Abstract
Background
The increasing incidence of oral cavity squamous cell carcinoma (OSCC) is challenging the capacity to treat patients efficiently. The aim of this study was to evaluate the impact of time to treatment initiation (TTI) on overall survival (OS) and recurrence free survival (RFS) for patients with primary OSCC.
Material and methods
All patients with primary OSCC treated with curative intent at Rigshospitalet in the period 2000–2014 with known date of diagnosis and treatment initiation were included. Correlation analyses between TTI and Charlson comorbidity index (CCI), UICC stage, and year of diagnosis were performed in addition to uni- and multivariate Cox proportional hazard regression analyses. Further, interaction analysis of TTI and UICC stage were conducted.
Results
Eight hundred and sixty-two patients (64% men) with a median age at diagnosis of 62 years (range: 28–95 years) were included. The median TTI was 31 days (range: 2–137 days). Correlation analyses showed correlations between TTI and CCI, TTI and UICC stage, and TTI and year of diagnosis (rho = −0.10, p-value = <.01; rho = 0.16, p-value = <.001; rho = −0.47 p-value = <.001). Univariate analyses showed a statistically significant increase in hazard ratio for both OS and RFS with a five-day increase in TTI (HR = 1.05, 95%CI: 1.02–1.07 and HR = 1.04, 95%CI: 1.02–1.07). However, when adjusting for age, sex, smoking, UICC stage, tumor sublocation, CCI, and year of diagnosis in a multivariate analysis, the increase in HR with TTI was not statistically significant. There was no statistically significant interaction between TTI and UICC stage.
Conclusion
Survival of OSCC patients decreased with increasing TTI, yet not statistically significant in multivariate analysis. There was no difference in the effect of TTI between patients diagnosed in low or advanced stages.
Introduction
There are many possible reasons for delay in the treatment of head and neck cancer. Regarding delay in diagnosis, this might differ between the tumor subsites partly because the symptoms experienced by the patients are different. Glottic cancers are often diagnosed early because symptoms such as hoarseness develop quickly and often lead to medical examination when persistent. This is opposed to nasopharyngeal and hypopharyngeal cancer, which is associated with relatively common and nonspecific symptoms, often resulting in a late diagnosis. Oral cavity cancer seems to be somewhere between this and is most commonly associated with symptoms such as non-healing sores and persistent pain in the mouth [Citation1]. Reasons for delay in treatment after diagnosis differs mostly in relation to the tumor’s anatomical subsite and the different treatment approaches and planning for different locations.
The increase seen in most head and neck cancers, including oral cavity squamous cell carcinoma (OSCC), in the past decades has contributed to the added workload of health care systems worldwide [Citation2,Citation3]. Prolonged time to treatment has shown to impact survival for patients with head and neck squamous cell carcinoma (HNSCC); however, the results are inconsistent [Citation4,Citation5]. Further, only few studies investigate the sublocations of the head and neck region specifically, here among the oral cavity [Citation4,Citation5]. Therefore, knowledge of the impact of TTI for OSCC patients remains insufficient.
Nevertheless, great effort is put into diagnosing and treating patients as quickly as possible. In Denmark, the government and public health services introduced a fast-track program to optimize diagnosis and treatment for head and neck cancer in 2007 [Citation6]. The program included time standards for all clinical steps and an organizational schedule to align procedures across centers, including that all patients with suspect malignancies are referred to centralized hospitals with minimum delay. Further, the Danish healthcare system provides free access to all diagnostics and treatment procedures from general practitioners to hospitals, financed by general taxes, for all citizens and legal residents in Denmark. The standards of treatment are uniform, and treatment is initiated when indicated, irrespective of the patient’s economic and insurance circumstances. By law, there is no private hospital alternative. Combined, this provides an ideal basis for investigation of the impact of time to treatment on the outcome for OSCC patients, as selection-bias and confounding effects are minimized.
The aim of this study was to evaluate the impact of time from diagnosis to treatment initiation (TTI) on overall survival and recurrence-free survival for patients with OSCC.
Material and methods
We included patients from the Copenhagen Oral Cavity Squamous Cell Carcinoma (COrCa) database, which holds information for all patients treated for an oral cavity squamous cell carcinoma in the period 2000–2014 at Rigshospitalet, Copenhagen University Hospital, Denmark [Citation7]. All patients with primary OSCC and known dates of diagnosis and treatment initiation were included. Information on the date of histologically verified diagnosis, age at diagnosis, year of diagnosis, sex, smoking status, tumor sublocation, T-stage, N-stage, UICC7 stage, treatment, and date of treatment initiation were retrieved from the database. For treatment, patients receiving primary radiotherapy and patients receiving primary radiotherapy with concomitant chemotherapy were categorized together in the group ‘radiotherapy’.
Time to treatment initiation (TTI) was defined as the number of days from the date of histologically verified diagnosis to the date of treatment initiation (i.e. surgery or radiotherapy). Patients not treated with curative intent were excluded. Further, patients with a TTI of 0 days were excluded, as it was assumed that the treatment of these patients was planned based on a clinical diagnosis rather than a histologically verified diagnosis. Data were validated for all patients with TTI above 90 days.
Charlson comorbidity index score (CCI) at diagnosis was calculated for each patient based on data from the Danish National Patient Register, which was linked with COrCa database by the patients’ unique personal identification number [Citation8]. CCI is a comorbidity summary measure, and is is based on 19 medical conditions weighted from one to six based on its potential to influence mortality which are summed in a score. The theoretically highest possible score is 33 (Table S1). The OSCC diagnosis of the patients was not included in the calculation of the CCI score. All patients with a CCI score of 3 or above were categorized in one group, resulting in the following groups: 0, 1, 2, and 3 or above.
Overall survival (OS) and recurrence-free survival (RFS) were defined as the time from diagnosis plus TTI to censoring or one of the defined endpoints. The defined endpoints were death from any cause for OS and recurrence or death from any cause for RFS. Follow-up data for patients with less than five years of follow-up where updated by review of medical charts, resulting in follow-up data until at least five years were available for all included patients.
Based on prior studies on HNSCC the hypothesis of this study was that an increase in TTI would impact survival of OSCC patients negatively, and that UICC stage would influence the effect of TTI on survival, with prolonged TTI having a greater negative impact on survival for patients diagnosed in advanced stage compared to patients diagnosed in low stage.
Statistical analysis
Statistical analyses were performed in R statistics version 3.6.1 [Citation9]. Spearman rank correlation analyses were performed for TTI and UICC stage, TTI and CCI, TTI and year of diagnosis, and UICC and CCI to test if UICC stage, CCI, or year of diagnosis affected TTI or each other. A strong statistically significant correlation would lead to exclusion of the variable(s) from the cox-regression analysis. Relation between TTI and treatment modality (i.e. surgery, surgery with adjuvant radiotherapy, and radiotherapy) was evaluated with a boxplot.
Multivariate Cox proportional hazard regression analyses were performed for OS and RFS. Variables included in the multivariate Cox regression analyses were sex, age at diagnosis, smoking (i.e. never smoker, current smoker, former smoker), UICC stage, tumor sublocation (i.e. floor of mouth, oral tongue, gingiva, and others), and CCI (i.e. 0, 1, 2, or ≤3). The analyses were stratified for the year of diagnosis divided in the two periods 2000–2007 and 2008–2014 (i.e. included with different baseline HR). Sex, Smoking, Tumor sublocation and CCI were included as categorical variables. The remaining variables were included as linear continuous variables. To investigate if UICC stage impacted the effect of TTI an interaction analysis of TTI and UICC stage was included as well. The proportionality of variables was evaluated with log minus log curves.
For investigation of non-linear effect of TTI, restricted cubic splines adjusted for the same variables included in the multivariate cox-regression analysis were performed for both OS and RFS. The median TTI were chosen as reference in both models.
Absolute risk estimates were calculated based on the multivariate Cox regression using the package ‘riskRegression’. The absolute risk estimates were calculated for two-year survival as recurrence of OSCC most often occur within two years. Further, the absolute risk estimates were calculated for patients low and high UICC stage, to compare the impact of TTI for patients with the best and the worst prognosis.
p-Values equal to or less than .05 were considered statistically significant.
Results
Eight hundred and sixty-two patients of the 1399 patients in the COrCa database were included in this study (). The majority of the included patients were men (64%, n = 549), and the median age at diagnosis was 62 years (range: 28–95 years). The most common tumor location was the oral tongue (39%, n = 333) followed closely by the floor of mouth (37%, n = 322). Most of the patients were treated surgically (87%, n = 752); 51% (n = 384) of these also underwent neck dissection and 41% (n = 311) received adjuvant radiotherapy. The overall median TTI was 31 days (range: 2–137 days) (). The median TTI decreased from 43 days (range: 6–137 days) for patients diagnosed in 2007 or before to 27 days (range: 2–87 days) for patients diagnosed after 2007, corresponding to a decrease of 37% ().
Figure 1. Depiction of the patients from the Copenhagen Oral Cavity Squamous Cell Carcinoma (COrCa) database included in this study, and reasons for exclusions of the remaining patients.
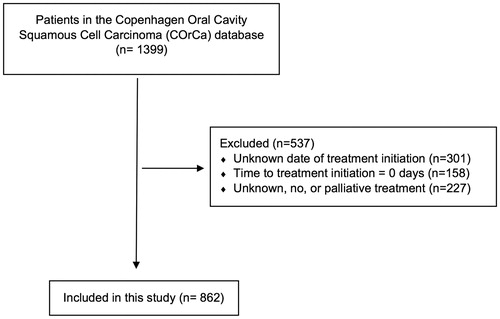
Figure 2. Median TTI per year for oral cavity squamous cell carcinoma patients diagnosed and/or treated at the University Hospital of Copenhagen in Denmark in the period 2000–2014, with trendline (blue line) and 95% confidence interval (grey area).
Table 1. Baseline characteristics for patients with oral cavity squamous cell carcinoma diagnosed or treated at the University Hospital of Copenhagen, Denmark in the period 2000–2014.
The correlation analysis showed a statistically significant but weak positive correlation between TTI and UICC (rho = 0.16, p-value = <.001). For TTI and CCI there was a weak negative correlation (rho = −0.10, p-value = <.01). The correlation was also negative for TTI and year of diagnosis, but in this case moderate (rho = −0.47 p-value = <.001). There was no statistically significant correlation between UICC and CCI.
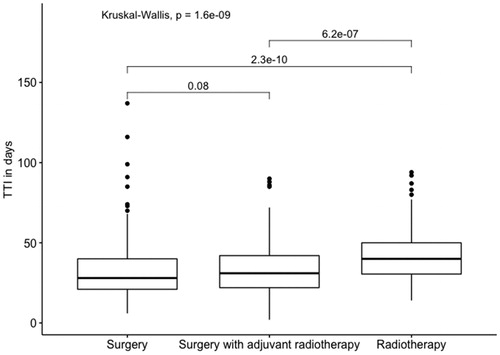
The relation between TTI and treatment modality showed a statistically significant longer TTI for patients receiving primary radiotherapy compared to patient treated surgically ().
Figure 3. Boxplot depicting relation between TTI and treatment modality for oral cavity squamous cell carcinoma patients diagnosed and/or treated at the University Hospital of Copenhagen in Denmark in the period 2000–2014.
Based on evaluation of log minus log plots no violation of proportionality were found for UICC stage why this variable was included as a continuous variable in the cox-regression analysis. However, year of diagnosis, tumor sublocation, and CCI was found non-proportional and the multivariate Cox regression analysis was therefore stratified by the year of diagnosis time periods 2000–2007 and 2008–2014, and tumor sublocation and CCI were included as categorical variables.
Univariate Cox regression analyses showed a statistically significant increase in HR for both OS and RFS with a five-day increase in the TTI (HR = 1.05, 95%CI: 1.02–1.07 and HR = 1.04, 95%CI: 1.02–1.07) (). However, when adjusting for age, sex, smoking, UICC stage, tumor sublocation, and CCI in multivariate analyses, the effect of TTI was not statistically significant, and the HR for TTI became very close or equal to 1. Further, there was no statistically significant interaction between TTI and UICC for either OS or RFS.
Table 2 . Uni- and multivariate cox regression analyses for patients with oral cavity squamous cell carcinoma diagnosed or treated at the University Hospital of Copenhagen, Denmark in the period 2000–2014, stratified for the time periods 2000–2007 and 2008–2014.
The restricted cubic spline showed no significant effect of TTI for either OS or RFS (Figure S1).
Absolute risk estimates were calculated based on the multivariate Cox regression analyses. A reference patient with the following data was defined: male gender, 60 years at diagnosis, diagnosed in the second half of the study period (i.e. 2008–2014), UICC stage I, CCI score 0, and a TTI of 14 days. This patient had a two-year OS of 89.4% (95% CI: 86.1–92.0). When increasing the TTI to 30 days, the two-year OS was 89.0% (95% CI: 85.7–91.6). When the UICC stage was increased to stage IV, the two-year OS for the 14-day TTI was 69.6% (95% CI: 61.4–76.4) and 68.6% (95% CI: 60.9–75.1) for a TTI of 30 days.
Discussion
It is undisputed that cancerous tumors of the head and neck grow with time and, simply put, large tumors are more difficult to treat and are associated with a worse outcome compared to small tumors. Nevertheless, the analysis of this study showed a decreasing survival with an increasing TTI, yet not statistically significant in a multivariate setting. Former reports on the impact of the TTI on recurrence and survival outcomes has been inconsistent, and only a few studies investigate OSCC specifically [Citation4,Citation5]. Among studies investigating HNSCC overall, numerous studies have found a statistically significant impact on outcome; however, there are also several studies reporting a statistical nonsignificant impact [Citation10–14]. Murphy et al. and van Harten et al. conducted the two largest studies on this topic. Investigating more than 50,000 and 13,000 patients, respectively. They found that increasing the TTI had a statistically significant negative impact on survival [Citation10,Citation12]. Murphy et al. found a statistically significant worse outcome among patients with a TTI above 61 days and above 90 days. Among the patients included in our study, 6.5% had a TTI above 60 days and only 0.7% above 90 days. The lack of statistical significance in the results of this study could, therefore, be explained partly by the fact that OSCC patients in Denmark, in general, receive treatment relatively fast.
Jensen et al. and Waaijer et al. investigated tumor volume doubling time (TDV) and reported a median of 99 days and 96 days respectively [Citation15,Citation16]. It is, however, important to note that both studies included relatively few patients and found a wide variance in the TDV between patients. Kowalski et al. found that the growth rate of tumors correlated with stage, with low stage tumors having a slower growth rate compared to tumors in advanced stages [Citation17]. A relatively high percentage (>50%) of patients included in our study were diagnosed in stages I and II. This can further add to the explanation of why the analysis of this study did not reach statistical significance, as a large part of the included patients were diagnosed in stages correlated to a slow tumor growth rate. The interaction analysis did not, however, find a statistically significant interaction between TTI and UICC stage at diagnosis; hence our data show no indication that TTI has a different impact on RFS or OS between the different UICC stages. This conflicts with the results from Murphy et al. who found that mortality risk according to the TTI was higher for patients diagnosed in stages I and II compared to patients diagnosed in stages III and IV [Citation10]. The authors explained this by noting that patients diagnosed in low stages had a higher risk of stage progression during the waiting time, due to the risk of developing lymph node metastases.
Even though the abovementioned explanations are feasible explanations of why the results of our analyses did not reach statistical significance, it should be noted that we included all patients diagnosed in the Eastern Denmark region in the period 2000–2014; hence there was no selection bias in our data. Therefore, it might still be true that TTI does not have a statistically significant impact on outcome in this population, as long as patients, in general, are treated relatively fast. This might allow loosening the strict time standards for initiation of treatment applied in the Danish health care system. Instead we could regain focus on other factors such as patients being affiliated with one physician who follows the patient throughout their process of diagnosis and treatment, which often is sacrificed in the effort to keep the TTI as short as possible.
As almost all citizens in Denmark are guaranteed payment during sick leave, there is almost no economic downside to longer waiting times for the patients. However, longer waiting times will inevitably be associated with psychological stress for the patients. This however, could perhaps be reduced with implementations that would be made possible when allowing longer waiting times.
The median TTI of the included patients diagnosed after 2007 (i.e. the year of implementations of the time standards for initiation of treatment in Denmark) was 27 days, and as shown in the studies by Murphy et al. and van Harten et al., we might allow up to two or three months without affecting the outcome significantly. However, we are hesitant to draw definitive conclusions from our results keeping in mind that absence of significance does not necessarily implies significance of absence.
Longer waiting times for patients with small tumors compared to large tumors have been reported previously and has been raised as an issue when evaluating the impact of the TTI on survival, that is, ‘the waiting time paradox.’ [Citation12,Citation18,Citation19] The opposite was evident in the data included in this study. Patients diagnosed in high UICC stages had a slightly longer TTI compared to patients diagnosed in low stages. Overall, however, there was very little difference in the median TTI between patients diagnosed in low stages versus advanced stages. This is plausible due to the fast-track programs introduced in Denmark in 2007, which ensure that all patients are treated as efficiently as possible independent of the stage at diagnosis [Citation6]. Why patients diagnosed in advanced UICC stages had longer TTIs compared to patients diagnosed in low stages has several explanations: Patients diagnosed in more advanced UICC stages often have more comorbidities, the surgical procedures needed are often of greater extent, and the patients more often receive primary radiotherapy. All are factors that lead to longer planning time before treatment can be initiated.
The introduction of the fast-track programs in 2007 further explains why the median TTIs for patients diagnosed after 2007 were 37% shorter than the median for patients diagnosed in 2007 or before. We additionally investigated if there was a difference in the effect of TTI for patients diagnosed before and after 2007. The only difference was that the effect of TTI was statistically significant in the univariate analysis for patients diagnosed before 2007 whereas it was not statistically significant for patients diagnosed after 2007. In the multivariate analysis, it was not statically significant in either time periods (Table S2). This further supports the view that there is not necessarily a benefit in decreasing TTI when patients already are treated relatively fast, as has been the case in Denmark at least since the new millennium, and especially after the introduction of fast track programs in 2007.
An important factor when evaluating treatment delay is the time from the patients’ first awareness of symptoms until contact with the health care system and diagnosis of the cancer, often referred to as patient delay. In this study, TTI was defined as the time from histologically verified diagnosis to initiation of treatment and did not include investigation of delay before diagnosis. Even though patient delay is difficult to investigate because it often relies on the patients’ recall of when they first became aware of symptoms, which is associated with substantial inaccuracy, it might have a large impact on the outcome. Schutte et al. conducted a review of the literature on all forms of delay. Among numerous other findings, they reported that, particularly for OSCC, delay was associated with increased stage at diagnosis [Citation4]. Together with the results presented in this study, this might indicate that the interval most important for the outcome of OSCC patients is not the time between diagnosis and treatment but perhaps the time until definitive diagnosis is established.
In conclusion, time to treatment of OSCC should be minimized without compromising the quality of treatment. However, to assure the latter, the results of this study indicate that, in terms of time from diagnosis to treatment, we might allow weeks instead of days. Within the coming decades, we hopefully will be able to individualize the TTI for patients to assure the shortest TTI for those who would benefit the most from it; however, it seems that UICC staging is insufficient in this matter.
Ethical approval
Permission to analyze the retrospective data included in this study were provided by Danish Patient Safety Authority (31-1521-175) and the Danish Data Protection Agency (P-2020-363).
The study was performed in accordance with the Declaration of Helsinki.
Supplemental Material
Download MS Word (16.2 KB)Supplemental Material
Download TIFF Image (2.7 MB)Disclosure statement
The authors declare no conflicts of interest.
Data availability statement
Data are available upon request to the corresponding author, however requires specific permission from the Danish Patient Safety Authority and the Danish Data Protection Agency which can be applied for.
References
- American Cancer Society. Signs and Symptoms of Oral Cavity and Oropharyngeal Cancer. [cited 2020 Mar 12]. Available from: https://www.cancer.org/cancer/oral-cavity-and-oropharyngeal-cancer/detection-diagnosis-staging/signs-symptoms.html. Published 2018.
- Simard EP, Torre LA, Jemal A, et al. International trends in head and neck cancer incidence rates: Differences by country, sex and anatomic site. Oral Oncol. 2014;50(5):387–403.
- Jakobsen KK, Grønhøj C, Jensen DH, et al. Increasing incidence and survival of head and neck cancers in Denmark: a nation-wide study from 1980 to 2014. Acta Oncol. 2018;57(9):1143–1151.
- Schutte HW, Heutink F, Wellenstein DJ, et al. Impact of time to diagnosis and treatment in head and neck cancer: a systematic review. Otolaryngol Head Neck Surg. 2020;162(4):446–457.
- Coca-Pelaz A, Takes RP, Hutcheson K, et al. Head and neck cancer: a review of the impact of treatment delay on outcome. Adv Ther. 2018;35(2):153–160.
- Danish Health Authority. Cancer Pathways. [cited 2019 Sep 24]. Available from: https://www.sst.dk/en/national-clinical-guidelines.
- Schmidt Jensen J, Jakobsen KK, Mirian C, et al. The Copenhagen Oral Cavity Squamous Cell Carcinoma database: protocol and report on establishing a comprehensive oral cavity cancer database. Clin Epidemiol. 2019;11:733–741.
- Thygesen LC, Daasnes C, Thaulow I, et al. Introduction to Danish (nationwide) registers on health and social issues: structure, access, legislation, and archiving. Scand J Public Health. 2011;39(7 Suppl):12–16.
- R Core Team. R: A language and environment for statistical computing. 2017. Available from: https://www.r-project.org/.
- Murphy CT, Galloway TJ, Handorf EA, et al. Survival impact of increasing time to treatment initiation for patients with head and neck cancer in the United States. J Clin Oncol. 2016;34(2):169–178.
- Grønhøj C, Jensen D, Dehlendorff C, et al. Impact of time to treatment initiation in patients with Human Papillomavirus-positive and -negative oropharyngeal squamous cell carcinoma. Clin Oncol. 2018;30(6):375–381.
- van Harten M, Hoebers FJP, Kross KW, et al. Determinants of treatment waiting times for head and neck cancer in the Netherlands and their relation to survival. Oral Oncol. 2015;51(3):272–278.
- Caudell JJ, Locher JL, Bonner JA. Diagnosis-to-treatment interval and control of locoregionally advanced head and neck cancer. Arch Otolaryngol Head Neck Surg. 2011;137(3):282–285.
- Barton MB, Morgan G, Smee R, et al. Does waiting time affect the outcome of larynx cancer treated by radiotherapy? Radiother Oncol. 1997;44(2):137–141.
- Jensen AR, Nellemann HM, Overgaard J. Tumor progression in waiting time for radiotherapy in head and neck cancer. Radiother Oncol. 2007;84(1):5–10.
- Waaijer A, Terhaard CHJ, Dehnad H, et al. Waiting times for radiotherapy: Consequences of volume increase for the TCP in oropharyngeal carcinoma. Radiother Oncol. 2003;66(3):271–276.
- Kowalski LP, Carvalho AL. Influence of time delay and clinical upstaging in the prognosis of head and neck cancer. Oral Oncol. 2001;37(1):94–98.
- Wildt J, Bundgaard T, Bentzen SM. Delay in the diagnosis of oral squamous cell carcinoma. Clin Otolaryngol Allied Sci. 1995;20(1):21–25.
- Crawford SC, Davis JA, Siddiqui NA, et al. The waiting time paradox: population based retrospective study of treatment delay and survival of women with endometrial cancer in Scotland. BMJ. 2002;325(7357):196.
