Abstract
Purpose
The aim of this study was to examine the risk of HPV-associated oral cavity, oropharyngeal or anal cancer in men with penile cancer to test the hypothesis of an increased risk to develop a second HPV-associated cancer later in life.
Material and methods
We conducted a population-based register study including all men in Sweden diagnosed with penile cancer between 2000 and 2012. For each patient, six men without penile cancer were matched based on age and county of residence. Data were retrieved from Swedish cancer and population registers, to assess the risk of oral cavity, oropharyngeal or anal cancer in patients with penile cancer. Cox proportional hazard models were used to calculate hazard ratios (HRs) with 95% confidence intervals (CIs). Risks in men with penile cancer were also compared with the background Swedish male population by use of standardized incidence ratios.
Results
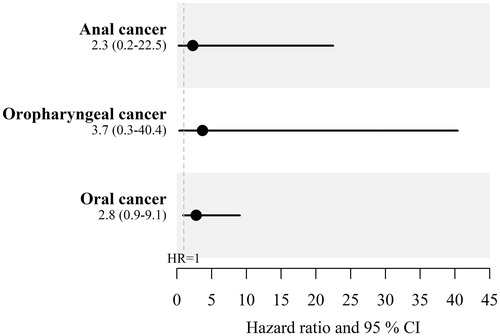
In total, 1634 men with and 9804 without penile cancer were included in the study. Among men with penile cancer, four men were subsequently diagnosed with oral cavity cancer, one with oropharyngeal cancer and one with anal cancer. Corresponding numbers among the penile cancer-free men were ten, two and three, respectively. There was evidence of an increased risks of all three cancers under study with an HR of 2.84 (95% CI 0.89–9.06) for oral cavity cancer, 3.66 (95% CI 0.33–40.39) for oropharyngeal cancer and 2.34 (95% CI 0.24–22.47) for anal cancer. When comparing the incidence of these malignancies between penile cancer patients and the background population, the patterns of association were similar.
Conclusions
Our findings indicate that men with penile cancer are at an increased risk of a second HPV-associated cancer of the oral cavity, oropharynx and anal canal. Considering that our study was based on small numbers reflecting the rarity of these cancers, larger studies are needed to confirm our findings.
Introduction
Penile cancer is a rare malignancy with a global incidence of 0.8 per 100 000 men [Citation1]. Although the incidence of penile cancer is low in most Western countries, higher incidence rates are reported from less developed parts of the world. In Sweden, about 150 new cases are diagnosed annually, corresponding to an incidence of approximately 2 per 100 000 [Citation2]. The aetiology of penile cancer is multifactorial; established risk factors include human papillomavirus (HPV) infection, chronic inflammatory conditions (e.g. penile lichen sclerosus), smoking and phimosis [Citation3].
HPV is a very common sexually transmitted infection, especially among sexually active young adults. Nearly all sexually active individuals will acquire an HPV infection at some point in life [Citation4,Citation5]. While most cases are cleared within 1-2 years by the immune system without causing any symptoms, persistent infection is associated with an increased risk of oncological transformation of the host tissue [Citation6–8].
Globally, HPV is the most common oncogenic virus, causing an estimated 5% of the total cancer burden worldwide [Citation4,Citation5]. HPV infections are associated with several cancer types such as anogenital (penile, anal canal), head and neck (oral cavity, oropharyngeal) and gynaecological (cervical, vaginal, vulvar) malignancies [Citation7,Citation9,Citation10]. Squamous cell carcinoma (SCC) constitutes 99% of penile cancer [Citation2] and 50% is related to HPV. HPV positivity is found more frequently in penile carcinoma in situ (Tis) compared to penile cancer [Citation4,Citation9,Citation11]. Approximately 25% of oral cavity and oropharyngeal cancers are HPV-associated with the remaining 75% being associated with alcohol and tobacco use [Citation12]. Anal cancer is HPV positive in nearly 90% of cases [Citation10,Citation13].
The risk of developing a second HPV-associated cancer after a primary HPV-associated cancer has been investigated previously [Citation14–17]. It is well established that women diagnosed with cervical cancer are at an increased risk of a second HPV-associated cancer because of persistent HPV infection and cross-infection at multiple anatomic sites [Citation18,Citation19]. However, less is known about the corresponding risk for men with penile cancer. If a similar association exists in penile cancer, improved access to early treatment and systematic follow-up is of importance since most of the precancerous and early-stage cancers caused by HPV can be treated successfully [Citation13,Citation14,Citation20]. Also, additional efforts are needed to reduce the prevalence of other factors associated with increased risks to develop anogenital and head and neck cancers, such as smoking [Citation2,Citation12,Citation21].
The aim of this study was to examine if men diagnosed with penile cancer are at an increased risk of a second cancer with a known HPV-association, i.e. oral cavity, oropharyngeal or anal cancer.
Material and methods
For the purpose of this study, we used data available in the Penile Cancer Data Base Sweden (PenCBaSe), a research database generated by record linkages between the Swedish National Penile Cancer Register (NPECR) and several other national registers, including the National Patient Register (NPR), the Swedish Cancer Register (SCR), the Cause of Death Register, the Register of the Total Population, the Swedish Prescribed Drug Register and the Longitudinal Integration Database for Health Insurance and Labour market studies (LISA).
A detailed description of the NPECR has been published previously [Citation2]. In short, the NPECR is a population-based, nationwide register initiated in the year 2000 to monitor the quality of penile cancer care. It contains information on date of diagnosis, county of residence, tumour characteristics according to the TNM classification, location and size of the tumour, primary treatment and lymph node management. HPV status is not routinely registered in the NPECR. The completeness of the NPECR is approximately 99% compared with the SCR to which reporting is mandated by law.
The study population was defined as men identified in the NPECR with a penile cancer diagnosis between 2000 and 2012 based on the ICD-7 code 179.0. No restrictions on morphological differentiation of neoplasms of the penis were applied. Thus, the study population included all manifestations of penile cancer including carcinoma in situ. This group will further be referred to as men with penile cancer. To each man registered with a penile cancer diagnosis (exposed) in the NPECR, six randomly selected men without a history of penile cancer (unexposed) were matched on age (± 1 year) and county of residence. Record linkages between registers were made possible by use of the individual personal identity number assigned to all residents of Sweden.
In this study we focussed on the subsequent risks of oral cavity, oropharyngeal and anal cancers since these are known to be associated with HPV infections [Citation9,Citation10]. The ICD-7 codes of cancer of the oral cavity (140.0–140.9, 141.7–141.9, 142.0–142.9, 143, 144 and 147), tongue base (141.0), tonsil (145.0), pharynx (145.7-145.9 and 148) and anus (154.1 and 154.8) were used for cross-linkage to the SCR to assess the occurrence of these malignancies in exposed and unexposed men [Citation22]. For oral, oropharyngeal and anal tumours morphological codes (8050-8078, 8083-8084, 8094 and 8123) representing SCC were used. In addition, using the same ICD-7 and morphological codes incidence rates of these HPV-associated malignancies in the general male population were estimated based on information from the National Board of Health and Welfare. The Charlson Comorbidity Index (CCI) [Citation23] was used to estimate the comorbidity burden in both exposed and unexposed men based on information in the NPR.
Statistical analyses
To examine the risk of HPV-associated oral cavity, oropharyngeal or anal cancer in men with penile cancer, Cox proportional hazard models were used to calculate hazard ratios (HRs) with 95% confidence intervals (95% CIs), comparing penile cancer patients with men free of penile cancer. The follow-up period started at the date of penile cancer diagnosis and ended at the date of an event, i.e. being diagnosed with either oral cavity, oropharyngeal or anal cancer; death, emigration or the end of the observation period (December 31, 2012), whichever came first.
In a separate step, the incidence of oral cavity, oropharyngeal or anal cancer were compared between penile cancer patients and the background population of Swedish men using standardized incidence ratios (SIRs), defined as the ratio of observed over expected number of cases. The observed number of these cancers was estimated in men with penile cancer by age (in 5-year categories) and time period (per year). On the basis of the number of cases per age-group (in 5-year categories) and calendar year obtained from the SCR, the expected number of cancers was calculated by multiplying person years at risk by the corresponding age- and period-specific incidence rates. Using Byar’s normal approximation, 95% CIs were estimated by the assumption that the observed cases had a Poisson distribution [Citation24].
All men diagnosed with oral cavity cancer, oropharyngeal cancer or anal cancer, prior to the inclusion date were excluded from the analyses. The calculation of person years at risk was performed using the Statistical Analysis Systems (SAS) release 9.4 (SAS Institute, Cary, NC). All other statistical analyses were performed using R version 3.4.2 (R Foundation for Statistical Computing, Vienna, Austria). The STROBE cohort reporting guidelines and checklist were used when writing our report [Citation25].
The study was approved by the Regional Ethics Committee in Uppsala (Approval #2012/021).
Results
Risk of developing HPV-associated cancer in men with and without a history of penile cancer
The final study cohort included 1634 men with penile cancer (exposed) and 9804 men free of penile cancer (unexposed). In the penile cancer group, 38% of men were diagnosed with non-invasive cancer (cTis, cTa/cT0), 56% with invasive cancer (cT1-4) and 6% with an unclassified tumour stage. The median age at diagnosis was 67 years, with 70% of men being older than 60 years. Detailed tumour characteristics are presented in and demographic characteristics of the study population are summarized in .
Table 1. Tumour characteristics of men with penile cancer.
Table 2. Demographic characteristics of men with and without penile cancer.
Six men with penile cancer had a record of a subsequent diagnosis of HPV-associated cancer. Four men (0.26%) were diagnosed with oral cavity cancer, one (0.06%) with oropharyngeal cancer and one (0.06%) with anal cancer. In the unexposed men, ten (0.10%) were diagnosed with oral cavity cancer, two (0.02%) with oropharyngeal cancer and three (0.03%) with anal cancer.
To compare the risk of developing HPV-associated cancer between exposed and unexposed men, hazard ratios (HRs) were calculated, yielding HR of 2.84 (95% CI 0.89-9.06) for oral cavity cancer, 3.66 (95% CI 0.33-40.39) for oropharyngeal cancer and 2.34 (95% CI 0.24–22.47) for anal cancer ().
Risk of developing HPV-associated cancer in men with a history of penile cancer compared with the male background population
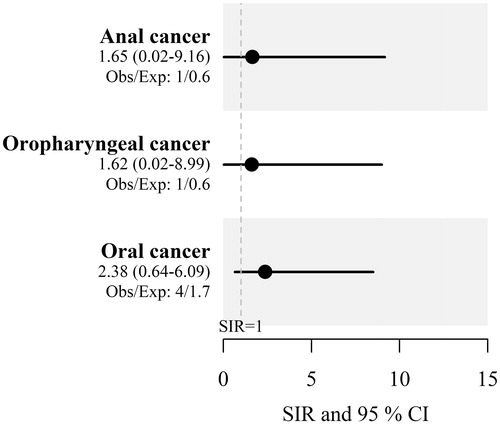
In a separate step, SIRs of oral cavity cancer, oropharyngeal cancer and anal cancer were calculated and compared between men with penile cancer and the Swedish male background population. The SIR was 2.38 (95% CI 0.64-6.09) for oral cavity cancer, 1.62 (95% CI 0.02-8.99) for oropharyngeal cancer and 1.65 (95% CI 0.02-9.16) for anal cancer, respectively ().
Discussion
It has been previously reported that patients diagnosed with primary HPV-associated cancers are at an increased risk of second HPV-associated malignancies although limited data exist regarding subsequent risks in men with penile cancer. In this study, we found indications of a 2-3-fold increased risks of oral cavity, oropharyngeal and anal cancer in men with penile cancer. Albeit based on small numbers yielding wide confidence intervals, our assessment of risk was based on two separate analytic approaches resulting in similar estimates. Taken together, our results provide further support of the hypothesis of an increased risk of a second HPV-associated cancer in patients with penile cancer [Citation14–16,Citation26].
Based on a large cohort of 113 272 men and women with primary cancers associated with HPV, Suk et al. investigated the risk of a second HPV-associated cancer [Citation14]. Compared with the general population, they reported a two-fold increased risk (SIR: 2.5; 95% CI 1.3-4.1) of oropharyngeal cancer in 2721 men with penile cancer, a risk estimate corresponding to the findings in this study. In contrast to our study, risk estimates for oral cavity and oropharyngeal cancer were not presented separately. Furthermore, the authors concluded that the overall risk of any HPV-associated cancer in both men and women was significantly higher after a first cancer associated with HPV compared to a primary non-HPV-associated cancer. Using population-based data, Nelson and Lai examined the risk of second HPV-associated malignancies in 10 537 patients diagnosed with SCC of the anal canal [Citation15]. In that study, a total of 416 patients diagnosed with a second HPV-associated cancer were identified, yielding a SIR of 21.5 (99% CI 19.0–24.2) when compared with the background population. Sex-specific analyses showed an elevated risk of a second HPV-associated malignancy in the male genitalia in men with anal cancer (SIR 19.6; 99% CI 8.7–37.6).
In a population-based retrospective cohort of 10 127 patients with a HPV-associated cancer including 205 men with penile cancer, Neumann et al. assessed the risk of any second cancer, i.e. independent of HPV-association, following a first HPV-associated cancer. Although, no excess risk of subsequent cancer was observed in men with penile cancer, possibly because of small numbers, the authors reported increased risks of a second HPV-associated malignancy in patients with primary head and neck, anal, cervical, vulvar and vaginal cancer, i.e. malignancies with a strong association with HPV [Citation16].
In addition, Gilbert et al. published a systematic review and meta-analysis based on 32 studies on the risk of second cancers at anogenital and oropharyngeal sites in patients with HPV-associated cancers. Two of these studies focussed on penile cancer with pooled results indicating that penile cancer patients are at increased risk of oropharyngeal cancer with a SIR of 3.88 (95% CI 2.21–6.81) [Citation26], estimates broadly in line with our results. The analysis was based on combined data from the anatomic sites of oral cavity and pharynx.
The benefit of HPV vaccination on cervical cancer in women is well established [Citation27]. HPV vaccination of men might prevent a considerable proportion of penile cancer and its precancerous lesions and possibly also reduce the risk of second HPV-associated malignancies at other sites. While there is no conclusive evidence that HPV vaccination can eliminate transformed cells, it has been reported that vaccination of patients treated for HPV-associated precancerous lesions reduces the risk of new lesions in the genital area [Citation27,Citation28].
The major strength of our study was the population-based setting with data retrieved from nationwide registers of high quality and completeness. The use of the personal identity number assigned to all residents of Sweden allowed for individual level record linkages and virtually complete follow-up.
The main limitation of our study was that our risk estimates were based on small numbers, reflecting the rarity of penile cancer and the other HPV-associated cancers under study. Another limitation was the lack of information on confirmed HPV status, precluding assessment of subsequent risks based on HPV status, possibly resulting in a dilution of observed associations. Furthermore, information on other established risk factors for HPV-associated cancers such as smoking history and sexual practices was unavailable [Citation2,Citation12,Citation29].
Conclusions
Based on nationwide register data, we found evidence of increased risks of a second HPV-associated cancer of the oral cavity, oropharynx and anal canal in men with penile cancer, supporting results from earlier studies and meta-analyses. Because of the rarity of these cancers, additional studies based on larger materials and longer follow-ups are needed. An improved understanding in this area can help decrease subsequent morbidity and mortality, for example by implementing monitoring strategies and preventive measures in men with penile cancer.
Supplemental Material
Download MS Word (28.7 KB)Acknowledgments
The study was made possible by the continuous work of the NPECR steering group.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Kirrander P, Sherif A, Friedrich B, et al.; Steering Committee of the Swedish National Penile Cancer Register. Swedish National Penile Cancer Register: incidence, tumour characteristics, management and survival. BJU Int. 2016;117(2):287–292. Feb
- Calmon MF, Tasso Mota M, Vassallo J, et al. Penile carcinoma: risk factors and molecular alterations. ScientificWorldJournal. 2011;11:269–282.
- Serrano B, Brotons M, Bosch FX, et al. Epidemiology and burden of HPV-related disease. Best Pract Res Clin Obstet Gynaecol. 2018;47:14–26.
- Chaturvedi AK. Beyond cervical cancer: burden of other HPV-related cancers among men and women. J Adolesc Health. 2010;46(4 Suppl):S20–S6.
- Kirrander P, Kolaric A, Helenius G, et al. Human papillomavirus prevalence, distribution and correlation to histopathological parameters in a large Swedish cohort of men with penile carcinoma. BJU Int. 2011;108(3):355–359.
- Tanaka TI, Alawi F. Human papillomavirus and oropharyngeal cancer. Dent Clin North Am. 2018;62(1):111–120.
- Bansal A, Singh MP, Rai B. Human papillomavirus-associated cancers: A growing global problem. Int J Appl Basic Med Res. 2016;6(2):84–89.
- Arbyn M, de Sanjose S, Saraiya M, et al. EUROGIN 2011 roadmap on prevention and treatment of HPV-related disease. Int J Cancer. 2012;131(9):1969–1982.
- de Martel C, Plummer M, Vignat J, et al. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–670.
- Olesen TB, Sand FL, Rasmussen CL, et al. Prevalence of human papillomavirus DNA and p16(INK4a) in penile cancer and penile intraepithelial neoplasia: a systematic review and meta-analysis. Lancet Oncol. 2019;20(1):145–158.
- Tumban E. A Current Update on Human Papillomavirus-Associated Head and Neck Cancers. Viruses. 2019;11(10):922.
- De Vuyst H, Clifford GM, Nascimento MC, et al. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis. Int J Cancer. 2009;124(7):1626–1636.
- Suk R, Mahale P, Sonawane K, et al. Trends in Risks for Second Primary Cancers Associated With Index Human Papillomavirus-Associated Cancers. JAMA Netw Open. 2018;1(5):e181999
- Nelson RA, Lai LL. Elevated risk of human papillomavirus-related second cancers in survivors of anal canal cancer. Cancer. 2017;123(20):4013–4021.
- Neumann F, Jegu J, Mougin C, et al. Risk of second primary cancer after a first potentially-human papillomavirus-related cancer: A population-based study. Prev Med. 2016;90:52–58.
- Saleem AM, Paulus JK, Shapter AP, et al. Risk of anal cancer in a cohort with human papillomavirus-related gynecologic neoplasm. Obstet Gynecol. 2011;117(3):643–649.
- Balamurugan A, Ahmed F, Saraiya M, et al. Potential role of human papillomavirus in the development of subsequent primary in situ and invasive cancers among cervical cancer survivors. Cancer. 2008;113(10 Suppl):2919–2925.
- Valari O, Koliopoulos G, Karakitsos P, et al. Human papillomavirus DNA and mRNA positivity of the anal canal in women with lower genital tract HPV lesions: predictors and clinical implications. Gynecol Oncol. 2011;122(3):505–508.
- Razzaghi H, Saraiya M, Thompson TD, et al. Five-year relative survival for human papillomavirus-associated cancer sites. Cancer. 2018;124(1):203–211.
- Daling JR, Madeleine MM, Johnson LG, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 2004;101(2):270–280.
- The Swedish National Board of Health and Welfare. The Swedish Cancer Register 2019 [April 5, 2020]. Available from: https://www.socialstyrelsen.se/en/statistics-and-data/registers/register-information/swedish-cancer-register/.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Breslow NE, Day NE. Statistical methods in cancer research. Volume II–The design and analysis of cohort studies. IARC Sci Publ. 1987;(82):1–406.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. [Internet]. 2020. Available from: https://www.goodreports.org/strobe-cohort/.
- Gilbert DC, Wakeham K, Langley RE, et al. Increased risk of second cancers at sites associated with HPV after a prior HPV-associated malignancy, a systematic review and meta-analysis. Br J Cancer. 2019;120(2):256–268.
- Lei J, Ploner A, Elfstrom KM, et al. HPV Vaccination and the Risk of Invasive Cervical Cancer. N Engl J Med. 2020;383(14):1340–1348.
- Swedish KA, Factor SH, Goldstone SE. Prevention of recurrent high-grade anal neoplasia with quadrivalent human papillomavirus vaccination of men who have sex with men: a nonconcurrent cohort study. Clin Infect Dis. 2012;54(7):891–898.
- Valvo F, Ciurlia E, Avuzzi B, et al. Cancer of the anal region. Crit Rev Oncol Hematol. 2019;135:115–127.