Abstract
Background
Breast cancer survivors are encouraged to be physically active. A recent review suggests that football training is an effective exercise modality for women across the lifespan, positively influencing health variables such as strength, fitness and social well-being. However, football is a contact sport, potentially posing an increased risk of trauma-related injury. Against this backdrop, breast cancer survivors are advised to avoid trauma or injury to the affected or at-risk arm in order to protect against lymphedema onset or exacerbation. The aim of this study was therefore to evaluate the feasibility and safety of Football Fitness training in relation to lymphedema and upper-extremity function after treatment for breast cancer.
Material and methods
Sixty-eight women aged 18–75 years, who had received surgery for stage I-III breast cancer and completed (neo) adjuvant chemotherapy and/or radiotherapy within five years, were randomized (2:1) to a Football Fitness group (FFG, n = 46) or a control group (CON, n = 22) for twelve months. Secondary analyses using linear mixed models were performed to assess changes in upper-body morbidity, specifically arm lymphedema (inter-arm volume % difference, dual energy X-ray absorptiometry; extracellular fluid (L-Dex), bioimpedance spectroscopy), self-reported breast and arm symptoms (EORTC breast cancer-specific questionnaire (BR23) and upper-extremity function (DASH questionnaire) at baseline, six- and twelve-month follow-up.
Results
We observed similar point prevalent cases of lymphedema between groups at all time points, irrespective of measurement method. At the six-month post-baseline assessment, reductions in L-Dex (extracellular fluid) were found in FFG versus CON. These significant findings were not maintained at the twelve-month assessment. No difference between groups was observed for inter-limb volume difference %, nor any of the remaining outcomes.
Conclusion
While superiority of Football Fitness was not observed, the results support that participation in Football Fitness training is feasible and suggests no negative effects on breast cancer-specific upper-body morbidity, including lymphedema.
Trial registration
The trial was registered at ClinicalTrials.gov. NCT03284567
Introduction
Breast cancer is the most frequent cancer among women in the world, representing approximately 25% of all cancers [Citation1]. While treatment modalities are increasingly effective in terms of survival, they are also associated with a range of treatment-specific adverse acute and late effects that negatively affect quality of life. Adverse effects include upper-body morbidities, such as restricted arm/shoulder mobility, pain and breast cancer-related lymphedema [Citation2–4], with the vast majority (up to 91%) of breast cancer survivors reporting some degree of arm/shoulder morbidity within the first two years post-treatment [Citation4]. Combined aerobic and resistance exercise has consistently been found to prevent and/or ameliorate adverse treatment-related effects including upper-body morbidity issues and fatigue, as well as improve fitness, strength and overall physical function [Citation5–9]. Indeed, recent guidelines from international organizations endorse participation in regular physical activity, and specifically exercise [Citation5,Citation7]. However, despite the beneficial effects of exercise, observational studies indicate that the majority of women experience declines in physical activity after breast cancer treatment or fail to meet recommended physical activity levels [Citation10–13]. To date, the majority of studies examining the effects of exercise have utilized traditional resistance and aerobic exercise. Against this backdrop, there is considerable rationale for investigating the safety, feasibility and effects of other types of exercise that support sustainable, regular participation.
Football Fitness training has emerged as a viable alternative to traditional exercise in non-cancer populations [Citation14,Citation15]. For example, results from randomized controlled trials examining the effect of Football Fitness in physically inactive women [Citation16–18] suggest that Football Fitness performed twice a week over a 12–15-week period can result in significant health gains that exceed the effects of other types of exercise (jogging, swimming and resistance exercise). Further, though predominantly targeting the lower-extremities, upper-body neuromuscular adaptations and increases in lean body mass are also associated with football training [Citation19,Citation20]. In the cancer setting, participation in 32 weeks of football provided significant health benefits (e.g. improved muscle-and bone mass, physical function and strength) in men undergoing androgen deprivation therapy for prostate cancer [Citation21–23]. In addition, a combination of psychosocial qualities, such as networking, positive competition and diversion supported retention beyond the intervention period [Citation24,Citation25].
However, football is characterized as a contact sport, potentially posing an increased risk of trauma or injury when compared to non-contact forms of exercise [Citation23]. This is especially relevant in breast cancer, as adoption of a range of risk-reducing behaviors is advised, including avoidance of trauma or injury to the affected or at-risk arm, in order to protect against lymphedema onset or exacerbation [Citation26]. Further, small-sided football games consist of multiple bouts of high-intensity exercise, and while aerobic exercise generally is recommended and considered safe in the breast cancer population, some physical activity guidelines indirectly warn against high intensity activities (‘gentle and moderate physical activity does not increase the risk of lymphedema’) [Citation27], or advise to exercise in a ‘safe manner’ [Citation28]. Indeed, among breast cancer survivors, fear of lymphedema onset/exacerbation has been identified as a barrier to participating in physical activity and exercise [Citation29].
Therefore, considering the potential positive effects of Football Fitness, we prospectively compared the effect of Football Fitness training with no intervention in breast cancer survivors who had completed active treatment (chemotherapy and radiotherapy). A full report detailing the results on aerobic capacity (the primary outcome), musculoskeletal effects and quality of life can be viewed elsewhere [Citation30]. The purpose of this manuscript is to report on explorative analysis of the feasibility and safety of Football Fitness training on upper-body morbidity (secondary outcomes), specifically lymphedema (extracellular fluid and arm volume), breast cancer-specific breast and arm symptoms, and upper-extremity function.
Material and methods
This was a parallel group randomized, controlled study conducted at the University Hospitals Center for Health Research, Copenhagen University Hospital, Rigshospitalet and the Department of Nutrition, Exercise and Sports, at the University of Copenhagen. The study was conducted in accordance with the Helsinki Declaration and approved by the Danish Capital Regional Ethics Committee (H-16029533). Written informed consent was provided by all participants before inclusion. The study is reported in accordance with the CONSORT guidelines (checklist Supplementary file) [Citation31].
Participants
Women were recruited by nursing staff at the Department of Oncology, Copenhagen University Hospital, Rigshospitalet, during control visits after completion of adjuvant treatment (chemotherapy and/or radiotherapy) for breast cancer. The study was also advertised on Facebook groups for breast cancer survivors, via public news outlets and on the Danish Cancer Society’s website. Women aged 18–75 years were eligible if they had a World Health Organization performance status of 0–1, could read and understand Danish and had received surgery for stage I-III breast cancer and completed neo- or adjuvant chemotherapy and/or radiotherapy within five years. Women were excluded from participation in the study due to osteoporosis (T-score < −2.5), serious cardiac morbidity (including ischemic heart disease), heart failure, poorly controlled hypertension, cardiac arrythmia, tendency to syncope, and pacemaker, or were receiving ongoing anticoagulant therapy. Further, women were excluded due to planned treatment with chemotherapy or radiotherapy in the intervention period.
Sample size, randomization and blinding
Sample size was based on expected differences in changes of the primary outcome, VO2max, which is reported elsewhere [Citation30]. After successful completion of all baseline assessments by research assistants or the primary investigator, women were randomized in a 2:1 ratio to either the Football Fitness training group (FFG) (n = 46) or the control group (CON) (n = 22). The unequal allocation ratio was chosen to increase the volume of data from the intervention group, as this study is the first of its kind in this patient group. Intervention allocation was determined using a list of random numbers generated in block sizes of three using a secure website, Sealed Envelope (London, UK). A researcher, not otherwise involved in the study, generated the randomization list by pairing the list of random numbers with the participants study identification numbers in the order by which the participants attended the baseline tests. Assessment of objective measures of arm volume and extracellular fluid were performed unblinded by study personnel, while questionnaires were delivered electronically through REDCap [Citation32]. All subsequent data entry, including calculation of L-Dex and inter-arm volume differences, were performed blinded to group allocation by personnel with no interventional activities.
Intervention
Women in the FFG group were invited to participate in a Football Fitness training regime twice weekly for 52 weeks. All training sessions were supervised by an exercise physiologist or physical therapists with football experience. Training sessions consisted of 10–15 min of warm-up (e.g. running, squats, sit-ups, core-strength and balance exercises) followed by 15 min of football drills (e.g. dribbling, passing, shooting) and 3–4 × 7 min of small sided (4–5 players per side) games on a 15-meter wide and 20-meter long natural grass pitch with two minute breaks in between games. From mid-October to late March, training was performed indoors on a 15-meter wide and 20-meter long pitch with boundaries. Participants were told to avoid hard tackles and other actions carrying a risk of injury. Attendance was recorded at each training session.
Participants in CON received no restrictions on physical activity and were invited to join Football Fitness training after the twelve-month intervention period.
Outcomes
All outcomes were assessed at baseline, six- and twelve-months post-baseline, by experienced research assistants. All assessments were performed at the same time of day (mornings), and participants were instructed to avoid strenuous exercise 48 h before the test and to avoid intake of alcohol 24 h prior to the test. Further, participants were instructed to avoid food intake eight hours prior to testing and to avoid smoking and intake of caffeine and medicine, including anti-hypertensive drugs, until after the tests were completed.
Arm lymphedema
Inter-arm volume % difference
Arm volume was determined using dual energy X-ray absorptiometry (DXA) (iDXA, Lunar Corporation, Madison, WI, USA). DXA provides a sensitive measure of tissue composition using a three-compartment model [Citation33,Citation34]. Total body scans were performed lying supine on the scan-table with arms slightly abducted and hands in a mid-prone position. From the total body scans, estimated arm volumes were calculated using previously derived densities (fat − 0.9 g/ml, lean mass −1.1 g/ml, bone mineral content − 1.85 g/ml)) with the region of interest extending from the gleno-humeral joint to the finger-tips [Citation33,Citation34]. Inter-arm volume % differences ((at-risk arm minus unaffected arm)/unaffected arm * 100) were then calculated. An inter-arm volume difference >5% was considered incident lymphedema [Citation35–38].
L-Dex
To obtain measures of extracellular fluid, bioimpedance spectroscopy (BIS) (SFB7, Impedimed, Brisbane, Australia) [Citation39,Citation40] was performed immediately after DXA scans. Participants were positioned in supine, with arms and legs slightly abducted and palms facing down. Using the principle of equipotential, four single tab electrodes were placed in a tetrapolar arrangement. The ratio of impedance between the at-risk and non-affected arm was calculated and converted into an L-Dex score, taking arm dominance into account. An L-Dex score >10 (or 3 standard deviations over normative data) was used to define incident lymphedema as this cutoff has been cited in previous literature [Citation36–38,Citation41]. An L-Dex >7.1 was also used as a diagnostic threshold, as it has been found to be more sensitive in discriminating between breast cancer survivors with- and at risk for lymphedema [Citation42,Citation43].
Patient reported outcomes
Breast and arm symptoms
The breast and arm symptom subscale of the European Organization for Research and Treatment of Cancer quality of life questionnaire breast cancer module (EORTC QLQ BR23), version 3.0, was used to assess breast cancer-specific arm and breast symptoms common to breast cancer patients [Citation44,Citation45]. The raw scores were summed and converted to a score out of 100, with higher scores representing greater symptom severity.
Upper-extremity function
The 30-item Disabilities of the Arm, Shoulder and Hand (DASH) outcome measure was used to assess region-specific impairment of the upper-extremities [Citation46]. This validated questionnaire assessed self-reported upper-extremity symptoms and ability to perform common functional activities. The raw scores were summed and converted to a score out of 100, with higher scores representing higher levels of upper-extremity disability.
Football-related injuries
Injuries occurring in relation to Football Fitness training were recorded by the instructor and, in addition, the participants in both FFG and CON filled out a questionnaire at the twelve-month follow-up assessment concerning musculoskeletal injuries sustained during the intervention period.
Statistical analysis
Descriptive statistics for continuous variables are presented as means (standard deviations (SD)) for normally distributed values and medians with interquartile ranges (IQR) for skewed distributed values at baseline. Categorical variables as well as lymphedema point prevalence are presented as counts (percentages). Differences at baseline of continuous variables were assessed by Welch Two Sample t-tests, while categorial variables were assessed by Chi-squared tests (R; RStudio Team, Inc., Boston, MA). Changes in outcomes, presented as means with 0.95 confidence intervals (CI’s), within- and between groups were assessed by linear mixed-model analyses, where an unstructured covariance was assumed (SAS software, version 7.1 (SAS Institute, Cary, NC)). Goodness of fit was assessed by residual diagnostics. A per-protocol analysis was also performed in participants with an adherence to the Football Fitness intervention of ≥50% versus participants from the control group who attended all three test days. A priori, as more extensive lymph node removal reflects the greatest risk factor for lymphedema, we also conducted sub-analyses using only data from participants who had ≥5 lymph nodes removed to better understand the effect of Football Fitness training in those participants considered to be at greatest risk of developing lymphedema. Statistical significance was considered as p < 0.05.
Results
Between March 2017 and October 2018, 68 of 81 (84%) eligible women who had completed primary treatment (chemotherapy and/or radiotherapy) for breast cancer were recruited (). Baseline characteristics () and outcome values were balanced between groups. Notably, a higher proportion of women in FFG had more than five lymph nodes removed and were, consequently, at higher risk of developing lymphedema than those in CON (41% vs. 27%, respectively).
Figure 1. Flow of participants from recruitment and through the trial including 68 women who had completed active treatment (chemotherapy/radiotherapy) for primary breast cancer.
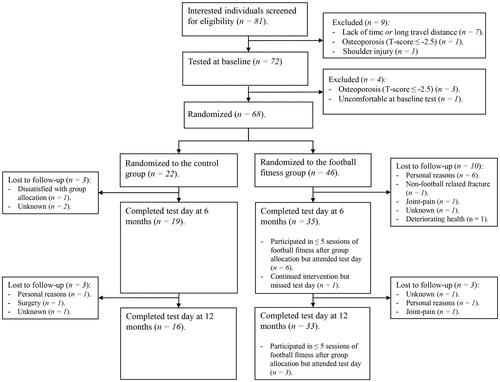
Table 1. Baseline characteristics of 68 women post-treatment (chemotherapy and/or radiation therapy) for breast cancer.
Seven participants had received bilateral breast surgery (5 FFG, 2 CON), however as all cases had unilateral axillary surgery, L-Dex and inter-arm volume measures were included for these participants.
Safety and feasibility
At twelve months post-baseline, outcome data were available for 16 (72%) and 33 (71%) participants from the CON and FFG, respectively. (). On average, FFG participants (n = 40) attended one Football Fitness session per week throughout the twelve-month intervention period, with an adherence rate of 45% (24 sessions) at six months and 50% (20 sessions) between six and twelve months. Seventeen (52%) of the women who contributed data at twelve months had attended at least 50% of all sessions. Six participants did not contribute data to adherence (one woman never partook in any training session and five participants attended less than five training sessions).
Fifteen participants incurred injuries during Football Fitness training (see Uth et al., in submission for details). All injuries were managed conservatively. Most injuries were minor and located in the lower extremities. One participant did not resume training after a fall during an indoor warm-up that resulted in a meniscus lesion. Two participants (one diagnosed with lymphedema prior to intervention start) experienced upper-extremity injuries related to falls during contact with the football (as opposed to contact with another player). One participant subluxed her fifth digit while another bruised her right side, including the shoulder, arm, and hand. Both returned to the intervention after a 2–3-week recovery period and neither reported an exacerbation in lymphedema or lymphedema symptoms.
Arm lymphedema
Point prevalence
While point prevalence at baseline varied depending on assessment method and cutoff, point prevalence rates were similar between FFG and CON at all time points for any given assessment method (). At baseline, 16 (35%) participants self-reported that they had lymphedema (), with a median time of 2.6 years from initial consultation with a lymphedema therapist to intervention start. One (5%) and three (7%) of these participants were lost to follow-up in CON and FFG, respectively. No participants were lost to follow-up due to lymphedema-related issues.
Table 2. Point prevalence (n (%)) of lymphedema as determined by L-Dex >10, L-Dex >7.1, and inter-arm volume difference > 5%.
Inter-arm volume % difference
No difference between groups was found at the six- and twelve-month assessments (). These findings remained consistent following analysis restricted to participants with more than five lymph nodes removed () and the per-protocol analysis (Supplementary table). Three women (6%) had no inter-arm volume data due to body dimensions exceeding the DXA scan table.
Table 3. Intention-to-treat analyses of lymphedema and upper-body outcomes at six- and twelve-months post-baseline (n = 68).
Table 4. Subgroup-analysis of lymphedema and upper-body outcomes at six- and twelve-months post-baseline in participants with over five lymph nodes removed (n = 25).
L-Dex
Between baseline and the six-month assessment, a significant decrease in L-Dex was found in FFG but not CON (). At twelve months post-baseline, no difference was observed between groups. When analysis was restricted to those with more than five lymph nodes removed, these findings remained consistent (). No difference between groups was seen at any time point following per-protocol analysis (Supplementary table). L-Dex data from two participants (3%) were not included at baseline due to unreliable measures.
Patient reported outcomes
No between group differences were observed for breast or arm symptom subscale scores nor for upper-extremity function at the six- and twelve-month assessments (). This is consistent with findings in participants with more than 5 lymph nodes removed () and the per-protocol analysis (Supplementary table). No data regarding breast or arm symptoms was available at baseline for one participant (CON).
Discussion
This is the first study to prospectively evaluate Football Fitness training in breast cancer survivors. We observed similar point prevalent cases of lymphedema between groups at all time points irrespective of measurement method. At six months, L-Dex data indicated a reduction in extracellular fluid in the Football Fitness group compared to the control group. While these significant findings were not observed for inter-limb volume difference, nor in the remaining outcomes, and were not maintained at the twelve-month assessment, the results do support that participation in Football Fitness training is feasible and suggests no negative effects on breast cancer-specific upper-body morbidity issues including lymphedema.
We found no additional benefit for upper-extremity function. It should however be noted that mean self-reported baseline levels of upper-extremity disability were low, indicating a study population that overall had mild or minimal upper-extremity concerns [Citation47], why the lack of change over time (in both groups) could be due to floor effects. Consequently, results from the present study cannot address whether participation in Football Fitness has a positive effect on upper-extremity function in individuals with higher levels of disability. Nonetheless, sub-analysis of participants with over five lymph nodes removed (n = 25) found favorable effects on extracellular fluid (L-Dex) at six months. While the sample size for this analysis was small (and therefore should be interpreted with caution), these results suggest that participation in Football Fitness also was well tolerated in participants at higher risk for upper-body morbidity issues, and specifically lymphedema. Further, no participants (including ten women with self-reported lymphedema at baseline) discontinued the intervention due to upper-body morbidities. As such, these results add to a growing and consistent evidence base suggesting that exercise is feasible for those with or at-risk of developing lymphedema [Citation5,Citation9,Citation48]. Finally, the risk of injuries related to Football Fitness training is not completely unavoidable given the nature of football involving physical contact between players, an uneven playing surface, and unexpected moves by teammates, opponents, and the ball [Citation23]. Nonetheless, benefits of training as well as the debilitating effects of physical inactivity (and subsequent risk of morbidity) should be weighed against potential training related adverse events.
On average, participants attended Football Fitness training once weekly throughout the twelve-month intervention. In contrast, regular exercise at least 2–3 times weekly is generally recommended in order to illicit optimal benefit from training [Citation49], why higher adherence would be considered more ideal. However, facilitating sustainable physical activity levels that meet recommended levels is a challenge [Citation11–13], as exemplified in a sample of 259 breast cancer survivors (on average three years post-diagnosis, which is similar to the present study), where just 16% were meeting physical activity guidelines [Citation10]. It could therefore be argued that, while not optimal, weekly participation over a twelve-month period attests to the feasibility of football fitness. Further, participants from the current trial continued training together after the trial was finalized (with a follow-up study planned to investigate long-term effects from participation in self-organized training). As such, this provides additional testament of the feasibility of this intervention as an exercise alternative to traditional resistance and aerobic training.
Limitations of this study should be considered when interpreting the findings. First, the primary endpoint of this study was VO2max, why all results represent exploratory analysis and should be regarded as such. Further, breast cancer survivors in Denmark are encouraged to be physically active and exercise. It is thus possible that a proportion of those in the CON group also participated in exercise during the intervention period. While there exists uncertainty as to the extent and specificity of this potential bias (as no physical activity data was collected for CON), the impact on findings would likely dilute differences between groups. Finally, three women (6%) of the sample are missing inter-arm volume data due to body dimensions exceeding the DXA scan table, why use of this measurement method in obese individuals is not recommended. For these individuals separate arms scans should be considered [Citation50].
Strengths of the study include the randomized design and multiple, well validated, objective measures of lymphedema and upper-extremity issues. Also, intention-to-treat analyses and per-protocol analyses were performed with consistency in findings. Further, the present study evaluated a novel exercise intervention in the breast cancer population, providing an evidence-based alternative to traditional exercise modalities that could potentially appeal to women who would not otherwise participate in exercise. This is especially relevant for the sub-group of breast cancer survivors who develop lymphedema as they are at an increased risk of declines in physical activity after treatment [Citation51]. Further, observational data indicate an association between exercise post breast cancer and reduced risk of chronic disease (such as cardiovascular disease and type II diabetes), breast cancer recurrence, cancer-related mortality and all-cause mortality [Citation10]. Therefore, interventions that can facilitate sustainable recommended physical activity levels are of utmost importance, with Football Fitness training as a viable option.
Conclusion
Football Fitness training was found to be safe and feasible for women who have completed active treatment (chemotherapy and/or radiotherapy) for stage I-III breast cancer. Participation did not lead to superior outcomes in lymphedema (extracellular fluid and arm volume), breast cancer-specific breast and arm symptoms, or upper-extremity function compared with no intervention.
Supplemental Material
Download MS Word (22.1 KB)Supplemental Material
Download MS Word (16.7 KB)Acknowledgments
We would like to thank Christian Lillelund for support in data collection and Frederik Sørensen for expert statistical advice, as well as Birgitte Rasmussen for the acquisition of data from medical records. We would also like to acknowledge the staff in the Department of Oncology, Rigshospitalet for their support with recruiting participants. Finally, we would like to thank all the participants, without whom this trial would not have been possible.
Disclosure statement
Peter Krustrup is the fitness coach for the Danish women’s national football team. All other authors report no conflicts of interest.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Bulley C, Gaal S, Coutts F, et al. Comparison of breast cancer-related lymphedema (upper limb swelling) prevalence estimated using objective and subjective criteria and relationship with quality of life. Biomed Res Int. 2013;2013:807569.
- Hayes SC, Johansson K, Stout NL, et al. Upper-body morbidity after breast cancer: incidence and evidence for evaluation, prevention, and management within a prospective surveillance model of care. Cancer. 2012;118(8 Suppl):2237–2249.
- Johansen S, Fossa K, Nesvold IL, et al. Arm and shoulder morbidity following surgery and radiotherapy for breast cancer. Acta Oncol. 2014;53(4):521–529.
- Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51(11):2375–2390.
- Dieli-Conwright CM, Orozco BZ. Exercise after breast cancer treatment: current perspectives. Breast Cancer (Dove Med Press). 2015;7:353–362.
- Hayes SC, Newton RU, Spence RR, et al. The exercise and sports science Australia position statement: exercise medicine in cancer management. J Sci Med Sport. 2019;22(11):1175–1199.
- Singh B, Spence RR, Steele ML, et al. A systematic review and meta-analysis of the safety, feasibility, and effect of exercise in women with stage II + breast cancer. Arch Phys Med Rehabil. 2018;99(12):2621–2636.
- Singh B, Disipio T, Peake J, et al. Systematic review and meta-analysis of the effects of exercise for those with cancer-related lymphedema. Arch Phys Med Rehabil. 2016;97(2):302.e13–315.e13.
- Boyle T, Vallance JK, Ransom EK, et al. How sedentary and physically active are breast cancer survivors, and which population subgroups have higher or lower levels of these behaviors? Support Care Cancer. 2016;24(5):2181–2190.
- Schmidt ME, Wiskemann J, Ulrich CM, et al. Self-reported physical activity behavior of breast cancer survivors during and after adjuvant therapy: 12 months follow-up of two randomized exercise intervention trials. Acta Oncol. 2017;56(4):618–627.
- Mason C, Alfano CM, Smith AW, et al. Long-term physical activity trends in breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2013;22(6):1153–1161.
- Irwin ML, Crumley D, McTiernan A, et al. Physical activity levels before and after a diagnosis of breast carcinoma: the Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer. 2003;97(7):1746–1757.
- Krustrup P, Williams CA, Mohr M, et al. The "Football is Medicine" platform-scientific evidence, large-scale implementation of evidence-based concepts and future perspectives. Scand J Med Sci Sports. 2018;28(Suppl 1):3–7.
- Krustrup P, Aagaard P, Nybo L, et al. Recreational football as a health promoting activity: a topical review. Scand J Med Sci Sports. 2010;20(Suppl 1):1–13.
- Helge EW, Aagaard P, Jakobsen MD, et al. Recreational football training decreases risk factors for bone fractures in untrained premenopausal women. Scand J Med Sci Sports. 2010;20(Suppl 1):31–39.
- Mohr M, Helge EW, Petersen LF, et al. Effects of soccer vs swim training on bone formation in sedentary middle-aged women. Eur J Appl Physiol. 2015;115(12):2671–2679.
- Mohr M, Lindenskov A, Holm PM, et al. Football training improves cardiovascular health profile in sedentary, premenopausal hypertensive women. Scand J Med Sci Sports. 2014;24:36–42.
- Randers MB, Nielsen JJ, Krustrup BR, et al. Positive performance and health effects of a football training program over 12 weeks can be maintained over a 1-year period with reduced training frequency. Scand J Med Sci Sports. 2010;20(Suppl 1):80–89.
- Pedersen MT, Randers MB, Skotte JH, et al. Recreational soccer can improve the reflex response to sudden trunk loading among untrained women. J Strength Cond Res. 2009;23(9):2621–2626.
- Uth J, Hornstrup T, Christensen JF, et al. Football training in men with prostate cancer undergoing androgen deprivation therapy: activity profile and short-term skeletal and postural balance adaptations. Eur J Appl Physiol. 2016;116(3):471–480.
- Uth J, Hornstrup T, Christensen JF, et al. Efficacy of recreational football on bone health, body composition, and physical functioning in men with prostate cancer undergoing androgen deprivation therapy: 32-week follow-up of the FC prostate randomised controlled trial. Osteoporos Int. 2016;27(4):1507–1518.
- Uth J, Hornstrup T, Schmidt JF, et al. Football training improves lean body mass in men with prostate cancer undergoing androgen deprivation therapy. Scand J Med Sci Sports. 2014;24(Suppl 1):105–112.
- Nielsen G, Wikman JM, Jensen CJ, et al. Health promotion: the impact of beliefs of health benefits, social relations and enjoyment on exercise continuation. Scand J Med Sci Sports. 2014;24(Suppl 1):66–75.
- Ottesen L, Jeppesen RS, Krustrup BR. The development of social capital through football and running: studying an intervention program for inactive women. Scand J Med Sci Sports. 2010;20(Suppl 1):118–131.
- NLN Medical Advisory Committee. Position statement of the National Lymphedema Network. Topic: lymphedema risk reduction practices [Internet]. San Francisco (CA): National Lymphedema Network; [updated May 2012; cited 2020 Nov 11]. Available from: https://klosetraining.com/wp-content/uploads/2015/05/NLNpractices.pdf.
- Prevention of Clinical Lymphoedema after cancer treatment: Early detection and risk reduction. A guide for health professionals. [Internet]. Dublin, Ireland: National Cancer Control Programme, An Clár Náisiúnta Rialaithe Ailse; 2015. Available from: https://www.hse.ie/eng/services/list/5/cancer/patient/leaflets/prevention-of-clinical-lymphoedema-after-cancer-treatment.pdf
- NLN Medical Advisory Committee. Position statement of the National Lymphedema Network. Topic: Exercise [Internet]. San Francisco (CA): National Lymphedema Network; [updated November 2013; cited 2020 Nov 11]. Available from: https://klosetraining.com/wp-content/uploads/2015/05/NLNexercise.pdf.
- Sander AP, Wilson J, Izzo N, et al. Factors that affect decisions about physical activity and exercise in survivors of breast cancer: a qualitative study. Phys Ther. 2012;92(4):525–536.
- Uth J, Fristrup B, Sorensen V, et al. Exercise intensity and cardiovascular health outcomes after 12months of football fitness training in women treated for stage I-III breast cancer: Results from the football fitness After Breast Cancer (ABC) randomized controlled trial. Prog Cardiovasc Dis. 63(6):792–799.
- Schulz KA, Moher D, for the CONSORT Group. CONSORT. Statement: updated guidelines for reporting parallel group randomised trials. 2010. Available from: https://bmcmedicine.biomedcentral.com/articles/10.1186/1741-7015-8-18/tables/1.
- REDCap. [cited 2020 Nov 11]. Available from: https://projectredcap.org/resources/citations/.
- Brorson H, Ohlin K, Olsson G, et al. Breast cancer-related chronic arm lymphedema is associated with excess adipose and muscle tissue. Lymphat Res Biol. 2009;7(1):3–10.
- Newman AL, Rosenthall L, Towers A, et al. Determining the precision of dual energy x-ray absorptiometry and bioelectric impedance spectroscopy in the assessment of breast cancer-related lymphedema. Lymphat Res Biol. 2013;11(2):104–109.
- Cheville AL, McGarvey CL, Petrek JA, et al. The grading of lymphedema in oncology clinical trials. Semin Radiat Oncol. 2003;13(3):214–225.
- Bloomquist K, Adamsen L, Hayes SC, et al. Heavy-load resistance exercise during chemotherapy in physically inactive breast cancer survivors at risk for lymphedema: a randomized trial. Acta Oncol. 2019;58(12):1667–1675.
- Buchan J, Janda M, Box R, et al. A randomized trial on the effect of exercise mode on breast cancer-related lymphedema. Med Sci Sports Exerc. 2016;48(10):1866–1874.
- Cormie P, Galvao DA, Spry N, et al. Neither heavy nor light load resistance exercise acutely exacerbates lymphedema in breast cancer survivor. Integr Cancer Ther. 2013;12(5):423–432.
- Ward LC, Dylke E, Czerniec S, et al. Confirmation of the reference impedance ratios used for assessment of breast cancer-related lymphedema by bioelectrical impedance spectroscopy. Lymphat Res Biol. 2011;9(1):47–51.
- Cornish BH, Jacobs A, Thomas BJ, et al. Optimizing electrode sites for segmental bioimpedance measurements. Physiol Meas. 1999;20(3):241–250.
- Hayes SC, Rye S, Disipio T, et al. Exercise for health: a randomized, controlled trial evaluating the impact of a pragmatic, translational exercise intervention on the quality of life, function and treatment-related side effects following breast cancer. Breast Cancer Res Treat. 2013;137(1):175–186.
- Fu MR, Cleland CM, Guth AA, et al. L-dex ratio in detecting breast cancer-related lymphedema: reliability, sensitivity, and specificity. Lymphology. 2013;46(2):85–96.
- Ridner SH, Dietrich MS, Spotanski K, et al. A prospective study of L-dex values in breast cancer patients pretreatment and through 12 months postoperatively. Lymphat Res Biol. 2018;16(5):435–441.
- Sprangers MA, Groenvold M, Arraras JI, et al. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol. 1996;14(10):2756–2768.
- Nguyen J, Popovic M, Chow E, et al. EORTC QLQ-BR23 and FACT-B for the assessment of quality of life in patients with breast cancer: a literature review. J Comp Eff Res. 2015;4(2):157–166.
- Gummesson C, Atroshi I, Ekdahl C. The disabilities of the arm, shoulder and hand (DASH) outcome questionnaire: longitudinal construct validity and measuring self-rated health change after surgery. BMC Musculoskelet Disord. 2003;4:11.
- Williams N. Dash. Occup Med (Lond). 2014;64(1):67–68.
- Baumann FT, Reike A, Reimer V, et al. Effects of physical exercise on breast cancer-related secondary lymphedema: a systematic review. Breast Cancer Res Treat. 2018;170(1):1–13.
- Michalsik L, Aerob t. In: Beyer N, Klinge K, editors. Traening I forebyggelse, behandling og rehabilitering. 3 udgave. Copenhagen (Denmark): Munksgaard; 2020.
- Gjorup C, Zerahn B, Hendel HW. Assessment of volume measurement of breast cancer-related lymphedema by three methods: circumference measurement, water displacement, and dual energy X-ray absorptiometry. Lymphat Res Biol. 2010;8(2):111–119.
- Meiklejohn J, Heesch K, Janda M, et al. Physical activity in the lives of those living with lymphoedema following cancer treatment. Lymphology. 2012;44:131–137.
