Abstract
Background
The role of adjuvant therapy in patients with oesophagogastric adenocarcinoma treated by neoadjuvant chemotherapy (NAC) and surgery is contentious. In UK practice, surgical resection margin status is often used to classify patients into receiving adjuvant treatment. This study aimed to assess any survival benefit of adjuvant therapy in patients with clear resection margins.
Methods
This was a retrospective collaborative cohort study combining two prospectively collected UK institutional databases of patients with oesophageal adenocarcinoma. Multivariable Cox regression and propensity matched analyses were used to compare overall and recurrence-free survival according to the adjuvant treatment.
Results
Of 374 patients with clear resection margins, 221 patients (59%) had no adjuvant treatment, 137 patients (37%) had adjuvant chemotherapy and 16 patients (4%) had adjuvant chemoradiotherapy. For patients who had received NAC (290, 76%), when adjuvant chemotherapy was compared to no adjuvant treatment, hazard ratios (HRs) favoured adjuvant chemotherapy but did not reach independent significance (overall survival [OS] HR 0.65 95% confidence interval [CI] 0.40–1.06; p .0.087). Responders to NAC (Mandard 1–3) were seemingly more likely to demonstrate a survival benefit from adjuvant chemotherapy (HR 0.42 95% CI 0.15–1.11; p .1.081).
Conclusions
Although no independent survival benefit was observed, the point estimates favoured adjuvant treatment, predominantly in patients with chemo-responsive tumours.
Introduction
Although survival rates have improved, oesophageal cancer is still an important cause of cancer-related deaths worldwide [Citation1]. Oesophageal adenocarcinoma is an aggressive disease and most patients will have locally advanced or metastatic disease at presentation [Citation2]. Despite advances in staging and oncological therapies, long-term outcomes following surgical treatment for oesophageal cancer remain relatively poor [Citation3].
Over the last two decades, UK practice has been influenced by a number of large clinical trials which have demonstrated a survival benefit for patients treated with neoadjuvant therapy compared to surgery alone [Citation4–8]. The MAGIC and FLOT trials both demonstrated a benefit for perioperative chemotherapy [Citation4,Citation8]. However, the specific gains afforded by adjuvant treatment remain unknown and only 50% of patients scheduled for post-operative treatment complete chemotherapy, largely due to the accumulation of acquired therapeutic toxicities [Citation4,Citation5].
According to the National Oesophago-Gastric Cancer Audit (NOGCA), most patients in the UK are offered neoadjuvant chemotherapy (NAC) rather than neoadjuvant chemoradiotherapy, although trials comparing the two strategies are in progress [Citation9,Citation10]. The decision to offer adjuvant treatment is multifaceted; it relies upon patient fitness and their tolerance of pre-operative chemotherapy. Individual tumour characteristics and resection margin status are also taken into account. For patients with clear resection margins (R0 resection) the decision for adjuvant treatment remains challenging. There is some evidence that post-operative systemic therapy should be considered, especially in patients with node-positive disease [Citation11,Citation12]. Recent studies have demonstrated that ‘responders’ to NAC, defined as Mandard tumour regression grade (TRG) 1–3, have a survival benefit from adjuvant chemotherapy when compared to ‘non-responders’ [Citation13]. Prospective clinical trials are still required to establish the benefit of adjuvant treatments in the context of neoadjuvant therapy and surgery.
The aim of this study was to assess the survival benefit of current adjuvant treatments in patients with clear margins (R0 resection) from two high-volume tertiary referral centres in the UK.
Patients and methods
Data source
This was a retrospective cohort study of patients with oesophageal and gastro-oesophageal junctional (GOJ) adenocarcinoma using an ethically approved, collaborative database, from two UK-based institutions; St Thomas’ Hospital and the Royal Marsden Hospital in London. Both are high-volume, tertiary referrals centres which provide specialist surgical and oncological care. The database combined two prospectively maintained, hospital-based operative registries of all patients undergoing oesophago-gastrectomy for cancer.
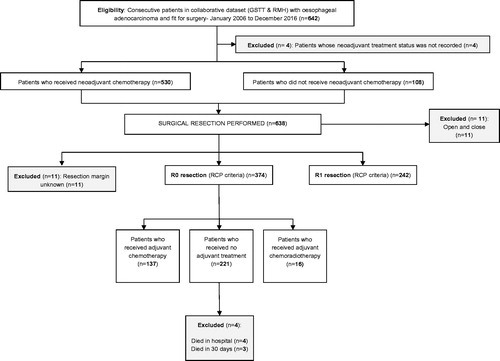
Study cohort
From the collaborative database, all patients who underwent surgical resection for oesophageal adenocarcinoma between 1 January 2006 and 31 December 2016 were identified. All included patients had at least twelve months of complete follow-up data. Clinical follow up continued for five years after resection and further investigations were performed according to suspicion of recurrent disease. Patients who died in-hospital or within 30 d of surgery were excluded from the analyses (). All patients were discussed in a specialist oesophago-gastric cancer multi-disciplinary team (MDT) meeting and standard staging investigations included endoscopy, computed tomography (CT), endoscopic ultrasound, positron emission tomography-computed tomography (PET-CT), and staging laparoscopy in selected cases. Transthoracic and transhiatal oesophageal resections were performed, dictated by tumour location, at the discretion of the individual surgeon. All resection specimens were examined by specialist gastrointestinal pathologists and the status of the resection margin was classified according to the Royal College of Pathologists criteria [Citation14]; an R0 resection having no involvement of any margin and an R1 resection showing tumour within 1 mm of any of the cut margins. All patients with clear margins (R0 resection) were included. All patients who received NAC were allocated a TRG according to the Mandard classification [Citation15]. Patients with a TRG of 1–3 were considered to have had a response to NAC (‘responders’) and those with a TRG of 4 or 5 were considered not to have responded (‘non-responders’). An alternative classification of responders (TRG 1 and 2) vs. non-responders (TRG 3–5) was also assessed.
Treatment and exposure
The NAC regimens included cisplatin and 5-fluorouracil (CF), epirubicin, cisplatin, and 5-fluorouracil (ECF), epirubicin, cisplatin, and capecitabine (ECX), and (epirubicin, oxaliplatin, and capecitabine (EOX) with most patients completing the standard 2–4 cycles as per randomised trial evidence available at the time of treatment. Patients with clear resection margins (R0) were categorised into three treatment groups; adjuvant chemotherapy, adjuvant chemoradiotherapy, or no adjuvant treatment. Tumour stage, response to neoadjuvant treatment, tolerance of chemotherapy, and patient fitness were taken into account by the MDT, however, all patients in the study cohort were deemed fit enough to tolerate peri-operative treatment. The treatment policies were identical in both units and those patients who did not receive adjuvant treatment left the designated treatment pathway. The adjuvant chemotherapy regimens included CF, ECF, ECX, and EOX with most patients completing three cycles or less. Radiotherapy in combination with chemotherapy (fluorouracil (5FU) ± capecitabine) was given as 45 Gray (Gy) in 25 fractions or 50 Gy in 28 fractions.
Outcomes
Overall survival (OS) was defined in days from the date of surgical resection until the date of death. Time to recurrence was calculated from the date of surgical resection until disease recurrence, defined as either histopathological or definitive radiological evidence of local recurrence, systemic recurrence, or both. Recurrence patterns were assessed as a secondary outcome. In the absence of recurrence, survival was calculated to the last confirmed attendance at a hospital or general practitioner clinic.
Statistical analysis
Basic demographic, surgical, and oncological data were evaluated using descriptive statistics. The Kaplan–Meier method was used to calculate and compare survival. Cox proportional hazards regression analysis (crude and adjusted) was used to calculate hazard ratios (HRs) with 95% confidence intervals (CI) to model the association between the study outcomes (all-cause mortality and recurrence) according to the study exposure (adjuvant treatment). Covariates included in the multivariable models were age (continuous), sex, tumour location (oesophageal, Siewert 1, Siewert 2), pathological T-stage (0–2, 3, and 4), pathological N-stage (0–3), lymphovascular invasion, post-operative differentiation (well/moderate and poor), operative approach (Ivor-Lewis oesophagectomy, trans-hiatal oesophagectomy, and left thoraco-abdominal oesophagectomy) and response to NAC (Mandard 1–3 and Mandard 4 and 5). To explore whether any effects of adjuvant treatment differed according to response to NAC, separate analyses, stratified for responders and non-responders, were undertaken. Cox regression analyses comparing OS and recurrence-free survival, according to different adjuvant treatments were also conducted using propensity score-matched cohorts. Propensity scores for receipt of adjuvant treatments were derived using logit models based on the same variables as the Cox regression models (Supplementary Table 1). Matching was performed using psmatch2 module in Stata, with calliper of 0.1 and non-replacement. All statistical analyses were performed using STATA version 15 (StataCorp. 2017. Stata Statistical Software: Release 15, StataCorp LLC, College Station, TX, USA) and a p value < .05 was used to define statistical significance for all outcomes.
Results
Study cohort
Patient demographics, clinical characteristics and neoadjuvant treatment details are summarised in . The database included 374 consecutive patients with oesophageal adenocarcinoma who had clear resection margins (R0) according to the Royal College of Pathologists criteria. This corresponded to an 85% R0 resection rate using the College of American Pathologists definition. The median age of the study cohort was 65 years (inter-quartile range 58–71) and the majority of patients were male (88%). More than half the patients (n = 220, 59%) had a clinically staged T3 (TNM7) tumour at the time of diagnosis. Median follow-up time was 24 months (Interquartile range 13–42 months).
Table 1. Patient demographics, clinical characteristics, and neoadjuvant treatment details for all R0 resection patients with oesophageal adenocarcinoma.
Of 221 (59%) patients received no adjuvant treatment compared to 137 (37%) patients who received adjuvant chemotherapy and 16 (4%) patients who received adjuvant chemoradiotherapy. All of the 16 (4%) patients who received chemoradiotherapy had either a serosal breach on the gastric side of the resection margin or local muscular infiltration of the diaphragmatic crura accounting for an individualised MDT decision to offer chemoradiotherapy. A total of 290 (76%) patients received NAC and of these 136 (47%) patients were deemed to be ‘responders’ (Mandard Grade 1–3) and 152 (52%) patients were ‘non-responders’ (Mandard 4 and 5). In this group, 164 (57%) patients had lymph node-negative disease and 126 (43%) patients had lymph node-positive disease.
Survival analyses
There were three deaths within 30 d of surgery (0.8%). In-hospital mortality following surgical resection was 1.1% (n of surgery (0.8%).
Overall survival
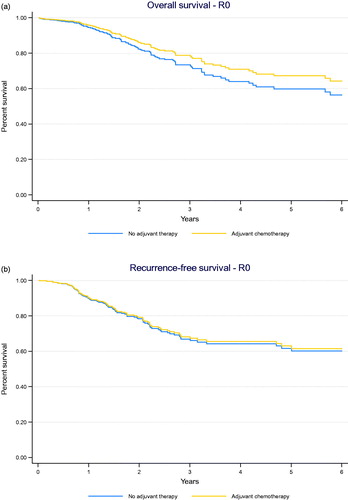
A Kaplan–Meier survival curve for adjusted OS comparing adjuvant chemotherapy to no adjuvant treatment is presented in .
Figure 2. (a) Adjusted Kaplan–Meier survival curve comparing overall survival between adjuvant chemotherapy and no adjuvant treatment in all patients with clear margins (R0 resection) (p = .154). (b) Adjusted Kaplan–Meier survival curve comparing recurrence-free survival between adjuvant chemotherapy and no adjuvant treatment in all patients with clear margins (R0 resection) (p = .732).
Results for crude and adjusted Cox regression analyses are presented in (see also Supplementary Table 2). Pathological nodal status, poor differentiation and poor response to NAC were independently associated with poor survival. In patients who received NAC, a benefit of adjuvant chemotherapy was indicated but did not reach independent significance when compared to no treatment (HR 0.65 95% CI 0.40–1.06; p = .087). When stratified by tumour response to neoadjuvant therapy, responders to NAC (Mandard Grade 1–3) were seemingly more likely to demonstrate a survival benefit from adjuvant chemotherapy (HR 0.42 95% CI 0.15–1.11; p = .081) than non-responders (Mandard Grade 4 and 5, HR 0.71 95% CI 0.39–1.32; p = .280). When stratified by lymph node status, no survival benefit was observed with adjuvant chemotherapy (lymph node-positive HR 0.78 95% CI 0.45–1.35; p = .357, lymph node-negative HR 0.57 95% CI 0.21–1.54; p = .270).
Table 2. Crude and adjusted analyses for overall survival and recurrence-free survival in all R0 resection patients with oesophageal adenocarcinoma stratified by response to neoadjuvant treatment and lymph node status.
Recurrence-free survival
A Kaplan–Meier survival curve for adjusted recurrence-free survival comparing adjuvant chemotherapy to no adjuvant treatment is presented in .
Pathological nodal status, poor differentiation and lymphovascular invasion were independently associated with poor recurrence-free survival in these patients. No statistically significant recurrence-free survival benefit was observed following adjuvant chemotherapy compared to no adjuvant treatment (HR 0.85 95% CI 0.54–1.36; p = .506). When stratified by tumour response to neoadjuvant therapy, no statistically significant recurrence-free survival benefit was observed following adjuvant chemotherapy, however, the HRs favoured treatment in responders (Mandard Grade 1–3, HR 0.68 95% CI 0.27–1.67; p = .395, Mandard Grade 4 and 5, HR 1.00 95% CI 0.55–1.81; p = .995).
Alternative definition of response
Similar results were observed using the alternative definition of responders (Mandard Grade 1 and 2) for OS (HR 0.10 95% CI 0.01–1.56; p = .101) and recurrence-free survival (HR 0.44 95% CI 0.08–2.53; p = .362). OS (HR 0.73 95% CI 0.42–1.27; p = .271) and recurrence-free survival (HR 0.99 95% CI 0.58–1.67; p = .971) were also comparable using the alternative definition for non-responders (Mandard Grade 3–5).
Recurrence patterns
The association between adjuvant treatment and risk of different recurrence patterns is presented in . When stratified by tumour response to neoadjuvant therapy, no survival benefit was observed for any type of recurrence for all R0 patients (responders and non-responders) with adjuvant chemotherapy (HR 0.85 95% CI 0.54–1.36; p = .506 – see ). A survival benefit was suggested for systemic recurrence following adjuvant chemotherapy in responders (Mandard 1–3, HR 0.34 95% CI 0.10–1.12; p = .077).
Table 3. Adjusted analysis for recurrence patterns in all R0 resection patients with oesophageal adenocarcinoma, stratified by response to neoadjuvant treatment.
Propensity matched analyses
The results from the propensity-matched analysis are presented in . The clinical characteristics of the propensity-matched cohorts are shown in Supplementary Table 1. No statistically significant decreased risk of mortality (HR 0.71 95% CI 0.42–1.18; p = .187) or recurrence (HR 0.88 95% CI 0.54–1.45; p = .636) was demonstrated when adjuvant chemotherapy was compared to no adjuvant treatment for all patients who received neoadjuvant therapy.
Table 4. Cox proportional hazards regression comparing effects of adjuvant chemotherapy vs. no adjuvant treatment on overall survival and recurrence-free survival in propensity-matched cohorts of patients with oesophageal adenocarcinoma.
Discussion
This study’s results have not demonstrated an OS benefit for adjuvant chemotherapy after R0 resection for oesophageal adenocarcinoma. However, the point estimates favoured adjuvant treatment, particularly in patients with chemo-responsive tumours, predominantly related to improvements in the rates of systemic recurrence. This raises the possibility that sub-groups of patients, if well selected, may benefit from adjuvant chemotherapy.
There are some methodological constraints which merit consideration. Ideally, prospective randomised data would guide therapeutic strategies in this patient group, yet few previous studies have specifically examined the role of adjuvant therapy in oesophageal adenocarcinoma. Although retrospective in nature, this was a large cohort that combined the results of consecutive patients treated in two high-volume specialist institutions. Whilst this may offer a more realistic reflection of contemporaneous practice, it remains impossible to completely eliminate bias in studies of this kind, despite adjustments for confounding factors and the use of propensity-matched cohorts. Patient numbers were dictated by the consecutive patients in the database, and although this represented a relatively large cohort in the context of the available literature, sub-group analyses were under-powered. Although two institutions’ data were included, both assumed the same MDT process using the same evidence-based protocols for perioperative treatment decisions. Whilst the neoadjuvant and adjuvant regimens varied slightly over the course of the study, this reflected real-time working practice and is unlikely to have affected the overall study conclusions.
One of the main considerations in this study is that patients given adjuvant chemotherapy might have had different characteristics to those who did not receive treatment and that this selection bias could have affected the results. For example, patients receiving adjuvant chemotherapy represented a younger and potentially fitter population, better able to tolerate treatment, which might have favoured this group in OS analyses. Surrogate evidence for this was a shorter post-operative length of stay in this group. Conversely, they had more advanced tumours than the no-treatment group, which would be expected to negatively impact on their cancer-related outcomes. The near significant benefit in the rates of systemic recurrence in patients treated by adjuvant chemotherapy may suggest a true oncological advantage to treatment that cannot be explained simply by patient fitness.
The histological Mandard TRG was used as a marker of response to NAC, and although this has been shown to be prognostic in oesophageal cancer patients treated with neoadjuvant therapy, it remains imperfect in identifying all true responders. Tumour down-staging and lymph node regression may provide important supplementary information that could be used to guide treatment decisions in these patients [Citation16,Citation17]. Classifying patients as responders or non-responders remains logical, although to which group patients with a Mandard TRG of 3 should be allocated, remains contentious [Citation13].
Currently, there is no clear evidence from prospective randomised clinical trials regarding the survival benefit of adjuvant treatment after surgical resection for oesophageal adenocarcinoma. Ongoing trials may help to determine whether post-operative treatment is beneficial for these patients. At present observational studies provide the available evidence to guide treatment decisions and these studies are mostly small and from single institutions.
A recent, large retrospective study, using propensity-matched cohorts, concluded that adjuvant chemotherapy was associated with significantly improved survival for patients with node-positive oesophageal adenocarcinoma after neoadjuvant therapy and complete resection [Citation11]. Similar findings were reported in another study from the United States, which demonstrated that adjuvant chemotherapy improved survival in patients with residual nodal disease after neoadjuvant chemoradiation and oesophagectomy [Citation18]. A European study of 134 patients treated with ECF, EOX, and FLOT demonstrated a survival benefit of continuing chemotherapy after surgical resection but this was limited to those patients with node-positive tumours with poor histological regression [Citation19]. In contrast, a single institution study in the UK demonstrated a significant OS benefit following adjuvant chemotherapy (MAGIC regimen) in patients with chemo-responsive cancers (Mandard 1–3) [Citation13]. They also reported no clear survival benefit in patients with non-responsive tumours (Mandard 4 and 5) and concluded that adjuvant chemotherapy in these patients could result in unnecessary morbidity [Citation13].
A number of studies have demonstrated the potential benefit of adjuvant treatment in patients with positive resection margins (R1 resection) following surgery [Citation3,Citation20,Citation21]. This raises an important point as a number of the aforementioned studies included patients with both positive and negative margins. In our study, patients with positive margins were excluded, as the intended treatment pathway for this group (adjuvant chemoradiotherapy) differed from margin negative patients (adjuvant chemotherapy). This may explain why no clear survival benefit was observed, as some high-risk patients were not included in the analysis. The limitations associated with retrospective observational work must also be taken into consideration when interpreting these studies. Until higher-level evidence is available, resection margin status, nodal disease, and response to neoadjuvant treatment should continue to guide adjuvant treatment decisions in these patients.
Post-operative treatment decisions in this patient group remain challenging. In this study, the HRs favoured adjuvant treatment in patients with chemo-responsive tumours, in keeping with results from earlier studies. Due to the significant morbidity associated with adjuvant treatments, careful decision making is imperative in those patients who are less likely to derive any survival benefit. Prospective clinical trials to establish the survival benefit of adjuvant treatments or new strategies focussing on novel therapies are needed to guide treatment decisions in these patients.
Ethics approval and consent
Ethics approval was gained for the clinical data used in this study. Ethics Committee- Northwest-Haydock Research Ethics Committee. REC Number 12/NW/0511.
Supplemental Material
Download MS Word (21.9 KB)Supplemental Material
Download MS Word (14.6 KB)Acknowledgments
The authors acknowledge RM Partners, Accountable Cancer Network for the Pan London Clinical Research Fellowship Grant received. The authors acknowledge that the work included in this article was presented in abstract form at the following scientific meetings:
13th International Gastric Cancer Congress, 8–11 May 2019, Prague, Czech Republic- Poster Presentation.
AUGIS 22nd Annual Scientific Meeting, 25–27 September 2019, Liverpool, UK- Poster Presentation.
European Society for Diseases of the Esophagus meeting, 20–22nd November, Athens, Greece- Poster Presentation.
Disclosure statement
Dr Nick Maisey – BMS, Servier. Professor David Cunningham – Amgen, Merrimack, Celgene, Bayer, Clovis, Janssen, Sanofi, AstraZeneca, MedImmune, 4SC, Eli Lily, Merck. Dr Elizabeth Smyth – Aptitude Health, Astra Zeneca, BMS, Celgene, Elsevier, Everest Clinical Research, First Word Group, Five Prime Therapeutics, Gritstone Oncology, Imedex, Merck, My Personal Therapeutics, Roche, Sai-Med, Servier, Zymeworks. All remaining authors have no conflicts of interest to declare.
Data availability statement
All data used in this study are available upon request to the corresponding author, Mr Andrew Davies. This article has not been deposited on a pre-print server.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Nevala-Plagemann C, Francis S, Cavalieri C, et al. Benefit of adjuvant chemotherapy based on lymph node involvement for oesophageal cancer following trimodality therapy. ESMO Open. 2018;3(5):e000386.
- Papaxoinis G, Kamposioras K, Weaver JMJ, et al. The role of continuing perioperative chemotherapy post surgery in patients with esophageal or gastroesophageal junction adenocarcinoma: a multicenter cohort study. J Gastrointest Surg. 2019;23(9):1729–1741.
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20.
- Allum WH, Stenning SP, Bancewicz J, et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27(30):5062–5067.
- van Hagen P, Hulshof MCCM, van Lanschot JJB, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084.
- Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090–1098.
- Al-Batran SE, Hofheinz RD, Pauligk C, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO. Lancet Oncol. 2016;17(12):1697–1708.
- Hoeppner J, Lordick F, Brunner T, et al. ESOPEC: prospective randomized controlled multicenter phase III trial comparing perioperative chemotherapy (FLOT protocol) to neoadjuvant chemoradiation (CROSS protocol) in patients with adenocarcinoma of the esophagus (NCT02509286). BMC Cancer. 2016;16:503.
- Reynolds JV, Preston SR, O'Neill B, et al. ICORG 10-14: NEOadjuvant trial in Adenocarcinoma of the oEsophagus and oesophagoGastric junction international study (Neo-AEGIS). BMC Cancer. 2017;17(1):401.
- Drake J, Tauer K, Portnoy D, et al. Adjuvant chemotherapy is associated with improved survival in patients with nodal metastases after neoadjuvant therapy and esophagectomy. J Thorac Dis. 2019;11(6):2546–2554.
- Greally M, Ku GY. Adjuvant chemotherapy for poor pathologic response after pre-operative chemoradiation in esophageal cancer: infeasible and illogical. J Thorac Dis. 2019;11(15):S1855–S1860.
- Saunders JH, Bowman CR, Reece-Smith AM, et al. The role of adjuvant platinum-based chemotherapy in esophagogastric cancer patients who received neoadjuvant chemotherapy prior to definitive surgery. J Surg Oncol. 2017;115(7):821–829.
- The Royal College of Pathologists. Dataset for the histopathological reporting of oesophageal carcinoma (2nd edition). Document G006. 2007. Available from: https://www.rcpath.org/uploads/assets/f8b1ea3d-5529-4f85-984c8d4d8556e0b7/068e9093-0aea-4316-bdd49771564784b9/g006-dataset-for-histopathological-reporting-of-oesophageal-and-gastric-carcinoma.pdf
- Mandard AM, Dalibard F, Mandard J, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathol Correlat Cancer. 1994;73(11):2680–2686.
- Davies AR, Myoteri D, Zylstra J, et al. Lymph node regression and survival following neoadjuvant chemotherapy in oesophageal adenocarcinoma. Br J Surg. 2018;105(12):1639–1649.
- Davies AR, Gossage JA, Zylstra J, et al. Tumor stage after neoadjuvant chemotherapy determines survival after surgery for adenocarcinoma of the esophagus and esophagogastric junction. J Clin Oncol. 2014;32(27):2983–2990.
- Burt BM, Groth SS, Sada YH, et al. Utility of adjuvant chemotherapy after neoadjuvant chemoradiation and esophagectomy for esophageal cancer. Ann Surg. 2017;266(2):297–304.
- Glatz T, Bronsert P, Schäfer M, et al. Perioperative platin-based chemotherapy for locally advanced esophagogastric adenocarcinoma: postoperative chemotherapy has a substantial impact on outcome. Eur J Surg Oncol. 2015;41(10):1300–1307.
- Javidfar J, Speicher PJ, Hartwig MG, et al. Impact of positive margins on survival in patients undergoing esophagogastrectomy for esophageal cancer. Ann Thorac Surg. 2016;101(3):1060–1067.
- Gertler R, Richter J, Stecher L, et al. What to do after R1-resection of adenocarcinomas of the esophagogastric junction? J Surg Oncol. 2016;114(4):428–433.