Abstract
Background
Previous studies reported that cigarette smoking during radiation therapy was associated with unfavorable outcomes in various cancers using medical interviewing or monitoring of cotinine. Here, we evaluated the effect of smoking cessation on definitive radiation therapy for early stage glottic carcinoma by monitoring expiratory carbon monoxide (CO).
Material and methods
We enrolled 103 patients with early glottic carcinoma (T1N0/T2N0 = 79/24) who underwent conventional radiotherapy between 2005 and 2016. The median age was 70 years. Pathologically, all patients had squamous cell carcinoma. Since 2009, we confirmed smoking cessation before radiation therapy by medical interviews. Since 2014, we measured expiratory CO to strictly monitor smoking cessation. The patients were divided according to diagnosis years: ‘no cessation’ (2005–2008), ‘incomplete cessation’ (2009–2013), and ‘complete cessation’ (2014–2016). We retrospectively analyzed the local recurrence rate and disease-free survival (DFS).
Results
The median follow-up period was 60.1 months (range, 1.9–110.0 months). The 2-year local recurrence rate in the ‘complete cessation’ group was 5.3% and tended to be lower than that in the ‘incomplete cessation’ group (13.7%) and ‘no cessation’ group (21.2%). Multivariate analysis revealed that ‘no cessation’ was a risk factor for DFS (hazard ratio [HR] = 4.25) and local recurrence rate (HR = 16.5, p < .05) compared to ‘complete cessation.’
Discussion
We confirmed that the ‘complete cessation’ group had better prognosis than the ‘no cessation’ group by monitoring expiratory CO during radiation therapy for early stage glottic carcinoma. Moreover, monitoring expiratory CO was easier and more suitable than conventional methods for evaluating smoking cessation because it provided real-time measurements.
Background
Laryngeal carcinoma is the most common head and neck tumor with 3.9 new cases per 100,000 persons per year in Japan, and the proportion of male patients is 10-fold higher than that of female patients. Glottic carcinoma comprises majority of the cases of laryngeal carcinoma, and it is often diagnosed at an early stage due to the ease of detecting initial symptoms, such as hoarseness, and low frequency of lymph node metastasis [Citation1]. Radiation therapy is one of most common treatment methods for early glottic carcinoma because it is function sparing and radical.
Cigarette smoking is one of the strongest causes of glottic carcinoma, and more than 95% of the patients are active or former smokers. In general, cigarette smoking during radiation therapy for various cancers was reported to be associated with poor therapeutic results [Citation2,Citation3], and it was hypothesized that smoking induces hypoxia in the tumor environment and attenuates the effect of radiation on tumors [Citation4–6]. Especially for head and neck tumors, several studies reported that smoking was an important factor that worsens the prognosis for overall survival (OS) rate, local control rate, and complications [Citation7–10]. However, these studies included head and neck tumors of all regions, and the effects on tumors in individual regions remain unknown. Furthermore, recently, intensity-modulated irradiation (IMRT) has been used more commonly than conventional radiation therapy, especially for head and neck tumors, making it difficult to compare results obtained in recent studies with those in previous studies. Contrarily, for early glottic carcinoma, there has been no change in the treatment method, and it is easy to compare the current treatment results with the previous results.
We had no specific restrictions on smoking during irradiation until 2009, and at that time, almost all patients with glottic carcinoma in our area were active smokers, even when undergoing radiation therapy. We have been using smoking cessation guidance through medical interviews and did not offer radiotherapy to cancer patients who were active smokers since 2009. Additionally, we have been monitoring expiratory carbon monoxide (CO) since 2014. We can easily detect smoking in real-time by monitoring expiratory CO even when patients have smoked within a few days [Citation11]. Furthermore, the level of expiratory CO was reported to be associated with tissue hypoxia [Citation12]. In this study, we investigated the therapeutic effect of complete smoking cessation on radical radiotherapy for early glottic carcinoma by measuring expiratory CO.
Materials and methods
Patients
We included a total of 103 consecutive patients who underwent definitive radiation therapy for early stage (T1N0M0, or T2N0M0) glottic carcinoma between 2005 and 2016 at the Osaka General Medical Center in this retrospective study. Clinical staging was based on the 7th edition of the UICC TNM classification, and we performed re-staging for patients who underwent radiation therapy before 2009. All patients underwent baseline imaging studies including nasopharyngolaryngoscopy with biopsies, microsurgery of the larynx using direct laryngoscope, neck computed tomography (CT), and/or 18-fluorodeoxyglucose positron emission tomography-CT (FDG-PET/CT).
Two independent radiation oncologists collected relevant information from medical records. Written informed consent was obtained from all patients according to the Declaration of Helsinki, and the institutional ethics committee of Osaka General Medical Center approved this study (approved number: 28-S0705).
Radiation therapy
All patients were treated with three-dimensionally planned radiation therapy that consisted of a conventional opposing 2-field technique with a 15–45° wedge filter and irradiated field of 5–6 cm square (upper: inferior border of tongue bone, lower: inferior border of cricoid cartilage, and posterior: anterior border of vertebra) and extended by 1–2 cm to the cranial or caudal side according to the T2 index (Supplementary Figure 1). Standard fractionated irradiation (2 Gy/fraction [fr] and 5 fr per week) was performed for the majority of the patients, excluding 2 patients who were treated with mild hypofractionated irradiation (2.25 Gy/fr, up to 63 Gy and 65.25 Gy for 1 patient, respectively). The total dose range was 60–70 Gy, and it was determined by each attending physician after considering the clinical stage, extent of the disease, or response during irradiation. We used α/β index of 10 Gy for glottic carcinoma and calculated the equivalent dose in 2-Gy fractions (EQD2) to compare and estimate different fractions of radiation therapies. The median total dose with clinical stage was T1 = 66 Gy and T2 = 70 Gy (Supplementary Table 1). We administered radiation therapy to all patients using a linear accelerator, and the energy of X-ray was 4 MV (until 2010) or 6 MV (since 2011).
Concomitant chemotherapy
Concomitant chemoradiation therapy was administered to 36 patients. Most of these patients had clinical stage T2 and some had clinical stage T1 (Supplementary Table 1). Administration of chemoradiation therapy was determined by otolaryngologists after considering the disease status (i.e. T1 with extended disease field) in each patient. Typical chemotherapeutic regimens are listed in .
Table 1. Patient characteristics.
Monitoring of smoking cessation
We did not monitor the smoking status of patients during radiation therapy before 2009. Since 2009, we started monitoring smoking cessation from the initial visit and did not administer radiation therapy in patients who were active smokers. We checked the smoking status through medical interviews since 2009. Additionally, since 2014, we have been using Smokerlyzer®︎ (Bedfont Scientific Ltd. Kent. UK), which detects expiratory CO, to strictly monitor their smoking cessation during treatment. Smokerlyzer® can quantitatively measure the level of expiratory CO and detect whether the patient has been smoking within a few days [Citation13]. Therefore, after 2014, there were no active smokers among patients who were being treated for head and neck cancer. Complete smoking cessation was defined as the level of expiratory CO that was stably maintained at 0–2 ppm without an increase for 4 weeks. The level was strictly adhered to, and we considered it as smoking even if they were exposed to secondhand smoke [Citation14]. Therefore, educational recommendation was provided on avoiding secondhand smoke, which affected the body as much as the mainstream smoke.
Toxicities and follow-up
Complications occurring in <180 days since the start of radiation were defined as acute and those occurring later were defined as late toxicities. In most patients, toxicities and disease recurrence were assessed weekly during radiation therapy and every 3–4 months after treatment completion for up to 5 years. All recurrences were detected using a direct laryngoscope and/or CT.
Statistical analysis
The patient characteristics were assessed using the Fisher’s exact test. OS and cause-specific survival (CSS) were calculated as the time from the start of the radiation therapy to death from any cause and regional disease, respectively. Disease-free survival (DFS) was calculated as the time from the start of the radiation therapy to death from any cause or recurrence. Time to local relapse period was calculated as the time from the start of the radiation therapy to the locoregional recurrence, and death from any cause except locoregional recurrence was regarded as competing risk. The probabilities of OS, CSS, and DFS were estimated according to the Kaplan–Meier method and were compared among groups according to diagnosis year by the log-rank test. The probabilities of local recurrence rate were estimated on the basis of cumulative incidence, and the results were compared using Gray's test. The Cox proportional-hazards regression models and Fine and Gray model were used to evaluate the effect of the variables on DFS and local recurrence rate, respectively. Univariate analyses were performed with the following factors: age (≤70 vs. >70 years), anemia (hemoglobin value of >2 g/dL below the lowest limit of normal), Brinkman index (<1000 vs. ≥1000), with or without concurrent chemotherapy, invasion to anterior commissure, stage (T1 versus T2), multiple cancers, total dose of radiation therapy (≥66 vs. <66 Gy), and diagnosis year. Cutoff values of age, Brinkman index, total dose of radiation therapy, and days until the start of the radiation therapy were defined at median values, respectively. We did not include sex as a confounder because the number of female patients were too small to conduct statistical analyses. To evaluate the effects of smoking cessation among groups of diagnosis year on the occurrence of both DFS and local recurrence rate, we performed multivariate analyses with risk factors which were reported at meta-analysis for early glottic carcinoma (anemia, invasion to anterior commissure, and stage)[Citation15] and the factors with significant background differences between groups. Results were expressed as hazard ratios (HRs) with 95% confidence intervals (CIs). All tests were 2 sided, and p < .05 was considered to be statistically significant. All statistical analyses were performed using EZR ver.1.42 (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [Citation16] which is a graphical user interface for R 4.0.0 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
The patient characteristics in this study are shown in . A total of 103 patients were included, and the number of patients according to diagnosis years were 48, 31, and 24 patients from 2005 to 2008, 2009 to 2013, and 2014 to 2016, respectively. These groups were defined as ‘no cessation,’ ‘incomplete cessation,’ and ‘complete cessation,’ respectively. The median follow-up period was 60.1 months (range, 1.9–110.0), and that in each group was as follows, ‘no cessation’ 61.1 months, ‘incomplete cessation’ 60.2 months, and ‘complete cessation’ 46.8 months.
The smoking status prior radiation therapy was similar among the three groups. There were no active smokers during radiation therapy since 2014 due to the strict monitoring of expiratory CO. Although before 2014, the proportion of patients who smoked during the treatment was unclear, almost all smokers included in the ‘no cessation’ group during the initial visit continued smoking even during radiation therapy. Other patient characteristics, including stage and concomitant chemoradiation therapy, were not significantly different between groups except for total dose and treatment duration of radiation therapy. Furthermore, the duration from the initial visit to the start of radiation therapy was longer for ‘complete cessation’ group, which confirmed smoking cessation. Pathologically, all patients had squamous cell carcinoma.
Survival and local recurrence
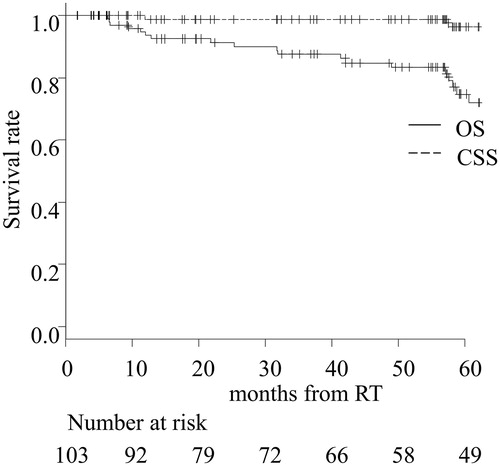
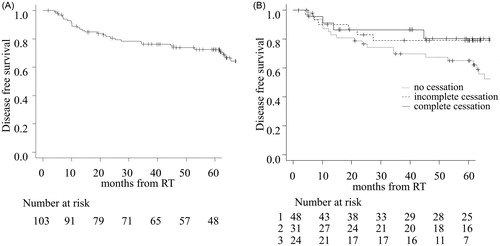
The 2- and 5-year OS in all patients was 91.3% and 83.3%, respectively. Both 2- and 5-year CSS was 98.8% (). There were no significant differences in OS and CSS among the groups (Supplementary Figure 2), and this may be because it was highly probable that salvage surgery had saved the patients with recurrent glottic carcinoma. The 5-year OS and CSS in patients with clinical stage (T1 vs. T2) was 85.0% vs. 75.6% (p < .05) and 98.6% and 100% (p = .41), respectively (Supplementary Figure 3). The 2- and 5-year DFS in all patients were 80.6% and 72.4%, respectively (). The 5-year DFS in patients was 65.0%, 79.2%, and 80.4% for the ‘no cessation,’ ‘incomplete cessation,’ and ‘complete cessation’ groups, respectively (). The ‘complete cessation’ group tended to have better prognosis than the other groups (p = .20).
Figure 2. Disease-free survival (DFS) in all patients (A) and each group (B). The number at risk for the three groups are designated as follows: 1 = no cessation, 2 = incomplete cessation, and 3 = complete cessation.
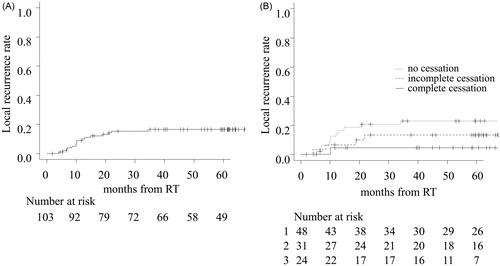
The 2- and 5-year local recurrence rates in all patients were 15.4% and 16.5%, respectively (). The 5-year local recurrence rate in the ‘complete cessation’ group was 4.5%, which was lower than that in the ‘incomplete cessation’ (13.7%) and in ‘no cessation’ group (23.5%) (, p = .21). The 5-year DFS in clinical stage T2 patients was significantly worse than that in T1 patients (54.2% vs. 77.0%, p < .01), and the local recurrence rate tended to be high (27.0% vs. 13.4%, p = .09) (Supplementary Figure 3).
Figure 3. Local recurrence rate in all patients (A) and each group (B). The number at risk for the three groups are designated as follows: 1 = no cessation, 2 = incomplete cessation, and 3 = complete cessation.
We conducted univariate and multivariate analyses for detecting the risk factors for DFS and local recurrence rate. In univariate analysis, clinical stage and anemia were significant risk factors for DFS. In addition, high total dose was an adverse risk factor for DFS in multivariate analysis. We did not include treatment duration and days until the start of treatment in variables used in multivariable analysis as it was highly correlated with total dose and cessation status, respectively. Furthermore, multivariate analysis revealed ‘no cessation’ was a risk factor for DFS and local recurrence rate as compared with ‘complete cessation’ (DFS: HR = 3.48, 95% CI, 1.07–11.3, p < .05; local recurrence rate: HR = 11.9, 95% CI, 1.05–136, p < .05) ().
Table 2. Univariate and multivariate analyses of risk factors for DFS and local recurrence rate.
Complications and second malignancies
We could not assess the acute complications in patients treated before 2007 because the respective detailed medical records were not available. Late complications, such as necrosis of the larynx, occurred in four patients, and was not different among the groups. However, the rate of voice preservation in the cessation groups tended to be higher than that in the ‘no cessation’ group due to late complications and local recurrence (96%, 90%, and 77% for ‘complete cessation,’ ‘incomplete cessation,’ and ‘no cessation’ group, respectively, p = .09). Of 103 patients, 21 developed second malignancies. Among them, the number in the ‘complete cessation’ group tended to be lower than that in other groups, and this may reflect the carcinogenesis prevention effect of smoking cessation ().
Table 3. Complications and second malignancies.
Discussion
This retrospective study analyzed the effect of the complete smoking cessation on definitive radiation therapy for early stage glottic carcinoma and confirmed there were significant differences in local control and survival between the complete smoking cessation and no smoking cessation patients by monitoring expiratory CO.
Several studies have assessed the effects of smoking on radiation therapy [Citation2,Citation7,Citation8,Citation10]. However, these studies used medical interviewing or monitoring of cotinine, which is a metabolite of nicotine, for evaluating smoking cessation. The monitoring of cotinine may be an incorrect method in the Japanese cohort because 20% of the population have gene polymorphism (homo- or heterozygous CYP2A6*4) in which the metabolism of cotinine is decreased and hence, we tend to underestimate the density of cotinine [Citation17]. In addition, medical interviewing may be incorrect due to the possibility of patients drafting false reports [Citation18].
Several hypotheses were proposed to explain why smoking during radiation therapy may lead to poor prognosis, such as smoking leading to chronic hypoxia in systemic and tumor tissues due to CO. The increase in expiratory CO concentration is a result of the increase in carboxyhemoglobin (CO-Hb) saturation degree in blood due to smoking. In general, the presence of CO results in a formation of CO-Hb, and since its affinity for hemoglobin is more than 200 times that of oxygen, CO-Hb is formed even at relatively low CO concentrations, reducing the ability of hemoglobin to carry oxygen to the tissues. In addition to the occurrence of reactions such as an increase in myocardial oxygen demand, it was shown to be involved in hypoxia in tumor tissue and result in radiotherapy resistance in in vivo models [Citation4,Citation5,Citation12]. Furthermore, anemia which is related to tissue hypoxia, was a risk factor for DFS in this study (). Moreover, monitoring expiratory CO is more suitable for clinical practice than the measurement of blood and urine cotinine because it provides real-time measurements at bedside. Therefore, we believe that it is the most useful smoking evaluation method considering the biological effects described above. On the contrary, the absorbance wavelengths of CO-Hb and oxyhemoglobin are similar, and the light source used in pulse oximeters is in the range of 660 nm; therefore, as hypoxemia is general does not get measured accurately by pulse oximeters due to inadvertent CO measurements, percutaneous oxygen saturation measurement is considered to be inaccurate in determining tissue hypoxia. Furthermore, since very small amount of oxygen gets dissolved in arterial blood when oxygen is administered under atmospheric pressure, it does not improve the hypoxia caused by CO (hyperbaric oxygen therapy may be required but is not realistic in the clinical situation). Therefore, smoking cessation is necessary to avoid hypoxia in tumor tissue. In the case of electronic cigarettes, as the level of expiratory CO is markedly decreased [Citation19], we may be unable to evaluate smoking cessation by monitoring expiratory CO [Citation20,Citation21]. However, the safety and hazards of electronic cigarettes are controversial, and their effect on radiation therapy is unclear.
Conventionally, radiation therapy for early stage glottic carcinoma has been administered in rectangular fields. Recently, although few studies have suggested that IMRT should be adopted as a new standard of care for early glottic carcinoma, there was no significant difference between conventional radiotherapy and IMRT for early glottic carcinoma, and therefore, it has been controversial [Citation22–24]. Contrarily, treatment for other head and neck carcinomas is dominated by IMRT or Volumetric Modulated Arc Therapy. Therefore, early glottic carcinoma is more suitable than other head and neck carcinomas for comparing the effect of smoking cessation on treatment outcomes of radiation therapy. Although this study has limitations inherent to a single center, retrospective analysis, the results sufficiently evaluate the effect of smoking cessation on radiotherapy due to the abovementioned reasons.
The prescription dose varied depending on the date, and before 2008, several patients were administered 60 Gy/30 fr. However, during this period, we finished the irradiation at 60 Gy/30 fr for patients with complete response during the course of treatment. Therefore, the prognosis in patients who underwent treatment with less than 66 Gy tended to be better than in those treated with more than 66 Gy (Supplementary Figure 4), and low total dose was a prognostic factor for improved DFS in multivariate analysis (). This may also reflect the outcome of the clinical stage (Supplementary Table 1). Although long total treatment duration and concomitant chemotherapy are generally associated with poor prognosis, these were not significant risk factors in our study (Supplementary Figure 5, Table 2). Additionally, an unfavorable prognosis due to an advanced clinical stage in the local recurrence rate may have been offset by the synergetic effect of chemotherapy, as the majority of T2 patients were administered concurrent chemoradiotherapy. Since 2009, we did not perform radiation therapy in patients who were active smokers until smoking cessation was confirmed. Thus, the average number of days from the first visit to the start of treatment was 1.2, 13.6, and 23.8 days for ‘no cessation,’ ‘incomplete cessation,’ and ‘complete cessation’ groups, respectively (). Nevertheless, median time until the start of irradiation (5 days) did not significantly affect the DFS and local recurrence rate, and the local recurrence rate tended to be lower in the group with more than 5 days than in the group with less than 5 days (Supplementary Figure 6). Therefore, the effect of complete smoking cessation on the treatment outcome may be more important than the delay in administration of radiation therapy.
In conclusion, we studied the effect of the complete smoking cessation on definitive radiation therapy outcome in early stage glottic carcinoma by monitoring expiratory CO and showed that patients with complete smoking cessation had low relapse rate and good prognosis. The monitoring of expiratory CO is useful in daily clinical practice and in addition, may reflect tissue hypoxia which is one of the most important assessments for radiotherapy sensitiveness. These results need to be validated in a large-scale multi-institution study.
Supplemental Material
Download Zip (28.5 MB)Acknowledgments
The authors acknowledge all the radiation technologist, nurses, and other staff who participated in the patients’ care in the Department of Radiology at Osaka General Medical Center. We would like to thank Editage (www.editage.com) for English language editing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- National Cancer Center Japan Center for Cancer Control and Information Services. [cited 2020 Oct 10]. Available from: https://ganjoho.jp/public/cancer/larynx/index.html.
- Waggoner SE, Darcy KM, Fuhrman B, et al. Association between cigarette smoking and prognosis in locally advanced cervical carcinoma treated with chemoradiation: a Gynecologic Oncology Group study. Gynecol Oncol. 2006;103(3):853–858.
- Steinberger E, Kollmeier M, McBride S, et al. Cigarette smoking during external beam radiation therapy for prostate cancer is associated with an increased risk of prostate cancer-specific mortality and treatment-related toxicity. BJU Int. 2015;116(4):596–603.
- Jensen JA, Goodson WH, Hopf HW, et al. Cigarette smoking decreases tissue oxygen. Arch Surg. 1991;126(9):1131–1134.
- Grau C, Nordsmark M, Khalil AA, et al. Effect of carbon monoxide breathing on hypoxia and radiation response in the SCCVII tumor in vivo. Int J Radiat Oncol Biol Phys. 1994;29(3):449–454.
- Harrison LB, Chadha M, Hill RJ, et al. Impact of tumor hypoxia and anemia on radiation therapy outcomes. Oncologist. 2002;7(6):492–508.
- Browman GP, Wong G, Hodson I, et al. Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N Engl J Med. 1993;328(3):159–163.
- Browman GP, Mohide EA, Willan A, et al. Association between smoking during radiotherapy and prognosis in head and neck cancer: a follow-up study. Head Neck. 2002;24(12):1031–1037.
- Fortin A, Wang CS, Vigneault E. Influence of smoking and alcohol drinking behaviors on treatment outcomes of patients with squamous cell carcinomas of the head and neck. Int J Radiat Oncol Biol Phys. 2009;74(4):1062–1069.
- Chen AM, Chen LM, Vaughan A, et al. Tobacco smoking during radiation therapy for head-and-neck cancer is associated with unfavorable outcome. Int J Radiat Oncol Biol Phys. 2011;79(2):414–419.
- Scherer G. Carboxyhemoglobin and thiocyanate as biomarkers of exposure to carbon monoxide and hydrogen cyanide in tobacco smoke. Exp Toxicol Pathol. 2006;58(2-3):101–124.
- Grau C, Khalil AA, Nordsmark M, et al. The relationship between carbon monoxide breathing, tumour oxygenation and local tumour control in the C3H mammary carcinoma in vivo. Br J Cancer. 1994;69(1):50–57.
- Deveci SE, Deveci F, Acik Y, et al. The measurement of exhaled carbon monoxide in healthy smokers and non-smokers. Respir Med. 2004;98(6):551–556.
- Laranjeira R, Pillon S, Dunn J. Environmental tobacco smoke exposure among non-smoking waiters: measurement of expired carbon monoxide levels. Sao Paulo Med J. 2000;118(4):89–92.
- Eskiizmir G, Baskin Y, Yalcin F, et al. Risk factors for radiation failure in early-stage glottic carcinoma: a systematic review and meta-analysis. Oral Oncol. 2016;62:90–100.
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458.
- Ino T, Ohtani T, Yoshimi I. Urinary biomarkers for secondhand smoke. J Clin Lab Anal. 2011;25(5):354–358.
- Hilberink SR, Jacobs JE, van Opstal S, et al. Validation of smoking cessation self-reported by patients with chronic obstructive pulmonary disease. Int J Gen Med. 2011;4:85–90.
- McRobbie H, Phillips A, Goniewicz ML, et al. Effects of switching to electronic cigarettes with and without concurrent smoking on exposure to nicotine, carbon monoxide, and acrolein. Cancer Prev Res (Phila)). 2015;8(9):873–878.
- Eltorai AE, Choi AR, Eltorai AS. Impact of electronic cigarettes on various organ systems. Respir Care. 2019;64(3):328–336.
- Goniewicz ML, Smith DM, Edwards KC, et al. Comparison of nicotine and toxicant exposure in users of electronic cigarettes and combustible cigarettes. JAMA Netw Open. 2018;1(8):e185937
- Samuels MA, Freedman LM, Elsayyad N. Intensity-modulated radiotherapy for early glottic cancer: transition to a new standard of care? Future Oncol. 2016;12(22):2615–2630.
- Cetinayak O, Dogan E, Kuru A, et al. Outcome of early-stage glottic laryngeal carcinoma patients treated with radical radiotherapy using different techniques. J Oncol. 2019;2019:8640549.
- Cho IJ, Chung WK, Lee JK, et al. Intensity-modulated radiotherapy for stage I glottic cancer: a short-term outcomes compared with three-dimensional conformal radiotherapy. Radiat Oncol J. 2019;37(4):271–278.