Abstract
Background
Pediatric Hodgkin lymphoma (pHL) is highly curable. However, a minority experience relapse and are subjected to toxic salvage regimens. Investigating the patterns of relapse could help to select the patients and/or the involved sites that would benefit from consolidating radiotherapy.
Material and methods
The Danish Childhood Cancer Registry was used to identify children <18 years with relapsed pHL from 1990–2018. The lymphoma volumes involved at diagnosis and at relapse were contoured on the patients’ original scans. Rigid image co-registration was used to merge the scans enabling a visual assessment of the anatomical relapse localization relative to the initially involved lymph nodes, and if irradiated, to the radiotherapy field.
Results
From 185 patients with pHL, 24 patients with relapse were available for analysis. All patients received combination chemotherapy and seven had consolidating radiotherapy. Relapses exclusively in initially involved sites occurred in 14 patients. Relapses exclusively in new sites were rare and only observed in three irradiated patients. Seven patients relapsed in both initially involved and new sites. The median time to relapse was 6 months (range 2–59 months), however, in-field relapses in irradiated patients occurred later (54 months, range 10–59 months). Neither risk group, initial bulky disease, early response, or metabolic activity seemed to be associated with the site of a later relapse.
Conclusion
The number of relapses were small, and conclusions regarding the selection of patients for radiotherapy could not be drawn. Relapse exclusively in initially involved sites were the most common, most often in the exact same initially involved lymph nodes. Hence, modern involved site radiotherapy, focusing on the initially involved lymphoma volume and minimizing the radiation doses to normal tissues, should be applied when consolidating radiotherapy is used in patients with pHL.
Introduction
Pediatric Hodgkin lymphoma (pHL) is highly curable with combination chemotherapy and consolidating radiotherapy [Citation1,Citation2]. Unfortunately, long-term survivors are at risk of treatment-induced late-effects that lead to increased morbidity and mortality [Citation3–5]. Hence, the focus of clinical trials is cure with minimum toxicity, commonly attempted with response adapted treatment reduction and/or omission of radiotherapy [Citation6–11]. As a result, event free survival (EFS) rates have decreased to approximately 90% in low risk patients and 80% in high and intermediate risk patients [Citation6–11]. However, the relative number of patients who relapse from pHL remains low making it challenging to investigate the patterns of relapse. More information on the patients who relapse and the anatomical relapse localization could help to distinguish the patients and/or the involved sites that would benefit from consolidating radiotherapy.
It is often stated that (1) irradiated patients relapse out-of-field and (2) patients with bulky disease relapse more often and benefit from radiotherapy to the bulky site. However, data to support the first theory come from an era where patients received radiotherapy alone and the exact relapse localization was not analyzed [Citation12]. And, although bulky disease, especially in the mediastinum, is an independent predictor of relapse [Citation8,Citation9,Citation13], it has not been established if the patients relapse within the bulky site nor if they benefit from radiotherapy, possibly to higher doses [Citation14].
The aim of this nationwide retrospective study is to characterize the Danish patients with pHL who relapsed over a 28-year period and analyze the anatomical relapse localization relative to the initially involved lymph nodes, and if irradiated, to the radiotherapy field.
Methods
Patients and treatment details
Children <18 years diagnosed with HL in Denmark from January 1990 to December 2018 and registered with a relapse and/or death in the Danish Childhood Cancer Registry were included in the study (last date of follow-up 1 April 2020). Four hospitals in Denmark treated pHL and individual medical records were retrieved and reviewed in order to validate a relapse. Patient characteristics, medical history, pathology reports (from the Danish Pathology Register), blood counts, clinical information, and treatment details (initially and at time of relapse) were obtained. Disease stage was defined according to the Ann Arbor classification, and patients were divided into low (IA/B, IIA,), intermediate (IE, IIEA, IIB, IIIA), and high risk (IIEB, IIIAE, IIIB, IV) treatment groups based on clinical stage (CS) and localized extranodal disease. Elevated erythrocyte sedimentation rate (ESR > 30 mm/hr) and bulky disease were not considered for treatment stratification in the pediatric protocols until 2012 (with the amendment of the EuroNet-PHL-C1 [European Network for Pediatric Hodgkin Lymphoma], NCT00433459) and were not used to divide patients into risk groups in the current study. Bulky disease was defined as a nodal mass of ≥10 cm or greater than one third of the transthoracic diameter (at any level of thoracic vertebrae) on computer tomography (CT) (according to the Lugano Classification [Citation15]). Imaging, together with the written reports from the local radiologists and nuclear medicine physicians, were obtained from the time of diagnosis, during follow-up, and at time of relapse. Imaging in 3D was imported into and viewed in the IMPAX system (AGFA HealthCare, Belgium). Imaging was acquired as CT, magnetic resonance imaging, or positron emission tomography/CT (PET/CT). The radiotracer 2-[18F]fluoro-2-deoxy-D-glucose (2-[18F]FDG) was used in all PET scans. The use of PET/CT varied over time and between the different centers. The first center implemented PET/CT for staging from 2004 but it was used earlier (from 2001) for response assessment and detection of relapse. From 2010 PET/CT was used consistently for imaging in patients with pHL in Denmark. In patients diagnosed before year 2000, the imaging could not be retrieved and only the written imaging reports were available.
Radiotherapy plans in 2D were collected manually, and the Eclipse treatment planning system (v. 13.7, Varian Medical Systems, USA) was used for 3D radiotherapy plans.
Relapse definitions
Time to relapse was defined as time from the date of last treatment till the date of validated relapse with imaging (increase of at least one initially involved mass and/or new lesions) and/or biopsy proven. Based on the disease-free interval, relapses were categorized as progressive disease (≤3 months), early (3–12 months), or late relapse (>12 months). Image-proven remission of disease in the intermediate period was a prerequisite. Patients with primary refractory disease who never achieved a complete response (CR), were excluded from the study.
The definitions of CR/CRu (CR unconfirmed) and partial response (PR) were not used consistently during the treatment decades. Also, tumor measurements were not consistently described on imaging reports, and metabolic activity on PET scans was primarily described as physiological or no uptake (PET-negative) or as pathological uptake (PET-positive). For the current study, we relied on the response to therapy stated on the imaging reports and in the medical records as this was the response which the treating physicians were guided.
Lymphoma volumes
For each patient, the lymphoma volumes were contoured on the diagnostic as well as on the relapse scan and named gross tumor volume initially (GTVi) and GTV relapse (GTVr). For patients who had both a diagnostic and a relapse PET/CT scan (from 2004 and onwards), the PET-positive lymphoma volume (GTVPET) was contoured on the PET scan on a Mirada XD® workstation (Mirada Medical, Oxford, UK) with standardized window level and display settings by a specialist in nuclear medicine with experience in PET/CT for radiotherapy planning. All PET-positive lesions we manually delineated with a visually adapted segmentation method according to departmental guidelines. No fixed thresholds were used in the delineation process. The finished GTVPET was then imported into Eclipse. The GTVi and GTVr contained both PET-positive and PET-negative parts of the lymphoma. Contouring was done by the same physician and subsequently validated by an experienced radiologist. Rigid image co-registration between the diagnostic and the relapse scans was done in Eclipse, cf. . The site of relapse was the focus of the fusion, and for patients with several sites (especially both above and below the diaphragm) several fusions were occasionally required. Relapse localizations were visually assessed and divided into three categories: initially involved sites, new sites, and bothin+new (concurrent initially involved and new sites). In patients with available imaging we were able to detect relapse, not just within the initially involved site, but within the initially involved lymph nodes. In irradiated patients a second co-registration was performed between the radiotherapy planning CT scan and the relapse scan. The GTVr (and GTVPETr) was transferred to the planning CT scan enabling detection of out-of-field and in-field relapses, as well as radiation dose to the site of relapse.
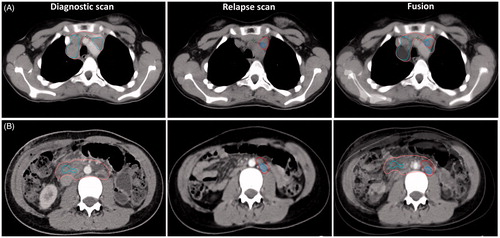
Figure 1. Lymphoma volumes for two patients shown on the diagnostic scan, the relapse scan, and together with the fusion. The fusion was done with rigid image co-registration in the Eclipse treatment planning system. On the diagnostic scan the gross tumor volume (GTV) initially is shown in pink and contains both the PET-positive (GTVPETi shown in cyan) and PET-negative parts of the lymphoma. On the relapse scan GTVr (relapse) is red and the GTVPETr is blue. All structures are shown on the fusion. Patient A (top): clinical stage IIA, relapsed after 6 months in previously involved lymph nodes. Patient B (bottom): clinical stage IIIB with paraaortic bulky disease, relapsed after 6 months within the previously bulky site only.
Summary statistics were reported. Analyses were performed using SPSS (v. 24, IBM).
Approvals
The study was approved by the Danish Patient Safety Authority (3-3013-2575/1), the Danish Data Protection Agency (VD-218-379), and The Danish Clinical Quality Program – National Clinical Registries (RKKP, DBCR-2019-05-28).
Results
Patients
A total of 185 children <18 years were diagnosed with HL during the 28-year period and 36 patients were registered with primary refractory disease, a relapse, and/or death. The median follow-up was 115 months (range 2–361). After a review of individual medical records, 26 patients were categorized as having experienced a relapse, four had primary refractory disease (excluded from this study), and six died with no evidence of a prior relapse or active HL making the crude refractory/relapse rate 16%. Two patients with relapse were excluded; one had a secondary HL, the other had no available medical records.
This study, therefore, includes the remaining 24 patients with relapsed pHL with a median follow-up time of 165 months (range 18–285). Patient characteristics at time of diagnosis are shown in . Two-thirds of the relapsed patients were male, 50% had nodular sclerosis histology, 50% were from the low risk treatment group, and 29% presented with bulky disease.
Table 1. Patient characteristics at time of diagnosis in all patients and divided by relapse localization.
Treatment details
All 24 patients received multidrug chemotherapy, but the drug combinations varied over time (cf. Supplemental material Table S1). Number of chemotherapy cycles depended on the patients’ treatment group (median number of cycles 4, range 2–11). Thirteen (54%) patients received the combination chemotherapy of the GPOH-HD 95 trial (German Pediatric Oncology Hematology Hodgkin Disease Group [Citation6]), three (13%) patients were treated according to the EuroNet-PHL-C1 trial (NCT00433459), and two (8%) patients were randomized within the trial. Patients treated before 1998 (six patients) did not adhere to specific protocols.
Both the GPOH-HD 95 [Citation6] and the EuroNet-PHL-C1 trial (NCT00433459) used a response adapted treatment approach omitting radiotherapy to patients with an adequate response after two cycles of induction chemotherapy. However, the protocol criteria for the omission of radiotherapy were not strictly followed, especially in the earlier years. In the 13 patients, who were treated according to the GPOH-HD 95, radiotherapy was omitted in 10 (77%), whereas, the radiotherapy omission rate for patients included in the GPOH-HD 95 protocol was 18% [Citation6].
PET/CT was used for staging in nine patients, for early response assessment in 11 patients, and relapse diagnostics in 16 patients. Nine patients had PET/CT scans at both time of diagnosis, response assessment, and relapse.
Not surprisingly, a favorable response assessment (PET-negative or CRu) after induction chemotherapy was more common in the non-irradiated patients (70%) whereas an unfavorable response (PET-positive or PR) was more common in irradiated patients (71%). Detailed information on irradiated and non-irradiated patients is shown in and , respectively.
Table 2. Relapse localization and detailed treatment information in irradiated patients.
Table 3. Relapse localization and detailed treatment information in non-irradiated patients.
In total, seven (29%) patients received consolidating radiotherapy; six had involved field radiotherapy (IFRT), one was irradiated to residual disease after chemotherapy completion. Six patients were planned with opposed anterior and posterior field configuration (2D radiotherapy: three; 3D conformal radiotherapy: three) and one with intensity-modulated radiotherapy. Median dose was 19.8 Gy (range 19.6–36.0 Gy) delivered in fractions of 1.5–1.8 Gy. A boost of 10 Gy was delivered to one patient.
Relapse localization
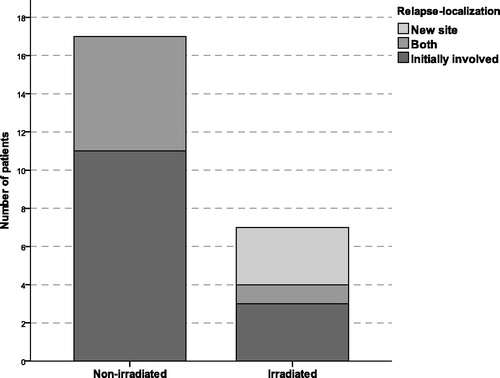
Relapse exclusively in initially involved sites was the most common and observed in 14 patients (58%) of whom three had been irradiated. Relapse exclusively in a new site was rare and observed in three patients (13%), all of whom had been irradiated. Concurrent relapse in bothin+new sites was observed in seven patients (29%), only one previously irradiated. The relapse localization between irradiated and non-irradiated patients is displayed in . In the seven irradiated patients, relapse localization was further divided into in-field and out-of-field relapse (cf. ). Three patients had an in-field relapse in lymph nodes irradiated to the full prescribed dose and four patients had an out-of-field relapse (new site: three, unirradiated initially involved lymph nodes in the patient irradiated to residual disease: one).
Figure 2. Stacked-bar count illustrating the relapse localization in patients treated with chemotherapy alone (non-irradiated) and in patients treated with chemotherapy and consolidating radiotherapy (irradiated). All but one of the irradiated patients received involved field radiotherapy.
For the 24 patients, a total of 118 nodal sites and six extranodal sites (in four patients) were involved at diagnosis. At the time of relapse 82 nodal sites and six extranodal sites (in six patients) were involved (cf. Supplemental material Table S2). Nodal relapse was the most common as 23 (96%) patients relapsed in a nodal site or in both a nodal and an extranodal site. Nodal relapses above the diaphragm (88%) affecting the neck (31%) or mediastinum (16%) were the most common. Two patients only relapsed in lymph nodes below the diaphragm, where the most common relapse site was the paraaortic lymph nodes (10%). The lungs were the most common extranodal site (83%).
Seven (29%) patients presented with bulky disease at diagnosis, most commonly in the mediastinum. Four non-irradiated patients and one irradiated patient (dose to bulk 19.8 Gy) relapsed within the site of initial bulky disease, whereas two irradiated patients relapsed outside the bulk and out-of-field (dose to bulk 29.8 and 36 Gy, respectively).
The median time to relapse for all patients was 6 months (range 2–59 months). Five (21%) patients had progressive disease, 14 (58%) an early, and five (21%) a late relapse. Median time to relapse for non-irradiated and irradiated patients were 6 months (range 2–30 months) and 8 months (range 2–59 months), respectively. However, in-field relapses occurred much later (54 months, range 10–59) than out-of-field relapses (3.5 months, range 2–8).
The metabolic response on the early response assessment PET scan did not seem to be associated with the relapse localization as the 11 patients with a response assessment PET relapsed in both PET-positive and PET-negative involved sites (cf. and , where initially involved sites in bold indicate PET-positive lymph nodes on the response assessment PET/CT). Six of the seven patients with bulky disease had a response assessment PET/CT. Likewise, time to relapse did not seem to be associated with the metabolic response on the early response assessment PET (relapse ≤12 months: 10 patients [five PET-positive, five PET-negative]; relapse >12 months: one PET-positive patient).
None of the patients who relapsed late (>12 months) had anemia, B-symptoms, or bulky disease initially and none relapsed in a new site. Patients from the high-risk group all relapsed in a previously involved site and four out of five relapsed within six months after chemotherapy.
Follow-up
Five patients had a second relapse and one patient had a third. Five of the 24 patients died from their relapse, all experienced an early relapse. Eleven patients received high dose chemotherapy and autologous stem cell transplant (HDCT/ASCT) (first relapse: seven, second relapse: four), one patient had an allogenic stem cell transplant, and 14 patients were irradiated (re-irradiated: three) as part of their salvage strategy. Among the 19 living patients, the following long-term toxicities have been reported: infertility: 11, hypothyroidism: seven, depression/anxiety: two, neuropathy: one, arthralgia: one, none: three. No secondary malignancies have been reported. Two female patients have given birth.
Discussion
This is the first study to investigate the patterns of relapse over three decades in an unselected nationwide cohort of pediatric patients with HL. We carefully analyzed the anatomic relapse localization and found that half of the relapses occurred exclusively in initially involved sites that had not been irradiated. A smaller number of relapses occurred in bothin+new sites. Relapses in a new site alone were rare (13%) and were only observed in irradiated patients. Relapse above the diaphragm affecting the mediastinum was the most common nodal site and the lungs were the most common extranodal site. Using rigid image co-registration we demonstrated that not only do the patients relapse in the initially involved lymph node regions, they relapse in the exact same lymph nodes which were initially involved, confirming that involved site radiotherapy (ISRT) or involved node radiotherapy [Citation16] rather than IFRT can be employed. However, the challenge is to select the patients who will benefit from radiotherapy.
In the era where all patients with HL received combination chemotherapy, other studies have demonstrated that the most common site of relapse was in initially involved lymph node regions regardless of whether consolidating radiotherapy was employed [Citation14,Citation17] or not [Citation18,Citation19]. Similar to our results, relapses in new sites only were more common for irradiated patients [Citation9,Citation18,Citation19].
Bulky disease, especially in the mediastinum, is an important prognostic factor [Citation8,Citation9,Citation13] and irradiating the bulky site to a higher dose has been investigated [Citation20]. Also, radiotherapy to initial bulky disease has been investigated for patients in the high-risk group despite a complete metabolic response after induction chemotherapy [Citation11]. However, only few studies have investigated if patients actually relapse within the bulky site. Krasin et al. [Citation17] found that mediastinal bulk was associated with a higher risk of relapse in previously involved sites but not necessarily within the bulky mediastinum as only 31% relapsed there (all patients were irradiated), whereas other studies have not found a difference in relapse rate between bulky and non-bulky sites nor a correlation between tumor volume and frequency of relapse [Citation14,Citation19]. In our study, only one third of the patients who relapsed had bulky disease initially. They relapsed early (within the first year) and, without consolidating radiotherapy, all relapses occurred within the originally bulky site whereas irradiated patients in two out of three cases relapsed outside of the bulk (like reported by Krasin et al. [Citation17]).
Response assessment with PET/CT is now considered standard of care in HL treatment [Citation15] and its ability to identify responders and non-responders is used to direct further treatment in pHL clinical trials. However, it has not been established that a CR after two series chemotherapy can predict EFS in low-risk patients [Citation9], nor after three series, but a very early PET-response after one series may possibly do so [Citation21]. Likewise, for adult patients with early stage HL, a negative response assessment PET/CT has not identified the patients for whom consolidating radiotherapy can be omitted without increasing the risk of relapse [Citation22–24]. In our study, time to relapse or site of relapse did not seem to be associated with the outcome of the response assessment or the metabolic response on PET. A recent study in adult patients with early stage HL did not find a correlation between initial lymph node characteristics (nodal size, 2-[18F]FDG-uptake, or CT abnormalities after chemotherapy) and the risk of relapse [Citation25]. Hence, irradiating metabolically active sites or bulky tumor sites only does not seem optimal and can only be recommended within clinical trials (currently investigated in AHOD1331 [NCT02166463], EuroNet-PHL C2 [NCT02684708] [Citation11]). In patients with advanced disease, ISRT or INRT would still lead to large treatment volumes and other treatment strategies should be explored. Brentuximab vedotin is currently investigated in the high-risk group as first line treatment in the Children’s Oncology Group AHOD1331 trial (NCT02166463).
Radiotherapy remains the most effective modality for achieving local tumor control in most types of lymphoma, including Hodgkin lymphoma, and omission of radiotherapy will decrease EFS rates [Citation6–11]. The use of consolidating radiotherapy has been limited in the treatment of Danish patients with pHL. In a comparison of pediatric patients with HL in Denmark and Sweden treated from 1990–2010, Englund et al. [Citation26] showed that 36% of the patients in Denmark were irradiated compared to 71% in Sweden. The 10-year EFS was lower in Denmark with 79% (95% CI, 70–86%) compared to 88% (95% CI, 83–92%) in Sweden. The only major difference in treatment approaches was a greater use of radiotherapy in Sweden. Consequently, consolidating radiotherapy might possibly have prevented a relapse in the 11 non-irradiated patients in our patient series who relapsed exclusively in initially involved sites. The risks of more intensive systemic treatment, including salvage regimens after relapse, should be weighed against the risks of modern, highly conformal radiotherapy [Citation27], especially in patients with limited disease. In our study, 46% of the patients with relapse were salvaged with HDCT/ASCT. Also, five of the 11 non-irradiated patients were from the high-risk group and had extensive and/or extranodal disease. For these patients, other systemic treatment options such as targeted agents should be considered [Citation28].
Interestingly, we found that radiotherapy delayed the time to in-field relapses. This was also observed in the Children’s Cancer Group 5942 trial where the median time to relapse for irradiated patients was 20 months (range 1–70 months) compared to 8.5 months (range 1–76 months) for non-irradiated patients [Citation8]. Time to relapse is an important prognostic factor for the outcome of salvage therapy (both with and without HDCT/ASCT) [Citation29,Citation30]. In the ST-HD-86 salvage trial, pHL patients with late relapse (>12 months after the end of therapy) had a significantly better EFS and overall survival (OS) compared to patients who relapsed within the first year [Citation29]. The patients in our study who died from relapsed HL all experienced an early relapse. Apart from being irradiated more, patients who relapsed late did not present with B-symptoms, bulky disease, nor anemia at initial diagnosis.
A limitation of our study was the small number of relapses in a cohort subjected to different treatment strategies, which prevented the ability to find statistically significant differences of relapse characteristics among the relapsed patients. Especially, the low-risk group and the patients with clinical stage IIA constituted a very heterogeneous patient population during the era where prognostic factors were not incorporated in the treatment stratification. Of the 12 low-risk patients, a minimum of three patients would have been categorized as intermediate risk if initial bulky disease and elevated ESR had been included as risk factors at the time. Other limitations are the long time period and the retrospective nature of the study. Imaging modalities changes over time and a detailed anatomical relapse localization of the exact involved lymph nodes were only available for 58% of the patients, for the remaining we relied on the written imaging reports to identify the site of relapse.
The current study describes the patterns of relapse in an unselected nationwide cohort, and although protocol treatment strategies were followed, they were not strictly adhered to and treatment decisions were based on the treating physician’s discretion which is the reality for many patients worldwide.
It is, however, of concern that the 10-year EFS observed in Danish patients with pHL is lower than for Swedish patients and lower than that reported in the GPOH-HD95 trial [Citation6,Citation26]. Also, it underscores the importance of participating in clinical trials which all Danish patients with pHL are offered today. Although, no difference was reported in the OS between the Danish and Swedish patients [Citation26] a recent registry-based study did report a reduction in OS for early stage patients with pHL when radiotherapy was omitted [Citation31]. And, it must be remembered that a very long observation time is needed to detect the influence of the long-term effects of both radiotherapy and intensive salvage therapy.
In conclusion, neither treatment group allocation, bulky disease, early response, nor metabolic activity seemed to be associated with the site of a later relapse in a cohort of consecutively treated patients with pHL in Denmark between 1990 to 2018. Consequently, we argue that the use of these factors to select patients for consolidating radiotherapy should be applied with caution and, preferably, within a randomized clinical trial. Radiotherapy provided local tumor control and prolonged the time to in-field relapses. Relapse in initially involved sites only was the most common, most often in the exact same lymph nodes that were involved at diagnosis. Hence, modern ISRT, focusing on the initially involved lymphoma volume and minimizing the radiation doses to normal tissues, should be applied when consolidating radiotherapy is used in patients with pHL in order to achieve optimal tumor control with minimal toxicity.
Supplemental Material
Download MS Word (15.4 KB)Supplemental Material
Download MS Word (25.1 KB)Acknowledgments
We would like to acknowledge the clinicians and hospital staff who made it possible to collect the data for this study, especially Karen Ottosen Møller from the Department of Pediatric and Adolescent Health, Aarhus University Hospital; Akmal Safwat from the Department of Oncology, Aarhus University Hospital; and the Department of Pediatrics and Department of Oncology, Aalborg University Hospital.
Disclosure statement
Lena Specht is on advisory board and has received speaking honoraria from Takeda and Kyowa Kirin, and she has research agreements with Varian and ViewRay. The remaining authors indicate no conflict of interest.
Additional information
Funding
References
- Schellong G, Brämswig J, Ludwig R, et al. Kombinierte Behandlungsstrategie bei über 200 Kindern mit Morbus Hodgkin: Abgestufte Chemotherapie, Involved-Field-Bestrahlung mit erniedrigten Dosen und selektive Splenektomie. Ein Bericht Der Kooperativen Therapiestudie DAL-HD-82*. Klin Pädiatrie. 1986;198:137–146.
- Kelly KM, Sposto R, Hutchinson R, et al. BEACOPP chemotherapy is a highly effective regimen in children and adolescents with high-risk Hodgkin lymphoma: a report from the Children's Oncology Group. Blood. 2011;117(9):2596–2603.
- Castellino SM, Geiger AM, Mertens AC, et al. Morbidity and mortality in long-term survivors of Hodgkin lymphoma: a report from the Childhood Cancer Survivor Study. Blood. 2011;117(6):1806–1816.
- Bhakta N, Liu Q, Yeo F, et al. Cumulative burden of cardiovascular morbidity in paediatric, adolescent, and young adult survivors of Hodgkin’s lymphoma: an analysis from the St Jude Lifetime Cohort Study. Lancet Oncol. 2016;17(9):1325–1334.
- Veiga LH, Curtis RE, Morton LM, et al. Association of breast cancer risk after childhood cancer with radiation dose to the breast and anthracycline use. JAMA Pediatr. 2019;173(12):1171.
- Dörffel W, Rühl U, Lüders H, et al. Treatment of children and adolescents with Hodgkin lymphoma without radiotherapy for patients in complete remission after chemotherapy: final results of the multinational trial GPOH-HD95. J Clin Oncol. 2013;31(12):1562–1568.
- Mauz-Körholz C, Hasenclever D, Dörffel W, et al. Procarbazine-free OEPA-COPDAC chemotherapy in boys and standard OPPA-COPP in girls have comparable effectiveness in pediatric Hodgkin's lymphoma: the GPOH-HD-2002 study. J Clin Oncol. 2010;28(23):3680–3686.
- Wolden SL, Chen L, Kelly KM, et al. Long-term results of CCG 5942: a randomized comparison of chemotherapy with and without radiotherapy for children with Hodgkin's lymphoma-a report from the Children's Oncology Group. J Clin Oncol. 2012;30(26):3174–3180.
- Metzger ML, Weinstein HJ, Hudson MM, et al. Association between radiotherapy vs no radiotherapy based on early response to VAMP chemotherapy and survival among children with favorable-risk Hodgkin lymphoma. JAMA. 2012;307(24):2609–2616.
- Friedman DL, Chen L, Wolden S, et al. Dose-intensive response-based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate-risk Hodgkin lymphoma: a report from the Children’s Oncology Group Study AHOD0031. J Clin Oncol. 2014;32(32):3651–3658.
- Kelly KM, Cole PD, Pei Q, et al. Response-adapted therapy for the treatment of children with newly diagnosed high risk Hodgkin lymphoma (AHOD0831): a report from the Children's Oncology Group . Br J Haematol. 2019;187(1):39–48.
- Horwich A, Specht L, Ashley S. Survival analysis of patients with clinical stages I or II Hodgkin’s disease who have relapsed after initial treatment with radiotherapy alone. Eur J Cancer. 1997;33(6):848–853.
- Charpentier A-M, Friedman DL, Wolden S, et al. Predictive factor analysis of response-adapted radiation therapy for chemotherapy-sensitive pediatric hodgkin lymphoma: analysis of the Children's Oncology Group AHOD 0031 Trial. Int J Radiat Oncol Biol Phys. 2016;96(5):943–950.
- Dieckmann K, Pötter R, Hofmann J, et al. Does bulky disease at diagnosis influence outcome in childhood Hodgkin’s disease and require higher radiation doses? Results from the German-Austrian Pediatric Multicenter Trial DAL-HD-90. Int J Radiat Oncol Biol Phys. 2003;56(3):644–652.
- Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol. 2014;32(27):3059–3067.
- Specht L, Yahalom J, Illidge T, et al. Modern radiation therapy for Hodgkin lymphoma: field and dose guidelines from the international lymphoma radiation oncology group (ILROG). Int J Radiat Oncol Biol Phys. 2014;89(4):854–862.
- Krasin MJ, Rai SN, Kun LE, et al. Patterns of treatment failure in pediatric and young adult patients with Hodgkin's disease: local disease control with combined-modality therapy. J Clin Oncol. 2005;23(33):8406–8413.
- Dhakal S, Biswas T, Liesveld JL, et al. Patterns and timing of initial relapse in patients subsequently undergoing transplantation for Hodgkin's lymphoma. Int J Radiat Oncol Biol Phys. 2009;75(1):188–192.
- Dharmarajan KV, Friedman DL, Schwartz CL, et al. Patterns of relapse from a phase 3 study of response-based therapy for intermediate-risk Hodgkin lymphoma (AHOD0031): a report from the Children’s Oncology Group. Int J Radiat Oncol Biol Phys. 2015;92(1):60–66.
- Hudson MM, Krasin M, Link MP, et al. Risk-adapted, combined-modality therapy with VAMP/COP and response-based, involved-field radiation for unfavorable pediatric Hodgkin's disease. J Clin Oncol. 2004;22(22):4541–4550.
- Keller FG, Castellino SM, Chen L, et al. Results of the AHOD0431 trial of response adapted therapy and a salvage strategy for limited stage, classical Hodgkin lymphoma: a report from the Children's Oncology Group. Cancer. 2018;124(15):3210–3219.
- André MPE, Girinsky T, Federico M, et al. Early positron emission tomography response-adapted treatment in stage I and II Hodgkin lymphoma: final results of the randomized EORTC/LYSA/FIL H10 Trial. J Clin Oncol. 2017;35(16):1786–1794.
- Radford J, Illidge T, Counsell N, et al. Results of a trial of PET-directed therapy for early-stage Hodgkin's lymphoma. N Engl J Med. 2015;372(17):1598–1607.
- Fuchs M, Goergen H, Kobe C, et al. Positron emission tomography-guided treatment in early-stage favorable Hodgkin lymphoma: final results of the international, randomized phase III HD16 trial by the German Hodgkin Study Group. J Clin Oncol. 2019;37(31):2835–2845.
- Nielsen K, Maraldo MV, Berthelsen AK, et al. Involved node radiation therapy in the combined modality treatment for early-stage Hodgkin lymphoma: analysis of relapse location and long-term outcome. Radiother Oncol. 2020;150:236–244.
- Englund A, Glimelius I, Rostgaard K, et al. Hodgkin lymphoma in children, adolescents and young adults–a comparative study of clinical presentation and treatment outcome. Acta Oncol. 2018;57(2):276–282.
- Specht L. Radiotherapy for Hodgkin lymphoma: reducing toxicity while maintaining efficacy. Cancer J. 2018;24(5):237–243.
- Mottok A, Steidl C. Biology of classical Hodgkin lymphoma: implications for prognosis and novel therapies. Blood. 2018;131(15):1654–1665.
- Schellong G, Dörffel W, Claviez A, et al. Salvage therapy of progressive and recurrent Hodgkin's disease: results from a multicenter study of the pediatric DAL/GPOH-HD study group. J Clin Oncol. 2005;23(25):6181–6189.
- Rimner A, Lovie S, Hsu M, et al. Accelerated Total lymphoid irradiation-containing salvage regimen for patients with refractory and relapsed Hodgkin lymphoma: 20 years of experience. Int J Radiat Oncol Biol Phys. 2017;97(5):1066–1076.
- Jhawar SR, Rivera-Núñez Z, Drachtman R, et al. Association of combined modality therapy vs chemotherapy alone with overall survival in early-stage pediatric Hodgkin lymphoma. JAMA Oncol. 2019;5(5):689.
