Abstract
Background
Comparative studies often cannot be conducted for cancer types with a small patient population. We reviewed the efficacy evaluations of new drug approvals in Japan based on the results of single-arm clinical trials.
Methods
We reviewed review reports on anticancer agents approved in Japan between 2006 and 2019 that are publicly available on the website of the Pharmaceuticals and Medical Devices Agency, Japan.
Results
The number of single-arm trial-based approvals increased, with a total of 43 drugs approved during the study period. Compared with comparative trial-based approvals, single-arm trial-based approvals had a tendency toward more biomarker-related indications (37.2% vs 22.6%, p = .053), as well as more approvals for hematological malignancies, orphan designation, and response-related outcomes as the primary endpoint. Only 13 of the pivotal trials of single-arm trial-based approvals had a predefined threshold for efficacy based on the same target population as the pivotal trial, and nearly half of the trials did not have an appropriate predefined threshold for efficacy. In particular, the efficacy thresholds for clinical trials for 4 molecular targeted agents were set based on the results of the nonbiomarker-selected population.
Conclusions
Evidence on standard cancer therapies for rare molecular subtypes is lacking. External control data from registries might support the efficacy evaluations of new drugs for newly established rare molecular subtypes.
Introduction
Anticancer drugs have been extensively developed in recent decades [Citation1], which can be attributed to advances in science and the increased needs of patients. Scientific advancements include an improved understanding of the molecular biology of cancer and tumor immunity. Multiple molecular subtypes within a tumor previously assumed to be of a single histological type can be identified through protein expression by immunohistochemistry or genomic alterations through in situ hybridization or next-generation sequencing [Citation2,Citation3]. The discovery of these molecular subtypes has led to the development of molecular targeted agents for each subtype. In addition, immune checkpoint inhibitors have dramatically changed cancer therapy within the last decade. The clinical development of immune checkpoint inhibitors has been simultaneously conducted for common cancer types such as melanoma, bladder cancer, and lung cancer, as well as for specific rare cancer types (including Merkel cell carcinoma and microsatellite instability-high and mismatch repair deficient solid tumors) based on their immunological characteristics. For these tumors, immune checkpoint inhibitors consequently demonstrated a higher response rate in single-arm clinical trials than response rates in other cancer types [Citation4,Citation5]. Furthermore, there has been a growing need for drug development for rare diseases and early access to new drugs that have shown promising efficacy in early-stage clinical trials. To meet these needs, various expedited programs such as the conditional approval and orphan drug designation have been introduced in a number of countries and regions [Citation6]. Under these circumstances, numerous drugs have been developed or are being developed for cancer types whose incidence is quite low.
The gold standard for determining the superior efficacy and safety of a new drug compared with the standard therapy has been randomized comparative clinical trials [Citation7]. In terms of drug development for cancer types with a small patient population, however, it is often not possible to conduct such trials, which can require several hundred patients or more. Consequently, a significant proportion of drugs for these cancer types are reviewed and approved based on the results of single-arm trials in which direct comparisons between the new drug and the standard treatment are not performed [Citation8,Citation9]. To overcome this problem, there has been an interest in using real-world evidence such as registry data to support the evaluation of the clinical utility of a new drug, especially its efficacy. Registry data have been referred and evaluated in the regulatory review of several recently approved drugs [Citation10,Citation11]. However, as these registries were not developed for the purpose of regulatory reviews, information obtained from the registries has been limited. If there are specific cancer types in which registry data could be used for the regulatory review, registries could be prospectively developed for this purpose.
In Japan, the Clinical Innovation Network project was launched in 2016, led by the Ministry of Health, Labor and Welfare (MHLW) in collaboration with the Pharmaceuticals and Medical Devices Agency (PMDA) as the regulatory agency, the Japan Agency for Medical Research and Development as the funding agency, and academic institutions to promote drug development through registry-based clinical trials and reviews [Citation12]. In this study, we analyzed the review reports from the PMDA to clarify the cancer types for which registry data could support the efficacy evaluation of new drugs in single-arm clinical trials.
Material and methods
Materials
Using the PMDA website (https://www.pmda.go.jp/review-services/drug-reviews/review-information/p-drugs/0010.html), we identified new drug applications (NDAs) and supplemental NDAs (sNDAs) of oncology agents reviewed by the PMDA and approved by the MHLW between 2006 and 2019. We gathered publicly available review reports on these NDAs and sNDAs from the PMDA website (https://www.pmda.go.jp/english/review-services/reviews/approved-information/drugs/0001.html#select15, https://www.pmda.go.jp/PmdaSearch/iyakuSearch/). In the present study, we excluded NDAs and sNDAs approved without clinical trial data but as a special category based on the notification ‘NDAs based on public knowledge’[Citation13], those not related to an indication such as a new administration route and new dosage, and those for supportive care therapy with no anticancer effect. In addition, we reviewed summaries of the submitted documents also available on the PMDA website and journal articles on clinical trials included in those NDAs and sNDAs as needed.
Review of the review reports
We collected the details of each NDA and sNDA from the review reports according to the following criteria: approval type (NDA or sNDA), indication, orphan designation, and drug category (cytotoxic agent, molecular targeted agent, hormone drug, immune checkpoint inhibitor, or others). Among the multiple clinical trials included in a review report, the trial identified as the ‘most important for evaluating the efficacy and safety’ was defined as the ‘key trial’ in the present study. In cases in which no trial or multiple trials were mentioned as the ‘most important,’ the later phase trial that enrolled the most patients was defined as the ‘key trial’ in the present study.
We also collected the following details of the key trials from the review reports: clinical trial design (comparative or single-arm), endpoints, and the threshold for efficacy determination. In addition to the review reports, we reviewed the summaries of submitted documents also available on the PMDA website and journal articles on the key clinical trials if necessary to determine the rationale for the threshold. The threshold determination was categorized as follows: (i) results of clinical trials with the same population as the key trial, (ii) results of clinical trials with a similar but not the same population as the key trial, (iii) clinical trial plans of other agents for the same population as the key trial, (iv) a threshold of 5%–10% given that no standard therapy was available for the target population of the key trial, (v) no detected rationale for the threshold in the reviewed materials, (vi) threshold not set in the key trial, and (vii) no detected information on the threshold in the reviewed materials.
Statistical analysis
Given that the present study was conducted in a retrospective manner for exploratory purposes, the results were narratively described. We employed the chi-squared test to compare two groups without any predefined hypothesis.
Results
Single-arm clinical trials
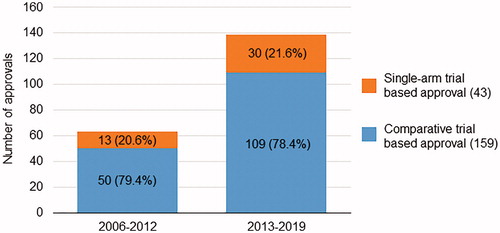
shows the flow diagram for the item selection. Of the 291 oncology agents approved in Japan between 2006 and 2019, 89 were excluded from the present study based on the following criteria: review report not available (n = 1); public knowledge-based application without clinical trial data (n = 41); agents for supportive care therapy or agents with no anticancer effect (n = 18); and sNDA not related to its indication but related to its dosage or administration route (n = 37). A total of 202 anticancer agents with an initial NDA or sNDA related to its indication were approved based on data from clinical trials and included 159 agents approved based on the results of comparative clinical trials and 43 agents approved based on the results of single-arm clinical trials. shows the chronological progression of these 202 agents according to the type of pivotal trial. Although the number of single-arm trial-based approvals varied each year, the proportions of such approvals were 20.6% in the first half (2006–2012) and 21.6% in the latter half (2013–2019). In accordance with the increased number of total approvals, there were more single-arm trial-based approvals in the latter half of the study (4.3/year) than in the first half (1.9/year).
Comparison between comparative trial-based approvals and single-arm trial-based approvals
shows the comparison between the comparative trial-based approvals and single-arm trial-based approvals. Single-arm trial-based approvals included the following categories significantly more often than the comparative trial-based approvals: agents for hematological malignancies (51.2% vs 24.5%, p < .001), orphan designation-granted agents (83.7% vs 33.3%, p < .001), and response-related primary endpoints in the pivotal trial (95.3% vs 10.1%, p < .001). In addition, single-arm trial-based approvals tended to include more agents with a biomarker-related indication (37.2% vs 22.6%, p = .053).
Table 1. Characteristics of comparative trial-based approvals and single arm trial-based approvals.
Details of single-arm trial-based approvals
shows details of the indicated tumor types in the 43 single-arm trial-based approvals. Among the 22 indications for hematological malignancies, 3 agents were approved for peripheral T-cell lymphoma and cutaneous T-cell lymphoma, as well as 1 agent for both peripheral T-cell lymphoma and cutaneous T-cell lymphoma. Three agents were approved for acute lymphoblastic leukemia, and 1 agent was indicated for both acute lymphoblastic leukemia and chronic myeloid leukemia. Tumor types other than those mentioned had 1 or 2 indications.
Table 2. Tumor types approved based on single arm trials.
Among the 21 agents indicated for solid tumors, 8 were indicated for lung cancer, all of which were indicated for specific molecular subtypes: 4 for anaplastic lymphoma kinase (ALK) fusion, 2 for BRAF mutation, 1 for ROS1 fusion, and 1 for epidermal growth factor receptor (EGFR) T790M mutations. In terms of tumor types, all tumors other than lung cancer had only 1 indication. However, 2 approvals were granted for solid tumors in a tumor-agnostic manner.
Threshold setting in single-arm clinical trials
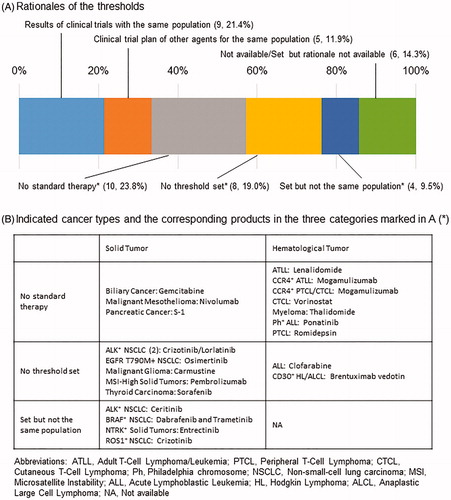
As most of the endpoints in single-arm trials leading to an approval were response-related (), we further investigated the efficacy thresholds in these trials. describes the rationales for the thresholds in the single-arm trials. Among 42 pivotal trials for 43 drugs, the thresholds of 9 trials (21.4%) were determined based on the results of previous clinical trials with the same population as the trial, and the thresholds of 5 trials (11.9%) were based on the clinical trial plans of other drugs for the same population. The thresholds for 10 trials (23.8%) were set at 5%–10% owing to the absence of standard therapies for each indication. There were also 8 trials (19.0%) for which an efficacy threshold was not set in the pivotal trials. In addition, the efficacy thresholds of 4 trials (9.5%) that enrolled patients with cancers harboring specific gene alterations were set based on the results of previous clinical trials without such biomarker specification. Among 16 agents with biomarker-related indications in single-arm trial-based approvals, 13 were in these 3 categories in which the efficacy endpoint was not determined based on information from the same population, while pivotal trials for only 2 drugs had predetermined thresholds based on information from the same population.
Discussion
In this study, we analyzed the efficacy evaluations of new drug approvals based on the results of single-arm clinical trials. We have shown that the number of approvals based on the results of single-arm clinical trials has increased in Japan and that nearly half did not have a predefined appropriate threshold for determining the efficacy in pivotal trials. The primary purpose of these single-arm trials was not always to obtain regulatory approval, and therefore it was not always necessary to set a threshold for efficacy evaluation. Nonetheless, the rationale for setting a threshold for efficacy evaluation was lacking in certain specific cancer types, especially in rare molecular subtypes.
The response rate was mostly used for the efficacy endpoint in single-arm clinical trials. Clinical trials on ceritinib (ALK inhibitor) and crizotinib (ROS1 inhibitor), which enrolled patients with non-small-cell lung cancer (NSCLC) harboring the corresponding gene alterations, had efficacy thresholds. However, these thresholds were not set based on the results of clinical trials for patients with the same specific gene alterations. In both trials, the rationale for the efficacy thresholds was the results of previous clinical trials enrolling patients with NSCLC without gene alteration profiles. The targeted gene alterations in those trials, the EML4-ALK fusion gene (which accounts for 3%–7% of NSCLC cases) and the ROS1 gene rearrangement (which also accounts for approximately 2% of NSCLC cases), were first reported in 2007 and 2012, respectively [Citation14,Citation15]. Given that the evidence of the efficacy of the standard therapy in patients with these specific gene alterations was not available at the time those clinical trials were planned, we inferred that the response rate of patients with NSCLC without gene alteration profiles was used as the rationale for the efficacy threshold. The circumstances of a combination of dabrafenib (BRAF inhibitor) and trametinib (MEK inhibitor) for BRAF-mutated NSCLC and entrectinib for NTRK gene fusion-positive solid tumors were also similar. However, the clinical characteristics of a specific molecular subtype are not always comparable with cancers without the molecular alteration; BRAF-mutated colorectal cancer is reportedly resistant to the standard therapies [Citation16–19]. It is therefore important to obtain data from patients with the same molecular subtype such as those enrolled in a single-arm clinical trial.
In addition to these molecular subtypes, many other rare molecular subtypes have been reported, such as RET fusion in lung cancer as well as BRAF mutation and HER2 amplification in colorectal cancer [Citation2,Citation3,Citation20]. For several rare molecular subtypes, comparative trials with the primary endpoint of survival outcomes can be conducted. Although pivotal trials with reviews in the PMDA for ALK inhibitors such as crizotinib, alectinib, and ceritinib were single-arm trials, phase 3 comparative trials were later conducted [Citation21–23]. In terms of these molecular subtypes, the early-stage approval of these drugs based on the results of single-arm trials was due to the extraordinarily high response rate and the resulting need for early access to those drugs. For a combination of BRAF inhibitor and EGFR inhibitor for BRAF V600E-mutated colorectal cancer, which demonstrated a response rate of 10%–20% in early-stage trials [Citation24,Citation25], a phase 3 comparative trial was then conducted and demonstrated a significantly prolonged overall survival compared with the standard therapies, leading to an NDA and approval [Citation26]. However, the number of patients with most of the molecular subtypes is too small to conduct comparative trials. Given that even a molecular targeted therapy for a specific gene alteration does not always show extraordinarily high efficacy in patients with the specific gene alteration, external control data would be helpful for evaluating the efficacy in single-arm trials.
The data included in the present study were limited to the information publicly available on the PMDA website. More comprehensive approaches, such as an analysis of data not publicly available and a review of review reports in other regulatory agencies, are warranted.
Given the current understanding of the genomic characteristics of cancer, more agents are expected to be developed for cancer types with a small number of patients. In conclusion, external control data from registries would support the efficacy evaluation of new agents for newly established cancer subtypes such as rare molecular and histological subtypes.
Acknowledgments
The authors were reviewers at the Pharmaceuticals and Medical Devices Agency, Japan during the following period: KH (2016–2018), CH (2016–2018), and YK (2016–2019). We are grateful to Dr. Takahiro Nonaka at the Office of New Drug V, PMDA, Japan for regulatory advice.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Beaver JA, Howie LJ, Pelosof L, et al. A 25-year experience of US Food and Drug Administration accelerated approval of malignant hematology and oncology drugs and biologics: a review. JAMA Oncol. 2018;4(6):849–856.
- Lee MKC, Loree JM. Current and emerging biomarkers in metastatic colorectal cancer. Curr Oncol. 2019;26(Suppl 1):S7–s15.
- Skoulidis F, Heymach JV. Co-occurring genomic alterations in non-small-cell lung cancer biology and therapy. Nat Rev Cancer. 2019;19(9):495–509.
- Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17(10):1374–1385.
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520.
- Lyerly HK, Ren J, Canetta R, et al. Global development of anticancer therapies for rare cancers, pediatric cancers, and molecular subtypes of common cancers. J Glob Oncol. 2018;4:1–11.
- General considerations for clinical trials E8. [cited 2021 Jan 5]. Available from: https://database.ich.org/sites/default/files/E8_Guideline.pdf.
- Chen EY, Raghunathan V, Prasad V. An overview of cancer drugs approved by the US Food and Drug Administration based on the surrogate end point of response rate. JAMA Intern Med. 2019;179(7):915–921.
- Ladanie A, Speich B, Briel M, et al. Single pivotal trials with few corroborating characteristics were used for FDA approval of cancer therapies. J Clin Epidemiol. 2019;114:49–59.
- BLA Multidisciplinary Review and Evaluation: BLA 761049 Bavencio (Avelumab). [cited 2021 Jan 5]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/761049Orig1s000MultidisciplineR.pdf.
- European Medicines Agency, Assessment Report: Mekinist/Tafinlar. [cited 2021 Jan 5]. Available from: https://www.ema.europa.eu/en/documents/variation-report/mekinist-epar-assessment-report-variation_en.pdf.
- Matsushita S, Tachibana K, Kondoh M. The Clinical Innovation Network: a policy for promoting development of drugs and medical devices in Japan. Drug Discov Today. 2019;24(1):4–8.
- Notifications no. 4 and 104. New drug applications based on public knowledge (Research and Development Division, Health Policy Bureau and Evaluation and Licensing Division, Pharmaceutical and Medical Safety Bureau, Ministry of Health, Labour and Welfare; 1999.
- Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30(8):863–870.
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–566.
- Karapetis CS, Jonker D, Daneshmand M, NCIC Clinical Trials Group and the Australasian Gastro-Intestinal Trials Group, et al. PIK3CA, BRAF, and PTEN status and benefit from cetuximab in the treatment of advanced colorectal cancer-results from NCIC CTG/AGITG CO.17. Clin Cancer Res. 2014;20(3):744–753.
- Kopetz S, McDonough SL, Lenz H-J, et al. Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG S1406). J Clin Oncol. 2017;35(15_suppl):3505–3505.
- Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371(17):1609–1618.
- Seligmann JF, Fisher D, Smith CG, et al. Investigating the poor outcomes of BRAF-mutant advanced colorectal cancer: analysis from 2530 patients in randomised clinical trials. Ann Oncol. 2017;28(3):562–568.
- Graham DM, Coyle VM, Kennedy RD, et al. Molecular subtypes and personalized therapy in metastatic colorectal cancer. Curr Colorectal Cancer Rep. 2016;12:141–150.
- Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N Engl J Med. 2018;379(21):2027–2039.
- Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390(10089):29–39.
- Shaw AT, Kim TM, Crino L, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017;18(7):874–886.
- Corcoran RB, Andre T, Atreya CE, et al. Combined BRAF, EGFR, and MEK inhibition in patients with BRAFV600E-mutant colorectal cancer . Cancer Discov. 2018;8(4):428–443.
- Tabernero J, Geel RV, Guren TK, et al. Phase 2 results: encorafenib (ENCO) and cetuximab (CETUX) with or without alpelisib (ALP) in patients with advanced BRAF-mutant colorectal cancer (BRAFm CRC). J Clin Oncol. 2016;34(15_suppl):3544–3544.
- Kopetz S, Grothey A, Yaeger R, et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N Engl J Med. 2019;381(17):1632–1643.