Abstract
Introduction
Recurrence of endometrial cancer is not routinely registered in the Danish national health registers. The aim of this study was to develop and validate a register-based algorithm to identify women diagnosed with endometrial cancer recurrence in Denmark to facilitate register-based research in this field.
Material and methods
We conducted a cohort study based on data from Danish health registers. The algorithm was designed to identify women with recurrence and estimate the accompanying diagnosis date, which was based on information from the Danish National Patient Registry and the Danish National Pathology Registry. Indicators of recurrence were pathology registrations and procedure or diagnosis codes suggesting recurrence and related treatment. The gold standard for endometrial cancer recurrence originated from a Danish nationwide study of 2612 women diagnosed with endometrial cancer, FIGO stage I–II during 2005–2009. Recurrence was suspected in 308 women based on pathology reports, and recurrence suspicion was confirmed or rejected in the 308 women based on reviews of the medical records. The algorithm was validated by comparing the recurrence status identified by the algorithm and the recurrence status in the gold standard.
Results
After relevant exclusions, the final study population consisted of 268 women, hereof 160 (60%) with recurrence according to the gold standard. The algorithm displayed a sensitivity of 91.3% (95% confidence interval (CI): 85.8–95.1), a specificity of 91.7% (95% CI: 84.8–96.1) and a positive predictive value of 94.2% (95% CI: 89.3–97.3). The algorithm estimated the recurrence date within 30 days of the gold standard in 86% and within 60 days of the gold standard in 94% of the identified patients.
Discussion
The algorithm demonstrated good performance; it could be a valuable tool for future research in endometrial cancer recurrence and may facilitate studies with potential impact on clinical practice.
Introduction
Surveillance for cancer recurrence is key in cancer follow-up. Insight into the patient pathway before a diagnosis of recurrence is crucial for the organisation of follow-up and to improve patient survival [Citation1]. However, little evidence exists to inform the surveillance for cancer recurrence and the organisation of follow-up. The majority of research on disease-free survival and cancer recurrence is based on clinical trials, and the results may be affected by selection bias since elderly and comorbid patients are often excluded [Citation2]. This limits the generalisability, and studies representing a broader population of cancer survivors are warranted [Citation3,Citation4].
Register-based research is challenged by the lack of information on cancer recurrence in registries [Citation5,Citation6]. In Denmark, it is mandatory for clinicians to report the diagnosis code to the Danish National Patient Registry (DNPR) [Citation7] when diagnosing a cancer recurrence. The Danish Gynaecological Cancer Database (DGCD) [Citation8] automatically imports the codes of recurrence from the DNPR. However, a previous study found that only 19% of endometrial cancer recurrences were registered in the DGCD [Citation9].
A recent Danish nationwide study of 2612 women operated for stage I–II endometrial cancer identified 183 (7%) women with recurrence of early-stage endometrial cancer; this finding was based on a review of the medical records of 308 women with pathology results raising suspicion of recurrence [Citation10]. However, reviewing medical records is time-consuming. Using algorithms to identify patients diagnosed with cancer recurrence will facilitate register- and population-based research on cancer recurrence [Citation5]. This is a particularly valuable research method in countries with high-quality population-based registers [Citation11]. Several register-based algorithms have recently been developed and validated in Denmark. They have proven to accurately identify patients diagnosed with recurrence of colorectal cancer [Citation6], breast cancer [Citation12,Citation13], and bladder cancer [Citation14]. A similar approach is needed to identify recurrence of endometrial cancer.
The aim of this study was to develop and validate a register-based algorithm to identify women diagnosed with endometrial cancer recurrence after primary treatment and to assess the accuracy of the recurrence diagnosis date derived from the algorithm.
Material and methods
Design
We conducted a cohort study based on data from Danish national health registers. The data were linked at the individual level using the unique personal registration number [Citation15].
Data sources
Data were collected from five Danish national registries: (1) the Danish Civil Registration System [Citation15] with daily updated information on vital status and migration; (2) the DGCD [Citation8], with information on all endometrial cancer patients treated at Danish hospitals since January 2005; (3) the Danish Cancer Register (DCR) [Citation16], with information on diagnosis codes, diagnosis dates and tumour stage for all incident cancer diagnoses; (4) the Danish National Patient Register (DNPR) [Citation7], with information on all somatic in-hospital contacts, contacts to emergency departments and outpatient clinics, including tumour stage, procedure codes, and diagnosis codes; (5) the Danish Pathology Register (DPR) [Citation17], with information on all pathology specimens analysed in Denmark.
Diagnosis codes registered in the DNPR follow the International Classification of Diseases, 10th revision (ICD-10). In the Danish version of ICD-10 coding, ‘M’ following ‘C549’ indicates a metastasis from endometrial cancer and ‘X’ following ‘C549’ indicates endometrial cancer recurrence. Registrations in the DPR follow the Danish version of the Systematized Nomenclature of Medicine (SNOMED) classification, which allows identification of malignant morphology (codes M8 and M9), and the fifth digit of the morphology code indicates behaviour (e.g., 4: ‘direct spread to surrounding tissue’, 6: ‘malignant metastasis’, and 7: ‘malignant recurrence’).
Definition of gold standard
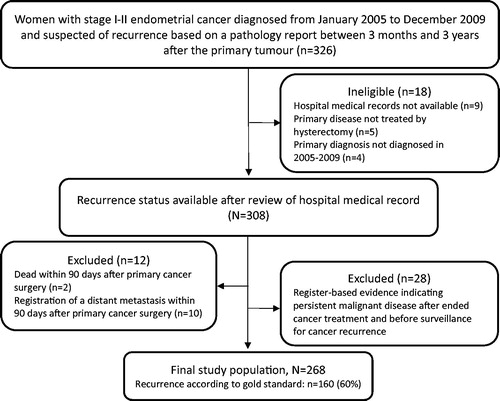
The gold standard information on cancer recurrence originated from a nationwide cohort study [Citation10] of 2612 women diagnosed with endometrial cancer in stage I–II in Denmark in 2005–2009. In this study, recurrence was suspected if a new tumour was registered in the pathology records, or if a diagnosis code of endometrial cancer recurrence ICD-10: C549X was recorded in the DNPR from three months to three years after the primary diagnosis (n = 326, ). A review of the medical record was conducted to confirm or disconfirm if the 326 women had been diagnosed with recurrence. After relevant exclusions, information was provided on recurrence status in 308 women; recurrence was confirmed in 183 women (7% of the original population) and rejected in 125 women. These 308 women served as the eligible gold standard population in the present study.
Definition of cancer recurrence
Cancer recurrence was defined in the algorithm as a sign of malignant disease originating from endometrial cancer, occurring from 90 days after completed endometrial cancer treatment, and with no signs of persistent malignant disease within these 90 days.
Study population
Women from the eligible gold standard population were excluded if registered in the DCR or the DNPR with distant metastasis within 90 days of cancer surgery or with distant tumour stage based on the tumour node metastasis (TNM) staging system of the Union for International Cancer Control (UICC) [Citation18] (). Women who emigrated or died within 90 days of the surgery for the primary cancer were excluded. Finally, women with register-based indicators of persistent disease within 90 days after completed cancer treatment and before surveillance for cancer recurrence were excluded (see details in section ‘Algorithm’).
Algorithm
The algorithm identified the date of endometrial cancer surgery in the DGCD and postoperative adjuvant oncological treatment in the DNPR (see Appendix for a detailed description). The algorithm deemed women to be in complete cancer remission if no evidence of persistent disease was identified during 90 days after surgery or during 90 days after completed oncological treatment in case of postoperative adjuvant treatment (). Indicators of persistent disease were (1) a registration of malignant morphology, (2) a procedure code for laparotomy, omentectomy, lymphadenectomy (except within 30 days after primary cancer surgery since this may have indicated surgery due to a complication or a secondary staging procedure), radiotherapy or chemotherapy combined with a malignant disease diagnosis (ICD-10: C00-C96, and D37-D48), (3) a distant tumour stage based on the UICC classification, or (4) a diagnosis code for malignant disease (except C54 and C44).
Figure 2. Schematic overview of the algorithm to identify women diagnosed with recurrence of endometrial cancer. *Hysterectomy and salpingo-oophorectomy or oophorectomy ±adjuvant chemotherapy and/or radiotherapy. **Until the last of the following dates: (1) 90 days after surgery or (2) 90 days after completed adjuvant therapy. Evidence of persistent malignant disease was evaluated based on registrations of pathology results, procedure codes, and diagnosis codes. Adapted from Rasmussen et al. [Citation12].
![Figure 2. Schematic overview of the algorithm to identify women diagnosed with recurrence of endometrial cancer. *Hysterectomy and salpingo-oophorectomy or oophorectomy ±adjuvant chemotherapy and/or radiotherapy. **Until the last of the following dates: (1) 90 days after surgery or (2) 90 days after completed adjuvant therapy. Evidence of persistent malignant disease was evaluated based on registrations of pathology results, procedure codes, and diagnosis codes. Adapted from Rasmussen et al. [Citation12].](/cms/asset/a231e4f3-9d80-42e3-9232-e6896414a4b6/ionc_a_1859133_f0002_c.jpg)
The algorithm was designed to search for indicators of recurrence from 90 days following completed cancer treatment and onwards. Three indicators of cancer recurrence were defined: (1) a diagnosis code of endometrial cancer recurrence (ICD-10: C549X) or a metastasis (ICD-10: CxxxM and C76-C79, except for C779 as this registration could refer to local lymph nodes at the time of primary diagnosis), (2) a procedure code of laparotomy, omentectomy, lymphadenectomy, radiotherapy, or chemotherapy combined with one of the following diagnosis codes: metastasis (ICD-10: C76-C79, CxxxM) and endometrial cancer (ICD-10: C54) including endometrial cancer recurrence (ICD-10: C549X), or (3) a malignant morphology registration coded as ‘recurrence’, or a malignant morphology registration similar to a morphology registered within 90 days of primary cancer surgery and coded as ‘direct spread to surrounding tissue’ or ‘metastasis’.
If a second primary cancer diagnosis was recorded in the DCR or the DNPR before or within 30 days of a diagnosis code for metastasis or a relevant morphology code, these registrations were disregarded as they might relate to the second primary cancer. If more than one indicator of cancer recurrence was present in the same woman, the first date of an indicator of recurrence was listed as the date of cancer recurrence.
Analyses
The concordant and discordant frequencies between recurrences identified by the algorithm and by the gold standard were used to compute the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) with 95% confidence interval (CI). Moreover, these analyses were performed separately for indicators of pathology data, procedure codes, and diagnosis codes.
The agreement between the date of recurrence identified by the algorithm and by the gold standard was measured by Lin’s concordance correlation coefficient (CCC) score [Citation19]. The agreement is considered ‘poor’ when CCC < 0.90, ‘moderate’ when CCC = 0.90–0.95, ‘substantial’ when CCC > 0.95 and ‘almost perfect’ when CCC > 0.99 [Citation20]. Furthermore, we analysed the proportion of recurrence dates estimated by the algorithm on the same date as the gold standard recurrence date, and within 7, 30, and 60 days of the gold standard recurrence date.
A series of sub-analyses were undertaken to test the robustness of the algorithm. First, to investigate if the algorithm performed similarly in a population with no previous history of cancer, we excluded women with a cancer diagnosis recorded in the DCR prior to the endometrial cancer diagnosis. Second, to investigate if the performance of the algorithm was affected by the presumption that all women with stage I–II endometrial cancer are curatively treated (regardless of registrations indicative of persistent malignant disease during the 90 days after ended cancer treatment and before recurrence surveillance), we reanalysed all 308 women eligible for inclusion in the study. Third, to investigate the dependency of the algorithm on clinical registry data, a sub-analysis was conducted; this required only registration of endometrial cancer in the DCR.
Results
A total of 308 women were potentially eligible for inclusion in the main analysis [Citation10]. After exclusions, 268 of these women served as the gold standard for endometrial cancer recurrence (). According to the gold standard, 160 (60%) had cancer recurrence and 108 did not. The algorithm identified 146 (91%) of the 160 recurrences and nine false positives (). Indicators of recurrence in the false positives were combinations of metastasis diagnosis codes, pathology codes, and procedure codes of radiotherapy and chemotherapy.
Table 1. Concordance of endometrial cancer recurrence between gold standard and algorithm.
The algorithm reached a sensitivity of 91.3% (95% CI: 85.8–95.1) and a specificity of 91.7% (95% CI: 84.8–96.1) (). The highest sensitivities were obtained through the use of indicators from pathology codes and procedure codes (). The specificities were identical across the three recurrence indicators in .
Table 2 . Performance of the algorithm to identify endometrial cancer recurrence in 268 women with early-stage endometrial cancer.
The agreement between the recurrence dates generated by the algorithm and the dates generated by the gold standard achieved a CCC score of 0.997 (95% CI: 0.996–0.998). The recurrence dates were estimated within 30 days of the gold standard in 86% and within 60 days of the gold standard date in 94% of cases (). The median difference between the recurrence date estimated by the algorithm and the recurrence date of the gold standard was 10 days (IQR: 6–19 days).
Table 3. Concordance of cancer recurrence date between gold standard and algorithm.
Sub-analyses
In the first sub-analysis, excluding 54 women with a history of cancer prior to the diagnosis of endometrial cancer, the performance of the algorithm was similar to the performance in the main analyses (Appendix, Table 1).
In the second sub-analysis, including all 308 eligible women from the gold standard population, the performance of the algorithm decreased slightly, by 1.7–3.7%, in sensitivity, specificity, PPV, and NPV (Appendix, Table 2).
In the third sub-analysis, 10 women with no registration of endometrial cancer in the DCR were excluded. Furthermore, the algorithm failed to identify registrations of curative cancer treatment in the DNPR in 41 women, and these women were excluded. After exclusions due to registrations indicating persistent malignant disease within 90 days after completed primary cancer treatment, 216 women were included; hereof 130 women (60%) had recurrence according to the gold standard. The sensitivity, specificity, PPV, and NPV of the algorithm in this population were similar to the corresponding figures from the main analyses (Appendix, Table 3).
Discussion
We developed and validated a register-based algorithm to identify women with recurrence of endometrial cancer in Denmark. The algorithm reached a sensitivity of 91.3% (95% CI: 85.8–95.1) and a specificity of 91.7% (95% CI: 84.8–96.1), and it estimated the cancer recurrence diagnosis date within 60 days in 94% of cases when validated on a population of women with early-stage endometrial cancer.
Strengths and limitations
To our knowledge, this study is the first to develop and validate a register-based algorithm to identify women diagnosed with recurrence of endometrial cancer. The algorithm is based on Danish national registers with high-quality records ensuring data of high validity [Citation7,Citation11,Citation17,Citation21]. Furthermore, the tax-funded healthcare system with free and equal access for all residents in Denmark limits the risk of selection bias [Citation11]. The inclusion of women with a cancer diagnosis prior to a diagnosis of endometrial cancer introduced a risk of confusing registrations related to recurrence of the previous cancer with registrations related to recurrence of endometrial cancer. However, the algorithm performed well and similarly in women with and without prior cancer. This broadens the applicability of the algorithm to cover also secondary cancer cases, which account for up to 16% of all cancer diagnoses [Citation22] and occur most often in cancers with shared risk factors, such as hormonal risk factors in breast and gynaecological cancers [Citation23]. In this study, 20% of the women had a previous cancer diagnosis, hereof 45% had a previous breast cancer. Finally, the use of pathology codes to identify women with recurrence was a great strength of the algorithm. Pathology codes alone identified more than 70% of women with recurrence and it accurately estimated the recurrence diagnosis date.
The study would have gained strength if all 2,612 women from the cohort study by Jeppesen et al. were included. However, reliable gold standard information on recurrence status was only available in the 326 women with hospital medical record review. Restrictions in the gold standard population might limit the generalisability of the results; the inclusion criteria were a tumour registration in the pathology records or an endometrial cancer recurrence diagnosis in the DNPR. Only women with early-stage cancer were included, and information on recurrence was only available within three years of follow-up. Thus, it remains unclear how the algorithm performs outside these criteria. International studies report that 98–100% of women diagnosed with recurrence from endometrial cancer were treated with surgery, chemotherapy, or radiotherapy [Citation24,Citation25], including women with distant recurrences. A high treatment rate in endometrial cancer recurrence increases the chance of the algorithm to identify recurrences, also in women not identified through biopsy tests and recurrence diagnosis codes. Furthermore, a diagnosis code for metastasis identified 27% of all recurrences, whereas only 3% were identified by a diagnosis code for recurrence. Although the present study does not allow any firm conclusions regarding the performance of the algorithm in women with stage III disease and recurrences occurring later than three years after primary cancer surgery, previous studies of bladder [Citation14], breast [Citation13], and colorectal [Citation6] cancers have proven that the indicators of recurrence performed well, also in patients with stage III cancer and in populations with follow-up beyond three years. Patients with recurrence after stage III cancer are registered with diagnosis codes and procedure codes similarly to patients with recurrence after early-stage cancer. Furthermore, studies have found that 90–99% of endometrial cancer recurrences occur within three years of follow-up [Citation26,Citation27]. Thus, we reason that the algorithm is applicable to the entire population of women treated for stage I–III endometrial cancer and with follow-up time beyond three years.
All eligible women (n = 308) had pathology results that raised suspicion of cancer recurrence. Consequently, 60% of the final study population had a verified recurrence of endometrial cancer, which is higher than previously reported in non-selected populations; 15–25% in stages I–III [Citation26,Citation28], 10% in stage I and 24% in stage II [Citation29]. When simulating this algorithm in a population with a recurrence rate of 10%, 15%, and 25%, the PPV decreases to 55%, 66%, and 79%, respectively. This defers more misclassification, which may blur outcome differences between patients with and without recurrence. More importantly, it raises ethical implications of the false positives, and the potential psychological harm it may cause if a study involves contacting patients with recurrence identified by the algorithm. Initiatives to avoid this should preferably be undertaken, such as validation of the recurrence in medical charts before contacting the patient.
Different definitions of persistent disease and cancer recurrence in the gold standard and in the algorithm caused exclusion of 13% of the eligible gold standard population. In the gold standard, surveillance for recurrence commenced 90 days after primary cancer surgery in all women. In the algorithm, surveillance for recurrence commenced 90 days after completed treatment, including adjuvant oncological treatment, and only in women with no registrations in the DNPR and the DPR indicating persistent malignant disease within these 90 days; malignant pathology codes, malignant diagnosis code and procedure codes combined with a malignant indication diagnosis. This resulted in the exclusion of women with an early recurrence according to the gold standard, and it might underestimate the absolute number of recurrences in future studies using the algorithm to identify recurrences. If the excluded women with an early recurrence represent a specific sub-group, i.e., women with more aggressive disease, there is a risk of bias in outcomes related to survival. In the third sub-analysis, the algorithm failed to identify procedure codes for primary cancer surgery in the DNPR in 13% of the gold standard population. These women were excluded, hereof 18 of the 183 with recurrence according to the gold standard. This increases the risk of underestimating the absolute number of recurrences and the risk of selection bias further, which underlines the benefit from using clinical registry data in the algorithm.
Comparison with the literature
The algorithm performed similarly to previously published algorithms developed to identify patients diagnosed with cancer recurrence based on Danish health registers [Citation6,Citation12–14]. This confirms that the Danish health registers provide robust and valid data to identify patients diagnosed with recurrence of cancer. Studies outside Denmark have developed algorithms to identify patients diagnosed with recurrence from lung, breast, colorectal, and prostate cancer based on administrative data combined with medical claims [Citation30–33] and administrative data from publicly funded health systems [Citation34]. Recently, a review on algorithms to identify breast cancer recurrence was published [Citation35]. Three of these 19 studies [Citation31,Citation34] provided algorithms demonstrating a sensitivity above 90%, PPVs above 73% and recurrence rates of 12–20%, which is comparable with our results.
Four studies investigated the accuracy of the diagnosis date of the identified cancer recurrence. One study [Citation36] provided an average prediction error of 4.5 months compared to 10 days in the present study. Two studies [Citation31,Citation33] identified 14–36% of the diagnosis dates within one month and 37–50% within three months, and the last study [Citation37] identified 82% within two months of the gold standard recurrence date. All Danish studies [Citation12,Citation14], including the current study, identified the diagnosis date more accurately, which may be driven by the recurrence indicator based on pathology registrations. Accurate estimation of the diagnosis date is important to increase the validity of research on disease-free survival. To our knowledge, the identified diagnosis dates for endometrial cancer recurrence are the most accurately estimated cancer recurrence diagnosis dates derived by a register-based algorithm.
Implications
The proposed algorithm provides an instrument to epidemiologic research in the field of endometrial cancer recurrence. The algorithm is a time-saving method for identification of cancer recurrence in large populations, which allows for detailed analyses with high statistical precision. The absence of exclusion criteria related to, e.g., comorbidity and age, the absence of underrepresented sub-groups due to active enrolment and the absence of loss to follow-up increases the generalisability to the entire population of women treated for endometrial cancer, although with a risk of excluding women with an early recurrence.
The proposed algorithm facilitates predictive and prognostic studies in endometrial cancer recurrence. Research in disease-free survival after cancer treatment is an important supplement to investigations in mortality and survival [Citation5]. The accuracy in the estimated recurrence date by the algorithm makes it a useful tool to investigate both disease-free survival after primary cancer and survival after recurrence. Furthermore, the algorithm offers an opportunity to fill a gap in the existing evidence on the diagnostic pathway for cancer recurrence; such new knowledge can be used to inform the organisation of cancer follow-up and recurrence surveillance [Citation1].
Conclusion
We developed an algorithm to identify women diagnosed with recurrence of endometrial cancer based on data in Danish health registries. The algorithm was validated in a population of women with early-stage endometrial cancer; it showed high sensitivity and high specificity, and it accurately estimated the recurrence date. The algorithm is assessed to be applicable for all women treated for non-metastatic endometrial cancer, and it may be a valuable resource for research in the field of endometrial cancer recurrence.
Ethical approval
The study is approved and registered as ‘The patient pathway for cancer recurrence’ (study 1, id 119) in the Record of Processing Activities at the Research Unit for General Practice in Aarhus in accordance with the provisions of the General Data Protection Regulation (GDPR). According to Danish law, the study does not require approval from the Committee on Health Research Ethics of the Central Denmark Region.
Supplemental Material
Download PDF (107.4 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Hewitt ME, Greenfield S, Stovall E. From cancer patient to cancer survivor: lost in transition. Washington (DC): The National Academies Press; 2006.
- Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112:228–242.
- Hovaldt HB, Suppli NP, Olsen MH, et al. Who are the cancer survivors? A nationwide study in Denmark, 1943–2010. Br J Cancer. 2015;112:1549–1553.
- Cohen AT, Goto S, Schreiber K, et al. Why do we need observational studies of everyday patients in the real-life setting? Eur Heart J Suppl. 2015;17(Suppl. D):D2–D8.
- Warren JL, Yabroff KR. Challenges and opportunities in measuring cancer recurrence in the United States. JNCI J Natl Cancer Inst. 2015;107(8):djv134.
- Lash TL, Riis AH, Ostenfeld EB, et al. A validated algorithm to ascertain colorectal cancer recurrence using registry resources in Denmark. Int J Cancer. 2015;136(9):2210–2215.
- Schmidt M, Schmidt SAJ, Sandegaard JL, et al. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490.
- Sørensen SM, Bjørn SF, Jochumsen KM, et al. Danish Gynecological Cancer Database. Clin Epidemiol. 2016;8:485–490.
- Juhl CS, Hansen ES, Høgdall CK, et al. Valid and complete data on endometrial cancer in the Danish Gynaecological Cancer Database. Dan Med J. 2014;61(6):A4864.
- Jeppesen MM, Jensen PT, Gilså Hansen D, et al. The nature of early-stage endometrial cancer recurrence—a national cohort study. Eur J Cancer. 2016;69:51–60.
- Schmidt M, Schmidt SAJ, Adelborg K, et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol. 2019;11:563–591.
- Rasmussen LA, Jensen H, Virgilsen LF, et al. A validated algorithm for register-based identification of patients with recurrence of breast cancer-based on Danish Breast Cancer Group (DBCG) data. Cancer Epidemiol. 2019;59:129–134.
- Cronin-Fenton D, Kjaersgaard A, Nørgaard M, et al. Breast cancer recurrence, bone metastases, and visceral metastases in women with stage II and III breast cancer in Denmark. Breast Cancer Res Treat. 2018;167(2):517–528.
- Rasmussen LA, Jensen H, Virgilsen LF, et al. A validated algorithm to identify recurrence of bladder cancer: a register-based study in Denmark. Clin Epidemiol. 2018;10:1755–1763.
- Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–549.
- Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39(7 Suppl.):42–45.
- Erichsen R, Lash TL, Hamilton-Dutoit SJ, et al. Existing data sources for clinical epidemiology: the Danish National Pathology Registry and Data Bank. Clin Epidemiol. 2010;2:51–56.
- Union for International Cancer Control. TNM classification of malignant tumours; 2018; [cited 2020 Feb 20]. Available from: https://www.uicc.org/resources/tnm
- Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45(1):255–268.
- McBride GB. A proposal for strength of agreement criteria for Lin’s concordance correlation coefficient. New Zealand: National Institute of Water & Atmospheric Research; 2005.
- Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(7 Suppl.):22–25.
- Travis LB. The epidemiology of second primary cancers. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2020–2026.
- Soerjomataram I, Coebergh JW. Epidemiology of multiple primary cancers. In: Clifton NJ, editor. Methods in molecular biology. Rotterdam: Department of Public Health, Erasmus MC; 2009. p. 85–105.
- Shim SH, Kim DY, Kim HJ, et al. Stratification of risk groups according to survival after recurrence in endometrial cancer patients. Medicine. 2017;96:e6920.
- Sahin H, Meydanli MM, Sari ME, et al. Recurrence patterns and prognostic factors in lymphovascular space invasion-positive endometrioid endometrial cancer surgically confined to the uterus. Taiwan J Obstet Gynecol. 2019;58(1):82–89.
- Miyahara D, Yotsumoto F, Hirakawa T, et al. Clinical features of recurrence in patients without residual tumour in endometrial cancer. Anticancer Res. 2019;39(8):4581–4588.
- Rasmussen LA, Jensen H, Virgilsen LF, et al. Time from incident primary cancer until recurrence or second primary cancer: risk factors and impact in general practice. Eur J Cancer Care (Engl). 2019;28(5):e13123.
- Ørtoft G, Lausten-Thomsen L, Høgdall C, et al. Lymph-vascular space invasion (LVSI) as a strong and independent predictor for non-locoregional recurrences in endometrial cancer: a Danish Gynecological Cancer Group Study. J Gynecol Oncol. 2019;30(5):e84.
- Ørtoft G, Høgdall C, Hansen ES, et al. Survival and recurrence in stage II endometrial cancers in relation to uterine risk stratification after introduction of lymph node resection and omission of postoperative radiotherapy: a Danish Gynecological Cancer Group Study. J Gynecol Oncol. 2020;31:e22.
- Hassett MJ, Ritzwoller DP, Taback N, et al. Validating billing/encounter codes as indicators of lung, colorectal, breast, and prostate cancer recurrence using 2 large contemporary cohorts. Med Care. 2014;52(10):e65–e73.
- Hassett MJ, Uno H, Cronin AM, et al. Detecting lung and colorectal cancer recurrence using structured clinical/administrative data to enable outcomes research and population health management. Med Care. 2017;55:e88–e98.
- Deshpande AD, Schootman M, Mayer A. Development of a claims-based algorithm to identify colorectal cancer recurrence. Ann Epidemiol. 2015;25(4):297–300.
- Ritzwoller DP, Hassett MJ, Uno H, et al. Development, validation, and dissemination of a breast cancer recurrence detection and timing informatics algorithm. JNCI J Natl Cancer Inst. 2018;110(3):273–281.
- Xu Y, Kong S, Cheung WY, et al. Development and validation of case-finding algorithms for recurrence of breast cancer using routinely collected administrative data. BMC Cancer. 2019;19(1):210.
- Izci H, Tambuyzer T, Tuand K, et al. A systematic review of estimating breast cancer recurrence at the population level with administrative data. JNCI J Natl Cancer Inst. 2020;112:djaa050.
- Uno H, Ritzwoller DP, Cronin AM, et al. Determining the time of cancer recurrence using claims or electronic medical record data. JCO Clin Cancer Inform. 2018;2:1–10.
- Chubak J, Onega T, Zhu W, et al. An electronic health record-based algorithm to ascertain the date of second breast cancer events. Med Care. 2017;55(12):e81–e87.