Introduction
Prostate cancer is the most common form of cancer in Dutch men [Citation1]. Chemical or surgical castration is a key strategy in patients with locally advanced or metastatic prostate cancer [Citation2]. Currently, chemical and surgical castration are considered equally effective modalities to achieve castration levels of testosterone [Citation3,Citation4]. Chemical castration is achieved by administering Luteinizing Hormone Releasing Hormone (LHRH) agonists on a regular basis (either intermittently or continuously) [Citation5–7].
In patients with advanced prostate cancer, the treatment aim is to maintain serum testosterone levels below castrate level (<1.7 nmol/L) [Citation8,Citation9]. However, after prescribing LHRH agonists physicians do not monitor the testosterone levels routinely. Moreover, current dosing regimens are manufacturer-recommended and not personalized per patient.
Several studies have shown that serum testosterone levels can remain at or below castrate levels for over 3 months after discontinuation of LHRH agonist injections [Citation10–15]. This creates opportunities for a personalized way of dosing LHRH agonists depending on the patient’s testosterone level and by monitoring the testosterone levels closely (scrutinization).
Current randomized, controlled trial was initiated to investigate the possibility to extend the dosing interval of goserelin 10.8 mg with a testosterone level-based dosing regimen and to determine whether this new regimen is cost-saving.
Patients and methods
Study design and participants
This study was a randomized (1:2), controlled trial. Patients were randomized to (1) standard treatment: 3-monthly depot injections of goserelin 10.8 mg (control group) or (2) injections based on testosterone-serum levels (interventional group). Included were males (≥18 year) diagnosed with locally advanced or metastatic prostate cancer with a clinical indication for ADT (≥2 years or permanently). Patients were included before or in the 2 months after the first injection of goserelin 10.8 mg. Patients received goserelin 10.8 mg injections by the home service of the outpatient pharmacy. All participants gave written informed consent before any study-related activity. Excluded were patients with a history of hypersensitivity to LHRH agonists, receiving anti-androgens (bicalutamide for 4 weeks around the first injection was allowed) and patients unable to measure testosterone levels at the hospital.
A serious adverse event was defined as a testosterone level above castration level (testosterone level > 1.7 nmol/L).
The study was performed at the Franciscus Gasthuis & Vlietland, Rotterdam and Schiedam (The Netherlands). The study was approved by Ethical Committee and registered in the Dutch Trial Registry (Nederlands Trial Register), number NTR6537 and conducted in accordance with the principles of the declaration of Helsinki. The final version of the study protocol is included as supplementary material.
Randomization and masking
Participants were randomly assigned (2:1) to either the intervention group or the control group. Stratification was done for concurrent use of chemotherapy. The randomization code was generated by Castor EDC [Citation16]. Blinding of participants and physicians was not applied.
Procedures
Control group: Patients were treated as usual being 3-monthly depot injections of goserelin 10.8 mg, regardless of the testosterone level. Testosterone levels were measured 2 weeks before the next depot injection of goserelin 10.8 mg.
Intervention group: At week 10 after an injection of goserelin 10.8 mg testosterone levels were measured. According to the following algorithm the next injection of goserelin 10.8 mg was postponed at week 12 if the testosterone level was <1.2 nmol/L and had increased maximal 0.5 nmol/L from the nadir.
When the next injection of goserelin 10.8 mg was postponed a new testosterone level was measured after 4 weeks, according to the study of Pathak et al., and checked with the algorithm [Citation11]. This cycle continued for every patient as long as the testosterone level did not meet the criteria for a repeat injection.
Testosterone levels were measured using a validated method on Abbott I2000SR analyzers in heparin plasma.
Outcomes
The primary outcome was median number of goserelin 10.8 mg injections per participants per strategy during follow-up. Secondary outcome was the difference in costs between the treatment strategies. Costs included costs for the goserelin 10.8 mg injections, laboratory tests, regular outpatient monitoring costs and costs due to treatment complications.
Statistical analysis
A sample size of 42 patients was planned for this study. To be able to reject the hypothesis that the median number of injections per patient during follow-up is equal between the treatment strategies the following assumptions were taken into account: during 24 months 6 injections of goserelin 10.8 mg in the interventional group and 8 injections of goserelin 10.8 mg in the control group, a standard deviation of 1.5, an alpha (2-sided) of 0.05, a power of 80% and a randomization ratio of 1:2.
The primary endpoint was analyzed using the two-sample Poisson test. Since the length of follow-up was different between the intervention and control group, the cumulative cost difference was estimated using the sum-limit method. Differences were tested using bootstrapping. Significance level was set at p < 0.05.
All data were collected in CastorEDC and statistical analyses were performed using IBM Statistics SPSS, version 25 (SPSS, Chicago, IL) and GraphPad Prism version 7.02 (GraphPad Software, LA Jolla, CA, USA).
Results
Between August 2017 and May 2018, 19 patients were enrolled. One participant randomized in the intervention group withdrew before start of the intervention due to mental health issues (Supplementary Figure 1).
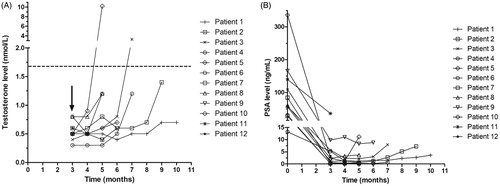
shows the baseline characteristics. Most patients included did not receive any previous prostate cancer therapy. All patients had a performance status of 0 or 1 on the Zubrod scale [Citation17]. Because of two SAEs in two participants in the interventional group the study was prematurely ended. The SAEs occurred in the participants 5 and 7 months after the first injection of goserelin 10.8 mg.
Table 1. Baseline characteristics.
Primary endpoint
The median number of goserelin 10.8 mg injections per patient during follow-up was 2 (IQR: 1.75–3.25) in the control group versus 1 (IQR: 1.00–2.00) in the interventional group. The number of goserelin 10.8 mg injections per patients was not different between the two groups (incidence density: 0.58 (0.28–1.18), p = 0.13). The median time to the next injection goserelin 10.8 mg in the intervention group was 22.8 (range 14–40) weeks. In the control and the interventional group, the median follow-up in weeks per patient was 21.5 (IQR: 14.4–30.1) versus 22.5 (19.3–25.0), the total follow up of all patients was 138.3 versus 277.7 weeks and the total number of given goserelin 10.8 mg injections was 14 versus 16.
shows the testosterone and PSA levels measured in the interventional arm during follow-up. One goserelin 10.8 mg injection was given in month zero to all patients shown. Except for two measurements, all testosterone levels are below castrate level (<1.7 nmol/L). Due to the premature end of the study, patients have differences in the follow-up time.
Secondary endpoint
In total, in the control and interventional group the mean costs per patient per day was €6.06 (5.99–6.13) versus €4.37 (4.29–4.46). The mean costs per patient per day are 27,9% lower in the intervention group compared with the control group (supplementary Table 1).
Discussion
This is the first randomized, controlled trial comparing a testosterone-based dosing regimen and usual treatment with goserelin 10.8 mg injections. The investigated dosing algorithm in this trial is unsafe due to a rapid and unexpected rise in the testosterone level above castrate level (> 1.7 nmol/L) in two participants treated with the testosterone-based dosing algorithm. Therefore, the study is prematurely ended because of these two events in the interventional group.
Our results show that the testosterone concentration stays longer below the castrate level as described in current literature. Dai et al. showed that, in LHRH-agonist naïve patients, 1 and 3 month(s) after cessation of monthly injections of goserelin acetate 3.6 mg or triptorelin acetate 3.75 mg, testosterone recovered to supracastrate level in 43.2% and 97.9% of the patients [Citation18]. Furthermore, Gulley et al. found that, in LHRH-agonist naïve patients, after 6 months of injections of leuprolide 22.5 mg and goserelin 10.8 mg, more than 80% of the patients achieved supracastrate serum testosterone level by 15 weeks. After a second cycle of 6 months of injections of leuprolide 22.5 mg and goserelin 10.8 mg, in the same patients, Gulley et al. found that more than 90% of the patients achieved supracastrate serum testosterone level by 18 weeks [Citation19]. We found a median time to the next injection goserelin 10.8 mg in the intervention group of 22.8 weeks. For all patients in the intervention group the next injection of goserelin 10.8 mg could be postponed safely for 4 months.
The difference in recovery time of the testosterone level to supracastrate levels compared with current literature might be explained by the fact that our patients had other patient characteristics. Several patient characteristics are possibly correlated to the recovery time of the testosterone levels to supracastrate levels after cessation of ADT, e.g. age, Gleason score, ethnicity, testosterone level before start ADT and treatment duration of ADT [Citation18,Citation19]. Our patients had received a different pretreatment, had higher Gleason scores and higher PSA levels at initiation of ADT in comparison with the trials performed by Dai et al. and Gulley et al.
The testosterone level appears to have a non-linear course in the time, which probably may have contributed to the occurrence of the two SAEs. We applied a rather conservative algorithm, however a sudden rise in testosterone will only be captured with a more intensive measurement strategy. After the administration of goserelin 10.8 mg, LHRH-receptors in the pituitary gland are downregulated. This causes a reduction in the production of FSH and LH and eventually a reduced testosterone production in the testis [Citation20]. However, a certain time after cessation of ADT the negative feedback loop will become ineffective and a rapid rise in the testosterone level will occur. Possible explanations are reactivation of the available LHRH-receptors, a fast upregulation of LHRH-receptors or a combination of both mechanism. These effects might be stronger in patients that only had a limited number of injections of LHRH. Therefore, investigating a testosterone based strategy in patients that have already been treated with ADT for a longer period of time (> 1 year) could be interesting.
There are several strengths to note in this MIDAS trial. First, this is the first randomized controlled trial testing the testosterone-based dosing regimen. Second, despite the fact that the study was prematurely ended we achieved a good median follow up in the control and interventional group. Treatment compliance among patients starting treatment was good due to the fact that the goserelin injections were administered at home. Finally, this trial adds evidence that prolonging the dosing interval to once every 4 months keeps testosterone low and prevents for antineoplastic medication overuse. There are also some limitation to our study. The most important limitation is the fact that we measured the testosterone levels every 4 weeks in the intervention group. If testosterone levels had been measured more frequently, we might have detected the rapid rise in testosterone level without reaching supracastrate levels. However, at this time the clinical opinion is that a short rise in testosterone level above the castrate level has no clinical implication. Furthermore, we did not measure patients satisfaction with the new dosing regimen.
Despite the fact that LHRH agonists have been available over the past 30 years, so far no research has been done to determine whether efficiently dosing LHRH agonists reduces costs. In the Netherlands, approximately 8000 patients per year are considered for starting ADT. The majority of these patients (95%) choose chemical castration by a LHRH agonist [Citation1]. Our study shows that for all patients in the intervention group the next injection of goserelin 10.8 mg could be postponed safely with at least 1 month. Based on the available literature and this study, if we start dosing goserelin 10.8 mg injections every 4 months instead of 3 months, total health care costs will drop with 2 million euros per year just in the Netherlands.
In summary, the investigated algorithm for testosterone level-based dosing of LHRH agonists, in which testosterone levels are monitored every 4 weeks when an injection is postponed, in patients with locally advanced or metastatic prostate cancer aiming for continuous testosterone suppression, in the first year of treatment, is unsafe due to the risk of a rapidly rising testosterone level.
Supplemental Material
Download MS Word (13.3 KB)Supplemental Material
Download MS Word (37.1 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Dutch Society for Urology. Prostate cancer guidelines, version 2.1. 2016. [cited 2019 April 17]. https://www.oncoline.nl/prostaatcarcinoom.
- Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1(4):293–297.
- Seidenfeld J, Samson DJ, Hasselblad V, et al. Single-therapy androgen suppression in men with advanced prostate cancer: a systematic review and meta-analysis. Ann Intern Med. 2000;132(7):566–577.
- Hedlund PO, Damber JE, Hagerman I, et al. Parenteral estrogen versus combined androgen deprivation in the treatment of metastatic prostatic cancer: part 2. Final evaluation of the Scandinavian Prostatic Cancer Group (SPCG) Study No. 5. Scand J Urol Nephrol. 2008;42(3):220–229.
- Hussain M, Tangen C, Higano C, et al. Evaluating intermittent androgen-deprivation therapy phase III clinical trials: the devil is in the details. J Clin Oncol. 2016;34(3):280–285.
- Verhagen PC, Wildhagen MF, Verkerk AM, et al. Intermittent versus continuous cyproterone acetate in bone metastatic prostate cancer: results of a randomized trial. World J Urol. 2014;32(5):1287–1294.
- Calais da Silva F, Calais da Silva FM, Goncalves F, et al. Locally advanced and metastatic prostate cancer treated with intermittent androgen monotherapy or maximal androgen blockade: results from a randomised phase 3 study by the South European Uroncological Group. Eur Urol. 2014;66(2):232–239.
- Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71(4):630–642.
- Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71(4):618–629.
- Oefelein MG. Serum testosterone-based luteinizing hormone-releasing hormone agonist redosing schedule for chronic androgen ablation: a phase I assessment. Urology. 1999;54(4):694–699.
- Pathak AS, Pacificar JS, Shapiro CE, et al. Determining dosing intervals for luteinizing hormone releasing hormone agonists based on serum testosterone levels: a prospective study. J Urol. 2007;177(6):2132–2135; discussion 2135.
- Pettersson B, Varenhorst E, Petas A, et al. Duration of testosterone suppression after a 9.45 mg implant of the GnRH-analogue buserelin in patients with localised carcinoma of the prostate a 12-month follow-up study. Eur Urol. 2006;50(3):483–489.
- Dijkman GA, Fernandez del Moral P, Plasman JW, et al. A new longer-acting LHRH analog depot: preliminary results of a Dutch open phase II clinical study on a 10.8 mg Zoladex 3-monthly depot. Eur Urol. 1990;18 Suppl 3:22–25.
- Hall MC, Fritzsch RJ, Sagalowsky AI, et al. Prospective determination of the hormonal response after cessation of luteinizing hormone-releasing hormone agonist treatment in patients with prostate cancer. Urology. 1999;53(5):898–902; discussion 902-893.
- Nejat RJ, Rashid HH, Bagiella E, et al. A prospective analysis of time to normalization of serum testosterone after withdrawal of androgen deprivation therapy. J Urol. 2000;164(6):1891–1894.
- Castor EDC. Castor Electronic Data Capture. 2019 Aug 28.
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655.
- Dai B, Qu YY, Kong YY, et al. Kinetics of testosterone recovery in clinically localized prostate cancer patients treated with radical prostatectomy and subsequent short-term adjuvant androgen deprivation therapy. Asian J Androl. 2013;15(4):466–470.
- Gulley JL, Aragon-Ching JB, Steinberg SM, et al. Kinetics of serum androgen normalization and factors associated with testosterone reserve after limited androgen deprivation therapy for nonmetastatic prostate cancer. J Urol. 2008;180(4):1432–1437; discussion 1437.
- Belchetz PE, Plant TM, Nakai Y, et al. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science. 1978;202(4368):631–633.