposure to modern breast cancer therapy. Cancer. 2019;125(16):2762–2771.
- D'Errico MP, Grimaldi L, Petruzzelli MF, et al. N-terminal pro-B-type natriuretic peptide plasma levels as a potential biomarker for cardiac damage after radiotherapy in patients with left-sided breast cancer. Int J Radiat Oncol Biol Phys. 2012;82(2):e239–e246.
- Bisoc A, Ciurescu D, Radoi M, et al. Elevations in high-sensitive cardiac troponin t and n-terminal prohormone brain natriuretic peptide levels in the serum can predict the development of anthracycline-induced cardiomyopathy. Am J Ther. 2020;27(2):e142–e150.
- Cameron D, Piccart-Gebhart MJ, Gelber RD, Herceptin Adjuvant (HERA) Trial Study Team, et al. 11 years' follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389(10075):1195–1205.
- Kosalka P, Johnson C, Turek M, et al. Effect of obesity, dyslipidemia, and diabetes on trastuzumab-related cardiotoxicity in breast cancer. Curr Oncol. 2019;26(3):e314–e321.
- Romond EH, Jeong JH, Rastogi P, et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2012;30(31):3792–3799.
- Mason JW, Badilini F, Vaglio M, et al. A fundamental relationship between intraventricular conduction and heart rate. J Electrocardiol. 2016;49(3):362–370.
- Gavazzi A, De Maria R, Grosu A, et al. Screening of asymptomatic left ventricular systolic dysfunction in a population sample at high cardiovascular risk in Lombardy (Italy): the DAVID-Berg study. G Ital Cardiol (Rome). 2014;15(5):313–322.
- Boffa U, McGrady M, Reid CM, et al. SCReening Evaluation of the Evolution of New Heart Failure Study (SCREEN-HF): early detection of chronic heart failure in the workplace. Aust Health Rev. 2017;41(2):121–126.
- McGrady M, Reid CM, Shiel L, et al. NT-proB natriuretic peptide, risk factors and asymptomatic left ventricular dysfunction: results of the SCReening Evaluation of the Evolution of New Heart Failure study (SCREEN-HF). Int J Cardiol. 2013;169(2):133–138.