Introduction
Several drugs have recently been shown to prolong life and improve quality of life in men with metastatic hormone sensitive (mHSPC) and castration resistant prostate cancer (CRPC) [Citation1]. Specifically, two randomized clinical trials have shown an increased survival for men with mHSPC who in addition to treatment with Gondadotropin Releasing Hormone agonist (GnRH) received abiraterone combined with prednisone or prednisolone as primary treatment [Citation2,Citation3]. An extension of the indication for abiraterone to treat de novo mHSPC in adult men in combination with androgen deprivation therapy (ADT) plus prednisone or prednisolone was approved by the European Medicines Agency (EMA) in November 2017 with the restriction that the patient should have high-risk mHSPC, i.e. at least two out of three of the following factors: Gleason score 8–10, three or more bone metastases, or visceral metastases. Reimbursement for this extended indication was approved by The Dental and Pharmaceutical Benefits Agency in Sweden in June 2018 with the additional restriction that the patient should not be suitable for docetaxel [Citation4]. Docetaxel is a chemotherapeutic drug with a lower cost than abiraterone that is recommended by the National Guidelines for use in men with de novo mHSPC, and there is no evidence that abiraterone is superior to docetaxel [Citation4,Citation5]. As abiraterone had already been approved by the EMA for treatment of CRPC in November 2012, and subsidized use on this new indication was approved on the June 2015 in Sweden, the uptake of the new indication cannot be monitored by data in the Prescribed Drug Registry as the only source of information since this registry does not include date of cancer diagnosis or the indication for treatment.
The aim of this study was to rapidly assess uptake in Sweden of the new indication for upfront use of abiraterone plus prednisolone in combination with GnRH in men with de novo high-risk mHSPC by use of a clinical cancer register and two other nation-wide health care registries and to assess adherence to the restrictions of the indication.
Material and methods
The National Prostate Cancer Register (NPCR) of Sweden
The National Prostate Cancer Register (NPCR) of Sweden captures 98% of all incident prostate cancer (PCa) cases in Sweden compared to The Cancer Registry to which reporting is mandated by law [Citation6,Citation7]. In NPCR detailed information on cancer characteristics, work-up, and primary treatment is registered, but no information from the subsequent disease trajectory is recorded, e.g. progression or secondary treatment [Citation6]. Data in NPCR were used to identify men with de novo mHSPC defined by bone metastases and/or visceral metastases on imaging at diagnosis. A dichotomous variable on the number of metastatic foci on bone imaging coded as one to three foci or four or more foci was added to NPCR in September 2018. High risk mHSPC was defined when at least two out of three of the following factors were fulfilled: Gleason score 8–10, four or more bone metastases, or visceral metastases. No impact on oncologic outcomes has been demonstrated when three or four bone metastases have been used as cut off criteria for high risk PCa [Citation8].
The Patient Registry
The Patient Registry holds data on hospital discharge diagnoses for all patients in Sweden since 1987 with a virtually complete capture and is updated monthly. The Charlson Comorbidity Index (CCI) was calculated on the basis of ICD codes for the discharge diagnoses within ten years preceding the date of PCa diagnosis [Citation6,Citation9].
The Prescribed Drug Registry
Since July 2005, all filled prescriptions in Sweden are registered in The Prescribed Drug Registry and data are available for analysis one month after a prescription has been filled [Citation6,Citation10]. All filled prescriptions for abiraterone (ATC code L02BX03) have been reported to this registry. We also retrieved filled prescriptions for opioids (ATC code N02A) within six months after date of PCa diagnosis in order to identify men who used a strong analgesic.
“The Statistics pathway” at The National Board of Health and Welfare
In order to provide a rapid ascertainment of drug use, The National Board of Health and Welfare (NBHW) provides a service in which data on subjects from a clinical register can be linked with data from other registers, e.g. The Prescribed Drug Registry and The Patient Registry, and only aggregated data are then returned to the researcher. Approval by the Research Ethics Board was obtained for a publication based such linkages.
Study population and procedure
On October 15, 2020, men with de novo mHSPC were identified in NPCR from the second quarter in 2018 to the third quarter in 2020 and a file with person identity number, cancer characteristics and region was submitted from NPCR to NBHW. At NBHW linkages to the Prescribed Drug Registry by use of the person identity number were performed to obtain data on filled prescriptions for abiraterone and opioids within six months after date of diagnosis and to the Patient Registry for ICD-10 codes to calculate CCI. Only data aggregated in a predefined table was then returned to the researchers.
Results
There were 2041 men registered in NPCR with de novo mHSPC, i.e. with bone metastases and/or visceral metastases on imaging, between June 1, 2018 and September 30, 2020 of whom 250 (12%) had filled a prescription for abiraterone within six months after the date of diagnosis (). For men diagnosed in the third quarter of 2020, only the fillings in that quarter were available. The proportion of men in NPCR who filled a prescription for abiraterone increased from 10/226 (4%) in the third quarter of 2018 to 58/223 (26%) in the first quarter of 2020, a quarter in which registration in NPCR is almost complete. Of note, in the second and third quarters of 2020, 17% of men with de novo mHSPC filled a prescription for abiraterone, despite the COVID-19 pandemic that affected Swedish health care.
Table 1. Number and proportion of men with de novo metastatic hormone sensitive prostate cancer (mHSPC) in the National Prostate Cancer Register of Sweden who filled a prescription for abiraterone within six months after date of diagnosis.
There was a higher proportion of men with four or more bone metastases among men who had filled a prescription (‘users’), than among men who had not filled a prescription (‘non-users’), users 140/250 (56%) vs non-users 706/1791 (39%). There were 41/250 (16%) men with visceral metastasis among users vs 364/1791 (20%) among non-users. Gleason was ≥ 8 in 209/250 (84%) users vs 1210/1791 (68%) non-users. There was a higher proportion of men with high risk mHSPC among users, 136/250 (54%) vs non-users, 676/1791 (38%). Median age at diagnosis was 73 years among users and 75 years among non-users. The proportion of men who had filled an opioid prescription was 38% for users and 35% for non-users, and CCI was 0 in 70% of users vs 60% of non-users.
The proportion of men with de novo mHSPC who had filled a prescription for abiraterone in each of the 21 Swedish regions ranged from 0 to 39%.
We compared the proportion of men who filled a prescription for abiraterone in men who were registered in NPCR soon after their diagnosis vs those who were registered late and found virtually no difference (data not shown).
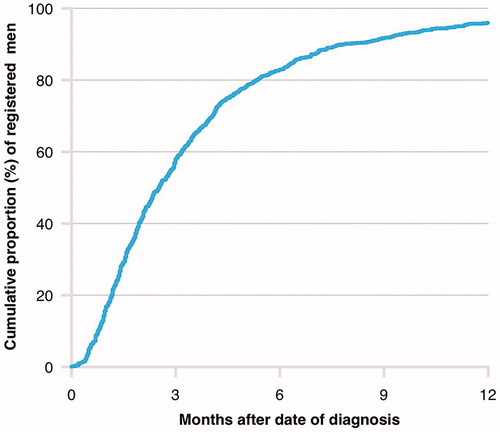
In 2018, the proportion of men with de novo mHSPC who had been reported to NPCR by use of the diagnostic and the work-up form, which both are necessary in order to identify men with bone or visceral metastases on imaging, were 50% at three months after date of diagnosis, 80% after six months, and 95% after nine months ().
Figure 1. Cumulative proportion of men with de novo metastatic prostate cancer registered in the National Prostate Cancer Register (NPCR) of Sweden according to time after diagnosis in 2018.
Legend: Registration was based on information in the diagnostic and in the work-up form, which both are necessary in order to identify men with bone metastases and/or visceral metastases on imaging.
Discussion
In this nationwide, population-based study in Sweden we found a slow but increasing uptake of the new indication of abiraterone, i.e. for treatment of newly diagnosed high-risk mHSPC in combination with GnRH plus prednisone or prednisolone. After reimbursement for this indication was approved by The Dental and Pharmaceutical Benefits Agency in Sweden in June 2018, overall 12% of all men diagnosed with de novo mHSPC had received abiraterone within six months after date of diagnosis. Uptake of the new indication for abiraterone varied from 0–39% in the 21 Swedish regions. Adherence to the restriction that only men with high-risk mHSPC and men not suitable for chemotherapy should receive abiraterone seemed low since only 54% of men who had filled a prescription for abiraterone had high risk mHSPC and 70% of these men had no comorbidities.
Strengths and limitations
The main strength of this cross-sectional study is that it is based on linkages of three nationwide, population-based registers with rapid and almost complete capture of data. NPCR provided data on the diagnosis of prostate cancer and occurrence and extent of metastases, which defined de novo high-risk mHSPC. Fillings in the Prescribed Drug Registry was used to define drug exposure and the Patient Registry provided data on comorbidity, used to assess if the drug was used in line with the indication approved for marketing authorization. These latter two registers are updated on a monthly basis.
Our study has some limitations. Neither NPCR nor the Patient Registry hold data on Eastern Cooperative Oncology Group/WHO Health organization Performance status (ECOG), a widely used index for assessment level of function in cancer patients. Instead we used the CCI based on hospital discharge diagnoses [Citation6,Citation9]. CCI has previously been shown to predict overall survival quite well in men in NPCR [Citation11]. The extent of metastases could be assessed for around two thirds of the men by data on number of bone and visceral metastases. Notably, a recent study has shown that men with low-risk mHSPC also benefit from abiraterone [Citation8]. The Prescribed Drug Registry does not capture data on drugs administered in-hospital, so this registry cannot be used to analyze docetaxel administration. Finally, there is some delay in reporting to NPCR and capture reaches 80% at six months and 95% around nine months after date of diagnosis. Thus, data for the second and third quarters in 2020 should be interpreted with caution since capture in NPCR was not complete and during that time period there was also an effect by the COVID-19 pandemic on cancer care.
National, regional, and local decisions with an impact on uptake of new drugs and new indications
Expenditure on cancer drugs in the EU has increased in both absolute and relative terms [Citation12]. Against that backdrop it is not surprising that there are several checkpoints before the pricing and reimbursement status of a novel expensive cancer drug is decided. After approval of subsidized use by The Dental and Pharmaceutical Benefits Agency, the uptake of a new treatment in clinical practice is also influenced by other factors. First, national guidelines have to recommend the use of the subsidized drug, and likely more important, there has to be adherence to these recommendations [Citation4]. Moreover, financing of the use of a new drug has to be decided on and there are different procedures for this step in different health care regions and regions.
Despite the efforts of the New Treatment council, a national working group that aims for fair, equal, and rational use of medical drugs by making recommendations on drug use to the county councils, there were large regional differences in uptake of abiraterone for use in men with de novo mHSPC. In other settings, uptake of new cancer drugs has been much quicker. For example, a US study of new treatments for multiple myeloma in 2010–2016 reported that uptake of new combinations of drugs occurred almost in parallel with their regulatory approval [Citation13].
Prescribed Drug Registries are also in existence in e.g. Denmark and Canada and somewhat similar rapid assessments of drug use have been performed. For example, a Danish study investigated the uptake of a new anticoagulant drug for treatment of atrial fibrillation [Citation14]. Notably, in that study there were no data on the indication for the drug in approximately one third of the study subjects as there were no data from a clinical register but only data from the National Prescription Registry, the National Patient Registry, and the Danish Civil Registration System.
Conclusion
In conclusion, uptake of the new indication for abiraterone in men with de novo mHSPC was 12% within 27 months after approval of the subsidized use and there seemed to be a low adherence to the restriction to high-risk mHSPC and men not suitable for docetaxel. After approval for subsidized use by the Swedish Dental and Pharmaceutical Benefits Agency, the uptake of a new treatment in clinical practice is also influenced by other national, regional and local factors, and this seems to have resulted in a slow uptake and large regional differences.
Disclaimer
Rolf Gedeborg is also employed by the Medical Products Agency (MPA) in Sweden. The MPA is a Swedish Government Agency. The views expressed in this article may not represent the views of the MPA.
Acknowledgments
Region Uppsala has, on behalf of NPCR, made agreements on subscriptions for quarterly reports for data in the Statistics pathway project with Janssen, quarterly reports from Patient-overview Prostate Cancer with Astellas, Sanofi, Janssen, and Bayer, as well as research projects with Astellas, Bayer, and Janssen.
We thank Lena Jörgensen at The National Board of Health and Welfare for timely quarterly cross linkages.
This project was made possible by the continuous work of the National Prostate Cancer Register of Sweden (NPCR) steering group: Pär Stattin (chair), Ingela Franck Lissbrant (deputy chair), Johan Styrke, Camilla Thellenberg Karlsson, Lennart Åström, Hampus Nugin, Stefan Carlsson, Marie Hjälm-Eriksson, David Robinson, Mats Andén, Ola Bratt, Magnus Törnblom, Johan Stranne, Jonas Hugosson, Maria Nyberg, Olof Akre, Per Fransson, Eva Johansson, Gert Malmberg, Hans Joelsson, Fredrik Sandin, and Karin Hellström.
Disclosure statement
Ingela Franck Lissbrant has received speaker´s honoraria from Astra Zeneca.
Additional information
Funding
References
- Mottet N, Cornford P, Bergh RVD, et al. EAU - EANM - ESTRO - ESUR - SIOG Guidelines on Prostate Cancer 2020. EAU - EANM - ESTRO - ESUR - SIOG Guidelines on Prostate Cancer [Internet]. Arnhem, The Netherlands; n.d. [cited 2020 Jul 12]. Available from: https://uroweb.org/guideline/prostate-cancer/#1.
- Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377(4):352–360.
- James ND, Bono JD, Spears MR, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377(4):338–351.
- National care program for prostate cancer [Internet]. 2020. Available from: https://kunskapsbanken.cancercentrum.se/diagnoser/prostatacancer/vardprogram.
- Barata PC, Sartor AO. Metastatic castration-sensitive prostate cancer: abiraterone, docetaxel, or…. Cancer. 2019;125(11):1777–1788.
- Hemelrijck MV, Wigertz A, Sandin F, et al. Cohort profile: the national prostate cancer register of Sweden and prostate cancer data base Sweden 2.0. Int J Epidemiol. 2012;42:956–967.
- NPCR - National Prostate Cancer Registry of Sweden [Internet]. n.d. Available from: http://npcr.se/.
- Hoyle AP, Ali A, James ND, et al. Abiraterone in “high-” and “low-risk” metastatic hormone-sensitive prostate cancer. Eur Urol. 2019;76(6):719–728.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Wettermark B, Hammar N, MichaelFored C, et al. The new Swedish Prescribed Drug Register-opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16(7):726–735.
- Hemelrijck MV, Folkvaljon Y, Adolfsson J, et al. Causes of death in men with localized prostate cancer: a nationwide, population-based study. BJU Int. 2016;117(3):507–514.
- Jönsson B, Hofmarcher T, Lindgren P, et al. The cost and burden of cancer in the European Union 1995–2014. Eur J Cancer. 2016;66:162–170.
- Jagannath S, Abonour R, Durie BGM, et al. Heterogeneity of second-line treatment for patients with multiple myeloma in the connect MM registry (2010–2016). Clin Lymphoma Myeloma Leuk. 2018;18(7):480.e3–485.e3.
- Pottegård A, Grove EL, Hellfritzsch M. Use of direct oral anticoagulants in the first year after market entry of edoxaban: a Danish nationwide drug utilization study. Pharmacoepidemiol Drug Saf. 2018;27(2):174–181.
