CDK4/6 inhibitors combined with endocrine therapy (ET) have become standard treatment for endocrine receptor (ER) positive, Her-2 negative metastatic breast cancer. Three agents, palbociclib, ribociclib, and abemaciclib, have been approved and all significantly increase progression-free survival when added to ET, compared with ET alone [Citation1]. These drugs have shown similar efficacy, but some differences have been observed in their side effect profiles [Citation2,Citation3]. Elevated levels of alanine and aspartate aminotransferases (ALT and AST) were observed in clinical trials on ribociclib. In the MONALEESA-2 study, grade 3 or 4 ALT elevation was observed in 9.3% of the treatment group compared with 1.2% of the placebo group [Citation1]. Liver dysfunction was dose-limiting in studies in Japanese patients [Citation4].
To our knowledge, only a single case report of drug-induced liver injury (DILI) associated with ribociclib has been published [Citation5]. Although elevated liver tests have been reported in clinical trials, no formal causal assessment has been undertaken in these trials and there are several other etiologies that can be associated with abnormal liver tests. Thus, the novelty of this report is a detailed phenotypic information on two well-characterized cases with clinically apparent liver injury, with proper exclusion of etiologies other than DILI.
Among breast cancer patients treated with ribociclib in Iceland during the first half of 2020, two developed clinically apparent DILI with significant liver impairment with elevation in serum bilirubin and INR. In total, 43 patients have been treated since the drug became accessible in the country at the beginning of 2019.
Case reports
Two cases of grade 4 hepatotoxicity due to ribociclib were observed among 43 patients treated.
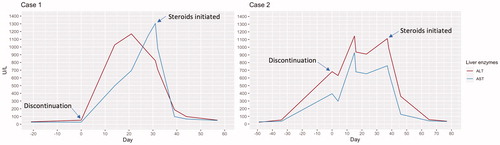
Case 1: 54-year old Caucasian woman was diagnosed in 2019 with ER-positive, Her-2 negative breast cancer with disseminated bone metastases. She received treatment with letrozole (2.5 mg daily) as well as eight cycles of cytotoxic chemotherapy (epirubicin and cyclophosphamide) because of extensive osteolytic bone metastases and threatening visceral impairment. Six months later, ribociclib was added to letrozole treatment. Ribociclib (600 mg) was given in 4-week cycles (21/7 schedule). Before initiation the patient had normal liver tests and there were no signs of liver metastases at the last imaging investigation.
After the first cycle of ribociclib for a total of 21 d of treatment she developed pruritus and an ALT elevation was observed (). Liver enzymes continued rising despite discontinuation of the drug with ALT > 1000 (U/L), concomitant jaundice, and coagulopathy (INR 2.1) but no encephalopathy. Ultrasound of the liver did not demonstrate any liver metastases and similarly a CT-scan of the liver was normal. As liver tests remained elevated despite cessation of therapy, treatment with prednisolone (40 mg) was initiated. Liver enzymes normalized a month later with prednisolone doses tapered off. shows the development of ALT and AST elevation after discontinuation of the drug and after starting corticosteroids.
Table 1. Results of laboratory tests.
Hepatitis serologies against hepatitis A, B, C, and E were negative as well as immunoglobulin M antibodies to cytomegalovirus and herpes simplex. She had no history of autoimmune disease, antinuclear (ANA) and smooth muscle (SMA) antibodies were negative. The patient did not have a history of alcohol or drug abuse. Past medical history apart from breast cancer was only notable for gastro-esophageal reflux disease and osteoarthritis. She did not use herbs or any alternative therapies. She was in addition to therapy with letrozole and denosumab treated with the following drugs: esomeprazole, long-acting morphine (Contalgin), paracetamol in therapeutic doses (1000 mg × 3), olanzapine, and prochlorperazine that were continued during the course of the liver injury.
Case 2: 66-year old Caucasian woman was diagnosed in 2007 with ER-positive, Her-2 negative breast cancer, and received treatment with partial mastectomy, axillary node dissection and adjuvant radiotherapy. She received adjuvant anastrozole therapy but discontinued after a few months due to side effects (not related to the liver). In 2017, she relapsed with bone lesions in the sternum and treatment with fulvestrant (500 mg every 28 d) was initiated as well as palliative radiotherapy to the sternum. In 2019, lung metastases were detected and ribociclib (600 mg) was added to ET in 4-week cycles. Before the start of this therapy, liver tests were normal. After three cycles liver enzymes were found to be significantly elevated (). Ribociclib was discontinued but liver enzymes continued to rise and remained elevated. The patient developed nonspecific symptoms of nausea and fatigue but no pruritus, jaundice, or encephalopathy (INR 1.3). Five weeks after discontinuation the liver enzymes peaked with ALT reaching 1112 (U/L). She was started on prednisolone (40 mg). Liver enzymes normalized after five weeks of corticosteroid treatment in doses that were tapered off.
Hepatitis serologies against hepatitis A, B, C, and E were negative but she had IgG antibodies against hepatitis B and E suggesting a previous infection with these viruses. She had negative autoimmune serology markers (ANA and SMA) and a CT scan of the abdomen showed no pathological changes in liver or biliary tree. The patient‘s past medical history was notable for hypothyroidism. Her recent medications consisted of losartan and escitalopram and she did not use alternative therapies. She did not have a history of alcohol or drug abuse.
After normalization of liver tests, both patients were started on treatment with another CDK4/6 inhibitor palbociclib (125 mg daily) with close monitoring of liver enzymes. During follow-up both patients currently have normal liver enzymes after receiving three and five cycles of palbociclib after three and five months of treatment, respectively.
Discussion
Background
CDK4/6 inhibitors interfere with retinoblastoma protein phosphorylation and thereby prevent cell-cycle progression from the G1 phase to the S phase [Citation1]. They are metabolized by CYP3A4 and eliminated by biliary clearance [Citation6]. Monitoring of liver enzymes is recommended during treatment with ribociclib due to the high frequency of elevated ALT and AST observed in clinical trials [Citation1].
Incidence of elevated aminotransferases and DILI due to ribociclib
In the current report, two clinically apparent cases of grade 4 hepatotoxicity were observed among 43 patients treated with ribociclib. In the MONALEESA-2, 3, and 7 clinical trials, grade 3 or 4 ALT elevation occurred in 1 out of 11 (9.3%), 12 (8.5%), and 19 (5.0%) patients, respectively [Citation1,Citation7,Citation8]. In a recently published study on the efficacy and safety of CDK4/6 inhibitors in 88 patients in Sweden, 10 patients (11.4%) discontinued treatment due to toxicity, thereof one due to hepatotoxicity after receiving ribociclib [Citation2]. Clinically apparent liver injury due to drugs is rare. For example, approximately 1 out of 2300 who were treated with amoxicillin–clavulanate developed DILI and among most other potentially hepatotoxic drugs the risk of DILI was less frequent [Citation9]. Thus, two out of 43 patients treated is a low number needed to harm.
Previous DILI case report due to ribociclib
In the only previously reported case of ribociclib induced liver injury, a 59-year old woman with relapsing metastatic breast cancer received ribociclib (600 mg) along with letrozole (2.5 mg daily) as first-line therapy. After 16 weeks of treatment she presented with a grade 2 ALT elevation which rapidly rose to grade 3. Ribociclib was immediately discontinued but the grade 3 ALT elevation persisted for 14 weeks. She had a grade 3 AST elevation as well but no other toxicity. She had no history of liver disease and common causes of aminotransferase elevation were excluded. Once liver enzymes normalized, she initiated treatment with palbociclib (100 mg) without further hepatic injury [Citation5].
Current report of DILI due to ribociclib
In the current report, ALT and AST remained elevated in both cases for a long period of time despite discontinuation of therapy. Although the patients did not have elevated autoimmune markers such as ANA, the clinical and biochemical phenotype was similar to that of seronegative drug-induced autoimmune hepatitis [Citation10–12]. One of the inclusion criteria for seronegative autoimmune hepatitis (AIH) is a requirement of corticosteroids in patients with a hepatocellular type of injury, where elevated ALT and AST do not improve after discontinuation of the implicated agent [Citation12]. In the MONALEESA-2 trial, there were four reported cases of Hy’s law (three related to study treatment) and two of those patients showed findings on biopsy suggestive of autoimmune hepatitis. None of these cases resulted in death or permanent hepatic failure and liver enzymes returned to normal within six months from discontinuation of treatment [Citation6]. Furthermore, there were four reports of autoimmune hepatitis after ribociclib use recorded in the FDA Adverse Event Reporting System, but one for palbociclib and none for abemaciclib [Citation6]. A liver biopsy in current cases might have revealed a histological phenotype compatible with AIH but would have been unlikely to change clinical management.
As the clinical status of the patients did not improve and liver tests did not normalize despite discontinuation of the implicated agent, they were treated with a course of corticosteroids with doses tapering off over eight weeks. Liver tests improved promptly after the first week of corticosteroids with complete normalization of liver tests at the last follow-up. Both received palbociclib without further elevation of liver enzymes. The fact that both patients in the current report were found to tolerate palbociclib supports the finding by Meynard and Grellety [Citation5] and argues that DILI caused by these drugs is not a class effect but due to specific properties of ribociclib. A recent paper by Raschi and De Ponti described strategies for assessment of drug-related risk factors, such as physiochemical and pharmacokinetic properties in order to predict the development of DILI with a particular focus on CDK4/6 inhibitors [Citation6]. Ribociclib has inhibitory effects on hepatic transporters, which might explain the higher frequency of liver-related adverse events compared with palbociclib. However, this pharmacological feature does not suggest an autoimmune mechanism of injury, such as those seen in MONALEESA-2 and the FDA reports. For palbociclib, grade 3 or 4 ALT elevation was less frequently observed during clinical trials, but two cases of hepatic failure were reported. Additionally, 17 reports of acute hepatic failure and 12 cases of pseudocirrhosis have been reported since the marketing of the drug [Citation6].
Conclusion
In conclusion, although CDK4/6 inhibitors are generally safe and well-tolerated, ribociclib seems to be able to induce liver injury. In this report, two patients out of 43 treated with ribociclib developed grade 4 hepatotoxicity and were considered to require corticosteroid treatment due to persistent ALT elevation, with prompt resolution of liver tests. Both patients have recovered and are currently receiving further treatment with another CKD4/6 inhibitor without signs of liver impairment.
Informed consent
Patients gave written consent to the inclusion of material pertaining to themselves, and it was acknowledged that they cannot be identified via the article; and they have been fully anonymized.
Disclosure statement
The authors report no conflicts of interest.
References
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375(18):1738–1748.
- Edman Kessler L, Wiklander O, Hamberg E, et al. Efficacy and safety of cyclin dependent kinases 4/6 inhibitors in the treatment of metastatic breast cancer: a real-world experience. Acta Oncol. 2020;59(11):1382–1386.
- Iwata H. Clinical development of CDK4/6 inhibitor for breast cancer. Breast Cancer. 2018;25(4):402–406.
- Yap Y-S, Chiu J, Ito Y, et al. Ribociclib, a CDK 4/6 inhibitor, plus endocrine therapy in Asian women with advanced breast cancer. Cancer Sci. 2020;111(9):3313–3326.
- Meynard L, Grellety T. CDK 4/6 inhibitor successful rechallenge after limiting hepatic toxicity. Breast J. 2020;26(2):255–257.
- Raschi E, De Ponti F. Strategies for early prediction and timely recognition of drug-induced liver injury: the case of cyclin-dependent kinase 4/6 inhibitors. Front Pharmacol. 2019;10:1–15.
- Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36(24):2465–2472.
- Tripathy D, Im SA, Colleoni M, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018;19(7):904–915.
- Bjornsson ES, Bergmann OM, Bjornsson HK, et al. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144(7):1419–1425.
- Gassert DJ, Garcia H, Tanaka K, et al. Corticosteroid-responsive cryptogenic chronic hepatitis: evidence for seronegative autoimmune hepatitis. Dig Dis Sci. 2007;52(9):2433–2437.
- Yilmaz B, Unlu O, Evcen R, et al. Acute onset seronegative autoimmune hepatitis: are simplified diagnostic criteria sufficient? Eur J Gastroenterol Hepatol. 2016;28(5):607–608.
- Bjornsson ES, Bergmann O, Jonasson JG, et al. Drug-induced autoimmune hepatitis: response to corticosteroids and lack of relapse after cessation of steroids. Clin Gastroenterol Hepatol. 2017;15(10):1635–1636.