Abstract
Background
Metronomic treatment is hypothesized to be less toxic and more effective as compared to standard maximal tolerable dosing treatment in metastatic cancer disease.
Material and methods
We tested the metronomic treatment principle with vinorelbine in a randomized phase 2 setting combined with standard capecitabine treatment in the XeNa trial with Clinical Trials.gov identifier number: NCT0141771. 120 patients with disseminated HER2 non-amplified breast cancer were included. Randomization was between Arm A: vinorelbine 60 mg/m2 day 1 + day 8 in the first cycle followed by 80 mg/m2 day 1 + day 8 in the following cycles or Arm B: vinorelbine 50 mg three times a week. Capecitabine 1000 mg/m2 twice a day for days 1–14 was administered in both arms.
Results
The treatment was generally well-tolerated. The response rate (RR) was 24% (arm A) versus 29% (arm B) (p = .67). The clinical benefit rate (CBR) 46.8% (arm A) versus 51.7% (arm B) (p = .72). We found a median progression-free survival (PFS) of 7.1 months (95% confidence interval [CI] 3.9–10.3) in arm A and 6.3 months (95% CI 4.1–8.5) in arm B (p = .25) whereas median overall survival (OS) was 23.3 months (95% CI 20.2–26.4) in arm A and 22.3 months (95% CI 14.3–30.3) in arm B (p = .76).
Conclusions
We confirmed that the combination of vinorelbine and capecitabine was well tolerated. Metronomic treatment can be used with acceptable adverse events (AEs), but we did not find significant difference in the effect compared to the standard treatment.
Introduction
Breast cancer is a chemosensitive disease and a combined strategy of surgery, chemotherapy, radiotherapy, antioestrogen, and antibody treatment is now used rather successfully both in early and advanced disease. Improvements in adjuvant treatment of breast cancer have changed the recurrence rate of the disease but patients still develop life-threatening distant metastases and recurrent disease, and a number of patients are diagnosed with a primary disseminated disease [Citation1,Citation2].
Once the disease becomes disseminated, the purpose of the treatment is to stabilize the disease, minimize the symptoms, and increase survival time [Citation3,Citation4]. Taxanes and anthracyclines are, when looking at the response rates (RRs), the most effective drugs but are also rather toxic, with a high rate of adverse events (AEs) including cardiac morbidity and febrile neutropenia. New ways to use the well-known drugs are needed as a more chronic and stable phase of the disease can sometimes be achieved.
Metronomic treatment could be one of these new ways. The treatment is defined as frequent administration of minimal effective dose (e.g. one third of maximum tolerable dose) without prolonged breaks [Citation5]. Metronomic treatment can in theory reduce the risk of development of resistant clones and influence angiogenesis in the tumor due to the continuously lower chemotherapeutic pressure. Preclinical data also suggest that the immune cells are affected, leading to a higher cytotoxic immune effect and better antigen presentation [Citation5–12].
Capecitabine [Citation13–15] and vinorelbine [Citation16–21] have been chosen for this study as both drugs are well known in the treatment of breast cancer, both drugs have an oral formulation; both drugs have an acceptable and not overlapping toxicity profile. The combination is considered ‘an ideal combination’ [Citation22,Citation23]. Administrating these drugs orally makes absorption, first-pass metabolism and distribution of the chemotherapy important to consider in order to determine the effective doses in the patient. The difference between intravenous and oral doses is well studied [Citation17,Citation24,Citation25] as well as the optimal maximal tolerable dose for the combined treatment [Citation26–28]. Vinorelbine belongs to the group of vinca alkaloids, which block cell division in G2/M phase of the cell cycle by inhibiting the assembly of microtubule in the cell division [Citation21]. Capecitabine is a Fluorouracil prodrug that needs to be activated in the body by the enzyme thymidine phosphorylase and thereafter act as an antimetabolite in the DNA synthesis [Citation14].
Metronomic treatment has been studied in metastatic breast cancer patients in the VICTOR-2 study with capecitabine and vinorelbine. The optimal schedule is recommended to be vinorelbine 40 mg thrice a week in combination with a fixed dose of capecitabine of 500 mg twice daily. Navalbine Oral® has a half-life of 40 h and administration three times a week is suggested as an optimal schedule. The study was carried out with limited toxicity [Citation29–31]. Several other studies have been carried out with different drugs, in combination or as single agents, primarily in Phase 1 and 2 trials [Citation12,Citation32].
To our knowledge this is the first randomized Phase 2 study with the combination of vinorelbine and capecitabine, comparing metronomic versus conventional maximal tolerable dosing. The primary endpoint was to decide the objective response rate (ORR) in both treatment arms. Secondary endpoints were to define the toxicity and safety profile of the combined treatment, define time to response, time to progression, and overall survival (OS).
Patients and methods
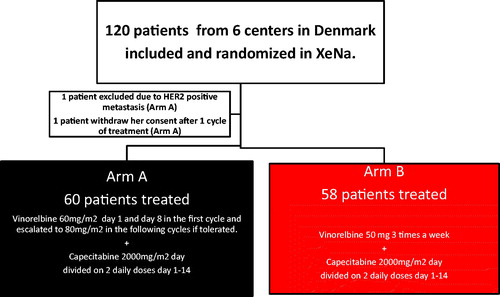
We have included 120 women, from 2012 to 2015, treated at six Oncology Outpatient Clinics in Denmark. All women had HER2 non-amplified metastatic breast cancer. The treatment was given as first or second-line chemotherapy treatment for metastatic disease. The patients had previously received adjuvant treatment with anthracyclines, and they must have progressed on or been considered unfit for taxanes.
The included patients had measurable disease according to RESIST, a performance status of at least 2, expected lifetime of at least 16 weeks and an age over 18 years. There had to be a signed informed consent, and the patients needed to be able to swallow the capsule. The exclusion criteria were prior treatment with capecitabine or vinorelbine, pregnancy, other current or prior malignant disease, inadequate liver, renal, or bone marrow function.
The study was designed as an open-labeled randomized Phase 2 study. The patients were randomized (1:1) between arm A and arm B. All patients in both arms were treated with oral capecitabine 1000 mg/m2 twice daily for day 1–14, and the randomization was between oral vinorelbine 60 mg/m2 given day 1 and day 8 increased to 80 mg/m2 in the second cycle if well tolerated or oral vinorelbine three times a week without treatment breaks. Patients aged 65 and older received treatment according to dose level −1. The study design is shown in .
Figure 1. Trial flowchart for the XeNa trial. Patients over 65 start at dose level −1 and are not increased. If one drug is reduced both drugs are given in the new dose. Blood samples were taken day 1 and day 8 in the first cycle, and only day 1 in the following cycle if satisfactory.
AEs were reported by the patients and collected at each visit, and reports were made in case of hospitalization. In case of unacceptable side effects (CTC grade 3 or 4) treatment dose was reduced as seen in and . Treatment was continued as long, as it was well tolerated and effective. Evaluation of the effect was done after every three cycles with CT of thorax and upper abdomen and MR scan of columnar.
Table 1. Standard dose packages ARM A.
Table 2. Standard dose packages ARM B.
The statistical analysis was performed using SPSS version 20 (SPSS Inc., Chicago, IL). All randomized patients are included in the intention-to-treat population. The NCI-CTC classification version 3.0 (Bethesda, MD) was used to classify AEs. The primary endpoint was to assess the ORR according to RESIST 1.0 criterion’s [Citation33] and to compare the RR for the two treatment arms. We calculated a clinical benefit rate (CBR) defining a clinical meaningful response as objective response (CR and PR) or stable disease (SD) for at least 6 months. We compared the CBR between the two treatment arms.
Secondary endpoints were to define the toxicity and safety profile of the combined treatment, progression-free survival (PFS), and OS and compare them between the two treatment arms.
Subgroup analysis of patients under versus over 65 years, patients with ER-negative versus ER-positive tumors, patients needed treatment in reduced dose versus full-dose treatment and patients in performance status 0–1 versus 2 are analyzed, as they represent clinically relevant subgroups in the outpatient population.
In the power calculation an assumption for ORR, a minimal desirable ORR, was 30% in the current population, and a desirable increase in ORRas an indication of further studies warranted, was 50%. We needed 50 patients in each arm and included 60 to ensure 50 evaluable patients in each arm. The null-hypothesis is equal ORR in the two treatment arms.
The study has the ClinicalTrials.gov Identifier NCT01941771 and was approved by the Danish Ethic authority (VEK) number: 1-10-72-84-12 and the Danish Medical authority (EUDRACT no. 2011-003564-72).
Results
The patient characteristics are shown in . The number of cycles ranged between 1 and 48 in both arms and 21% of the patients received more than 15 cycles equal to more than 1-year of treatment as seen in . Rather surprisingly only 54 (45%) patients managed to complete the full dose treatment. Significantly more patients in arm B started out at dose level −1 mostly due to the number of patients over 65 years old (<0.01). The number of subsequent dose reductions, on the other hand, was significantly higher in arm A than in arm B especially due to leukopenia during treatment (<0.01).
Table 3. Patient characteristics.
Table 4. Treatment response.
The RRs are as seen in . Our ORR was 24% (95% CI 13.4–34.6) in arm A and 29% (95% CI 17.3–40.7) in arm B and are not significantly different in the two treatment arms (p = .18). The CBR was calculated for the patients who have benefited with ORR or SD for more than 6 months and was 46.8% (95% CI 34.6–59.4) in arm A and 51.7% (95% CI 39.1–64.9) in arm B (p = .72).
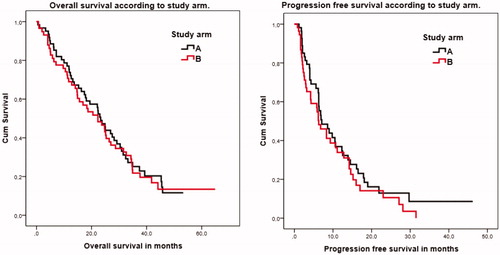
When we looked at the PFS, treatment time, and OS the two arms were comparable as seen in . The OS in arm A was 23.3 months (95% CI 20.2–26.4) and 22.3 months (95% CI 14.3–30.3) in arm B. PFS was 7.1 months (95% CI 3.9–10.3) in arm A and 6.3 months (95% CI 4.1–8.5) in arm B. The median follow-up time was 33.5 months. OS and PFS are shown in .
The frequency of reported AE in both treatment arms was rather low and both treatments were well tolerated and safe (). Considering all the AEs, the total amount of reported AE was lower for all grades of AE in the metronomic arm, but there was no significant difference between the side-effects in the different arms, when we compare the proportion of patients experiencing a particular side-effect in the two treatment arms as seen in . Looking at the hematological side effects in absolute numbers, leukopenia and neutropenia occurred more frequently in arm A and anemia in arm B, but yet no statistical differences were observed between arms. Only few patients experienced febrile neutropenia in both arms. Among the non-hematological side-effects, the reported classical side effects, such as nausea, vomiting, diarrhea, alopecia, and neutropenia and hand foot skin syndrome and stomatitis were equal in the treatment arms. The study was considered safe throughout the entire study. The serious AEs were equally represented and acceptable in both treatment arms.
Table 5. Worse adverse event in number of patients.
The patients in both treatment arms receiving reduced doses achieved the same efficacy, and surprisingly there was significantly better OS (p < .01) and PFS (p = .04) among the patients receiving dose reduced treatment in both arm A and arm B, but the group receiving full-dose treatment was relatively small. The better OS and PFS could also be biased by the age group as patients over 65 years were dose reduced per protocol, but there were no significantly different OS between patients below or over 65 years (p = .45) in arm A and arm B. Patients having triple-negative disease survived longer in the non-metronomic arm A (p < .01). Patients with performance status 0 did not have a longer PFS in arm A or B (p = .91)).
Discussion
The goal for treatment of patients with metastatic breast cancer is to relieve symptoms and prolong survival with the best possible quality of life. Palliation, therefore, needs to be balanced against survival and toxicity. Keeping the disease on a stable level and obtaining a more chronic phase of the disease must be the second-best option, when cure is impossible.
The frequencies of reported AE in both treatment arms were rather low and both treatments were well tolerated. In particular, the rate of febrile neutropenia was low in both arms. Looking at the hematological side effects in absolute numbers, leukopenia and neutropenia occurred more frequently in arm A and anemia in arm B, but yet no statistical differences were observed between arms as seen in . This observation could be due to the more continuous pressure on the bone marrow in the metronomic treatment arm.
When we compared our results in the non-metronomic arm to previous studies using the same dosing of the drugs in combination [Citation2,Citation34,Citation35] our results are in the middle. The OR, in the previous rather small studies, ranges from 20 to 56%, PFS from 3.4 to 10.5 months, and OS from 11.3 to 29 months. Overall, the combined regimen was also described as well tolerated in previous studies.
The effect of capecitabine alone in a previous study had an ORR of 26% [Citation13]. Vinorelbine as monotherapy was studied in rather small studies and the RRs were found in the range from 12 to 60% [Citation16–20,Citation36,Citation37]. The large range reflects the rather small sample sizes, and a direct comparison is therefore difficult.
Patients in the older age-group (over 65 years) tolerated the treatment well in our study, and the treatment seems suitable for all age groups. Surprisingly, we found a significant better PFS (p = .04) and OS (p < .01) in the dose reduced group, but it may be due to a low number in the patients receiving full treatment. Patients with triple-negative disease had shorter OS (p < .01) in the metronomic treatment arm, but PFS was not significantly different (p = .38).
Since the study was designed, capecitabine has successfully been used in a metronomic schedule [Citation29,Citation31]. We have only one variable in this study, but it could be interesting to examine how well a pure metronomic arm will perform in a randomized way. Currently, the randomized study NAME with metronomic daily vinorelbine as monotherapy is now recruiting (EUDRACT no 2016-002165-63).
Conclusion
To our knowledge, this was the first randomized Phase 2 study investigating the effect of metronomic treatment compared to standard treatment. When we compared our results to other investigators, our RRs were comparable in the non-metronomic arm.
The study has shown metronomic treatment is possible with low frequencies of AEs, and it is possible to receive good RRs and high CBRs.
However, metronomic treatment in this form is not more effective measured by RR, CBR, PFS, and OS as compared to standard dosing of chemotherapy. There was a significant shorter survival among the patients with a triple-negative tumor receiving metronomic treatment. Metronomic treatment might have a place in cancer treatment in the less aggressive breast cancer subtypes to keep the disease at chronic state, but in this study, we did not find a better effect of the metronomic treatment.
Acknowledgments
The authors thank all the medical staff and the employees at the clinical trials departments participating in the inclusion and treatment of the protocol patients in the Departments of Oncology in Aarhus, Aalborg, Esbjerg, Roskilde, Hilleroed, and Herning and thereby making the study possible. We thank the GCP authority for controlling the data and validating the CRF.
The author thanks the funding ‘Astrid Thaysen legat for Laegevidenskabelig grundforskning’ and Radiumstationens forskningsfond (AUH) for financial support. The study was an investigator-initiated study supported economically by Pierre Fabre and the authors thanks Pierre Fabre for the support. The preliminary data has been presented as a poster at European Breast Cancer Conference in Amsterdam 2016. Abstract no.384.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Harbek N, Penault-Llorca F, Cortes J, et al. Breast cancer. Nat Rev Dis Primers. 2019;5:66.
- Tubiana-Mathieu N, Bougnoux P, Becquart D, et al. All-oral combination of oral vinorelbine and capecitabine as first-line chemotherapy in HER2-negative metastatic breast cancer: an international phase II trial. Br J Cancer. 2009;101(2):232–237.
- Liedtke C, Kolberg HC. Systemic therapy of advanced/metastatic breast cancer - current evidence and future concepts. Breast Care (Basel). 2016;11(4):275–281.
- Cardoso F, Costa A, Senkus E, et al. 3rd ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 3). Breast. 2017;31:244–259.
- Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4(6):423–436.
- Scharovsky OG, Mainetti LE, Rozados VR. Metronomic chemotherapy: changing the paradigm that more is better. Curr Oncol. 2009;16(2):7–15.
- Briasoulis E, Pappas P, Puozzo C, et al. Dose-ranging study of metronomic oral vinorelbine in patients with advanced refractory cancer. Clin Cancer Res. 2009;15(20):6454–6461.
- Hanahan D, Bergers G, Bergsland E. Less is more, regularly: metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J Clin Invest. 2000;105(8):1045–1047.
- Hobson B, Denekamp J. Endothelial proliferation in tumours and normal tissues: continuous labelling studies. Br J Cancer. 1984;49(4):405–413.
- Cazzaniga ME, Camerini A, Addeo R, et al. Metronomic oral vinorelbine in advanced breast cancer and non-small-cell lung cancer: current status and future development. Future Oncol. 2016;12(3):373–387.
- Munzone E, Colleoni M. Clinical overview of metronomic chemotherapy in breast cancer. Nat Rev Clin Oncol. 2015;12(11):631–644.
- Montagna E, Cancello G, Dellapasqua S, et al. Metronomic therapy and breast cancer: a systematic review. Cancer Treat Rev. 2014;40(8):942–950.
- Blum JL, Dieras V, Lo Russo PM, et al. Multicenter, Phase II study of capecitabine in taxane-pretreated metastatic breast carcinoma patients. Cancer. 2001;92(7):1759–1768.
- Mackean M, Planting A, Twelves C, et al. Phase I and pharmacologic study of intermittent twice-daily oral therapy with capecitabine in patients with advanced and/or metastatic cancer. J Clin Oncol. 1998;16(9):2977–2985.
- Reigner B, Blesch K, Weidekamm E. Clinical pharmacokinetics of capecitabine. Clin Pharmacokinet. 2001;40(2):85–104.
- Bruno S, Puerto VL, Mickiewicz E, et al. Phase II trial of weekly i.v. vinorelbine as a single agent in first-line advanced breast cancer chemotherapy. The Latin-American experience. Am J Clin Oncol. 1995;18(5):392–396.
- García-Conde J, Lluch A, Martin M, et al. Phase II trial of weekly IV vinorelbine in first-line advanced breast cancer chemotherapy. Ann Oncol Off J Eur Soc Med Oncol. 1994;5(9):854–857.
- Fumoleau P, Delgado FM, Delozier T, et al. Phase II trial of weekly intravenous vinorelbine in first-line advanced breast cancer chemotherapy. J Clin Oncol. 1993;11(7):1245–1252.
- Terenziani M, Demicheli R, Brambilla C, et al. Vinorelbine: an active, non cross-resistant drug in advanced breast cancer. Results from a phase II study. Breast Cancer Res Treat. 1996;39(3):285–291.
- Langkjer ST, Ejlertsen B, Mouridsen H, et al. Vinorelbine as first-line or second-line therapy for advanced breast cancer: a Phase I-II trial by the Danish Breast Cancer Co-operative Group. Acta Oncol. 2008;47(4):735–739.
- Mathé G, Reizenstein P. Phase I pharmacologic study of a new Vinca alkaloid: navelbine. Cancer Lett. 1985;27(3):285–293.
- Dieras V, Extra JM, Bellissant E, et al. Efficacy and tolerance of vinorelbine and fluorouracil combination as first-line chemotherapy of advanced breast cancer: results of a phase II study using a sequential group method. J Clin Oncol. 1996;14(12):3097–3104.
- Saridaki Z, Malamos N, Kourakos P, et al. A phase I trial of oral metronomic vinorelbine plus capecitabine in patients with metastatic breast cancer. Cancer Chemother Pharmacol. 2012;69(1):35–42.
- Marty M, Fumoleau P, Adenis A, et al. Oral vinorelbine pharmacokinetics and absolute bioavailability study in patients with solid tumors. Ann Oncol. 2001;12(11):1643–1649.
- Variol P, Nguyen L, Tranchand B, et al. A simultaneous oral/intravenous population pharmacokinetic model for vinorelbine. Eur J Clin Pharmacol. 2002;58(7):467–476.
- Nolè F, Catania C, Sanna G, et al. Dose-finding and pharmacokinetic study of an all-oral combination regimen of oral vinorelbine and capecitabine for patients with metastatic breast cancer. Ann Oncol. 2006;17(2):322–329.
- Briasoulis E, Aravantinos G, Kouvatseas G, et al. Dose selection trial of metronomic oral vinorelbine monotherapy in patients with metastatic cancer: a Hellenic cooperative oncology group clinical translational study. BMC Cancer. 2013;13(1):263.
- Welt A, von Minckwitz G, Oberhoff C, et al. Phase I/II study of capecitabine and vinorelbine in pretreated patients with metastatic breast cancer. Ann Oncol. 2005;16(1):64–69.
- Cazzaniga ME, Torri V, Villa F, et al. Efficacy and safety of the all-oral schedule of metronomic vinorelbine and capecitabine in locally advanced or metastatic breast cancer patients: the phase I-II VICTOR-1 study. Int J Breast Cancer. 2014;2014:769790.
- Cazzaniga ME, Torri V, Riva F, et al. Efficacy and safety of vinorelbine-capecitabine oral metronomic combination in elderly metastatic breast cancer patients: VICTOR-1 study. Tumori. 2017;103(1):e4–e8.
- Cazzaniga ME, Cortesi L, Ferzi A, et al. Metronomic chemotherapy with oral vinorelbine (mVNR) and capecitabine (mCAPE) in advanced HER2-negative breast cancer patients: is it a way to optimize disease control? Final results of the VICTOR-2 study. Breast Cancer Res Treat. 2016;160(3):501–509.
- Liu Y, Gu F, Liang J, et al. The efficacy and toxicity profile of metronomic chemotherapy for metastatic breast cancer: a meta-analysis. PLoS One. 2017;12(3):e0173693.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247.
- Finek J, Holubec L, Svoboda T, et al. A phase II trial of oral vinorelbine and capecitabine in anthracycline pretreated patients with metastatic breast cancer. Anticancer Res. 2009;29(2):667–670.
- Jones A, O'Brien M, Sommer H, et al. Phase II study of oral vinorelbine in combination with capecitabine as second line chemotherapy in metastatic breast cancer patients previously treated with anthracyclines and taxanes. Cancer Chemother Pharmacol. 2010;65(4):755–763.
- Canobbio L, Boccardo F, Pastorino G, et al. Phase-II study of Navelbine in advanced breast cancer. Semin Oncol. 1989;16(4):33–36.
- Freyer G, Delozier T, Lichinister M, et al. Phase II study of oral vinorelbine in first-line advanced breast cancer chemotherapy. J Clin Oncol. 2003;21(1):35–40.