Abstract
Background
Neutrophil-to-lymphocyte ratio (NLR) has been studied as a biomarker for cancer prognosis, predicting survival in many tumors. The aim of this umbrella review was to combine the results from all systematic reviews and meta-analyses related to the prognostic role of the NLR in patients with urological tumors.
Methods
A PubMed, Scopus, Embase and Cochrane search was undergone from inception through September 2020 for systematic reviews and meta-analyses investigating the prognostic value of NLR in urological tumors, subdivided into prostate cancer, renal cell carcinoma, urothelial bladder and upper tract carcinomas PROSPERO (CRD42020216310).
Results
The results have shown, with a high level of evidence, that an elevated NLR predicts worse overall survival (OS), progression-free survival (PFS) and relapse-free survival (RFS) in prostate cancer, worse OS, PFS and RFS in renal cell carcinoma, worse OS, PFS, RFS and cancer-specific survival (CSS) in muscle invasive bladder cancer, worse PFS and RFS in non-muscle invasive bladder cancer, and worse OS, PFS, RFS and CSS in urothelial upper tract carcinoma.
Conclusion
NLR has a significant prognostic value in urological tumors and should be included in prognostic scores of these cancers.
Introduction
Urological neoplasms, including prostate, bladder, kidney, urinary tract, testicular and penile cancers, are common causes of cancer worldwide with disparities in incidence [Citation1]. Regardless of their frequency and etiology, morbidity and mortality of urological cancers depend on the stage at which they are diagnosed. Thus, since the burden of these malignancies is evolving around the world with population growth and aging, it is of interest to identify high risk and poor prognosis cancer patients, and therefore to identify prognostic factors in patients with urological tumors with the aim of predicting progression and survival.
Evidence has shown that inflammatory response is closely related to tumor development and progression [Citation2]. Moreover, different complex mechanisms were involved in the interactions between tumor and inflammation, leading to tumor cell proliferation, angiogenesis promotion, apoptosis inhibition and tumor metastasis [Citation3]. Accordingly, several blood parameters were studied to reflect systemic inflammatory response and therefore, were used to predict the prognosis of cancer patients, such as C-Reactive Protein (CRP) [Citation4], modified Glasgow Prognostic Score (mGSP) [Citation5], platelet-to-lymphocyte ratio [Citation6] and neutrophil-to-lymphocyte ratio (NLR) [Citation7]. Some authors have even suggested composite prognostic scores based on the measurement of these routine systemic biomarkers [Citation8].
The NLR has arisen as a possible biomarker of cancer prognosis and is of clinical interest due to its convenience and the ease of calculating it from the patient’s routine blood test [Citation9]. Recently, different studies have reported that an elevation in the NLR is associated with poor clinical outcomes in cancer patients, especially urological cancers [Citation10], while other studies have denied it [Citation11]. The EAU guidelines have suggested NLR as a prognostic factor, with however a low level of evidence limiting its clinical usability in prognostic scores. Taking into consideration the recent significant increase in the number of systematic reviews and meta-analyses studying NLR as a potential prognostic marker in urological tumors, there is a need for a new concept that can highlight if the evidence found around the prognosis of the NLR in urological tumors is consistent or if discrepancies exist.
Systematic review of existing reviews, also known as ‘umbrella review’, is currently being used to compile, compare and contrast published reviews and to consider an overall assessment of the highest levels of evidence for a given subject [Citation12]. Hence, we performed an umbrella review with the aim to summarize systematic reviews and meta-analyses and compile all the published evidence related to the prognostic evaluation of the NLR in patients with urological tumors.
Material and methods
Search strategy and eligibility criteria
The study was registered on PROSPERO (CRD42020216310). A systematic literature search was conducted by two independent authors (G.M. and R.C.) of PubMed, Scopus, Embase and Cochrane databases to identify systematic reviews and meta-analyses investigating the prognostic value of NLR in urological tumors, subdivided into prostate cancer, renal cell carcinoma, urothelial bladder and upper tract carcinomas, testicular cancer and penile cancer, prior to or after all treatment options (surgery and/or systemic therapy and/or radiotherapy) published in English or French from inception through 15 September 2020. Disparities between these two authors were solved by a third author (A.K.).
The following search query was used, with no filters applied:
((Neutrophil-to-lymphocyte) OR (Neutrophil lymphocyte ratio) OR (NLR)) AND ((systematic review) OR (meta-analysis)) AND ((urological tumors) or (urological cancers))
((Neutrophil-to-lymphocyte) OR (Neutrophil lymphocyte ratio) OR (NLR)) AND ((systematic review) OR (meta-analysis)) AND (prostate cancer)
((Neutrophil-to-lymphocyte) OR (Neutrophil lymphocyte ratio) OR (NLR)) AND ((systematic review) OR (meta-analysis)) AND ((bladder cancer) OR (upper tract) OR (urothelial carcinoma))
((Neutrophil-to-lymphocyte) OR (Neutrophil lymphocyte ratio) OR (NLR)) AND ((systematic review) OR (meta-analysis)) AND ((renal cell carcinoma) OR (kidney cancer))
((Neutrophil-to-lymphocyte) OR (Neutrophil lymphocyte ratio) OR (NLR)) AND ((systematic review) OR (meta-analysis)) AND ((testicular cancer) OR (testicular tumor))
((Neutrophil-to-lymphocyte) OR (Neutrophil lymphocyte ratio) OR (NLR)) AND ((systematic review) OR (meta-analysis)) AND (penile cancer)
Citation lists of retrieved articles were screened manually to ensure sensitivity of the search strategy.
Studies retrieved through search were stored using the reference manager Zotero® and duplicates were removed. The titles and abstracts of each study were independently reviewed and assessed for eligibility by two investigators (G.M. and R.C.) and disparities were solved by a third investigator (A.K.). Studies that are unclear from their title or abstract had full text reviewed, and thereafter assessed for eligibility.
Inclusion criteria were: 1) Systematic reviews and meta-analyses adhering to PRISMA guidelines; 2) Investigating the prognostic role of NLR in any of the urological tumors abovementioned prior to or after any kind of treatment, using survival outcomes, which include but are not limited to overall survival (OS), progression-free-survival (PFS), cancer-specific survival (CSS) and relapse-free-survival (RFS); 3) Written in English or French languages.
Exclusion criteria were: 1) Original studies, conference proceedings and letters to editors 2) Systematic reviews/meta-analyses targeting all cancers in general, or other non-urological tumors and 3) those tackling less than three original studies.
Data extraction
Two independent authors (G.M. and R.C.) accomplished data extraction from included meta-analyses and settled differences through discussion with a third author (A.K.).
The variables which were retrieved for each study were: first author’s name, publication year, country, type of cancer targeted in the study (all urological tumors or specifically prostate, RCC, bladder, upper tract, testicular or penile), disease stage and setting, number of included studies in the systematic review or meta-analysis, population size, NLR cutoff, hazard ratio (HR) and its 95% confidence interval. Qualitative summary was written in case of systematic review without a quantitative assessment by a meta-analysis.
Quality evaluation
The methodological quality of included studies was assessed using AMSTAR 2 (A MeaSurement Tool to Assess systematic Reviews), which constitutes a valid tool for umbrella reviews: after calculating a specific score, it describes the quality of a systematic review/meta-analysis as either high, moderate, low, or critically low grade [Citation13].
Main outcome
The main outcome was to evaluate the effect of NLR on survival outcomes (OS, RFS, PFS, CSS) of each urological tumor, prior to or after surgery/systemic treatment/radiotherapy. Subgroup analysis was done in each type of cancer (i.e., localized and Castrate Resistant Prostate Cancer (CRPC) in prostate cancer, metastatic and non-metastatic in RCC).
Statistical analysis
Extracted data were pooled using RevMan® 5.4.1 analysis software (Cochrane Collaboration, Copenhagen, Denmark). Results of included meta-analyses were reported in a Forest plot. A final summary HR with 95% confidence interval deduced from the results of all the meta-analyses was calculated for each type of cancers. Estimates for HRs were pooled and weighted by generic inverse variance. Interstudy heterogeneity was assessed using Cochran’s Q test and the I2 statistic. For each cancer site, the summary effect size and 95% confidence interval were calculated using fixed effects model when I2<50% or random effects model when I2>50% (indicating therefore a great heterogeneity). All statistical tests were two-sided, and statistical significance was defined as p < 0.05. Associations between NLR and cancer prognosis was categorized into strong, highly suggestive, suggestive, weak or uncertain according to pre-defined Grading of Recommendations, Assessment, Development and Evaluations (GRADE) criteria [Citation14].
Results
Included studies
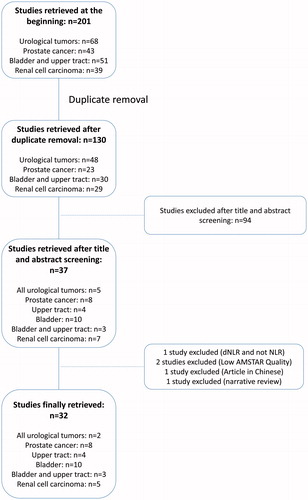
The flowchart of our review is illustrated in . The final number of included systematic reviews and meta-analyses was 31 [Citation10, Citation15–44] (urological tumors (n = 2), prostate cancer (n = 8), upper tract urothelial carcinoma (n = 4), bladder urothelial carcinoma (n = 9), bladder and upper tract urothelial carcinoma (n = 3), and renal cell carcinoma (n = 5)), three of which were only qualitative systematic reviews and the rest (n = 28) were quantitative meta-analyses. Based on the AMSTAR 2, 23 studies were considered as high quality, while 8 were considered as moderate quality. The vast majority of these studies included only retrospective studies or very few prospective ones. Years of publication ranged from 2014 to 2020.
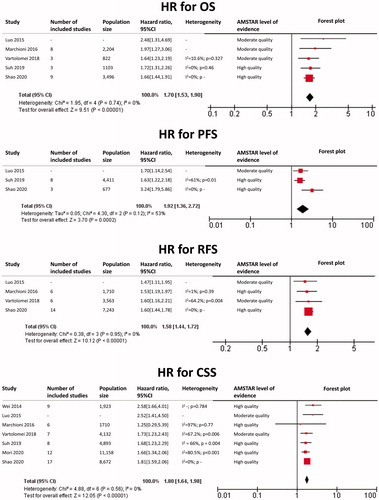
Prostate cancer
The results of each meta-analysis as well as our findings are shown in . The summary HR for OS overall was 1.49 (95%CI: 1.43 − 1.56; p < 0.0001), with a very low heterogeneity (I2 = 0%). The summary HR for PFS overall was 1.46 (95%CI: 1.26–1.70; p < 0.0001), with I2 = 64% and p = 0.03. Heterogeneity was due to some of the results of Guo et al. [Citation37]. Summary HR for PFS excluding these results was 1.37 (95%CI: 1.23–1.53; p < 0.0001), with I2 = 24% and p = 0.27. The summary HR for RFS overall was 1.18 (95%CI: 1.11–1.25; p < 0.0001), with a low heterogeneity (I2 = 41%).
Figure 2. Prostate cancer: HR for Overall Survival (OS), Progression-Free Survival (PFS) and Relapse-Free Survival (RFS) for each included meta-analysis as well as our findings.
In subgroup analysis, HR for OS in localized disease was 1.18 (95%CI: 1.00–1.39; p = 0.05) with a very low heterogeneity (I2 = 0%). HR for OS in CRPC was 1.50 (95%CI: 1.43–1.58; p < 0.0001), with a very low heterogeneity (I2 = 0%). HR for PFS in CRPC was 1.47 (1.33–1.62; p < 0.0001), with a very low heterogeneity (I2 = 0%).
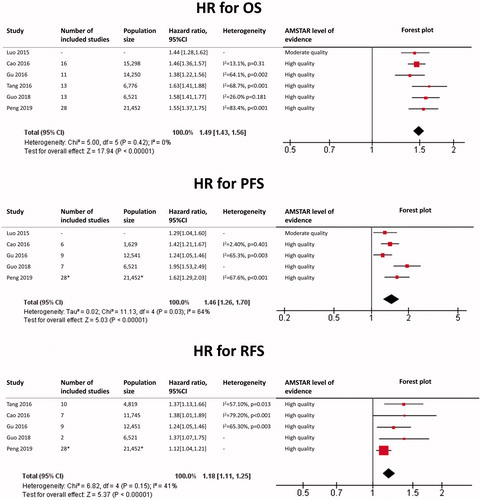
Renal cell carcinoma
The results of each meta-analysis as well as our findings are shown in . The summary HR for OS overall was 1.82 (95%CI: 1.70–1.94; p < 0.0001), with a very low heterogeneity (I2 = 0%). The summary HR for PFS overall was 1.89 (95%CI: 1.68–2.13; p < 0.0001), with a low heterogeneity (I2 = 16%). The summary HR for RFS was 1.86 (95%CI: 1.65–2.10; p < 0.0001), with a low heterogeneity (I2 = 34%).
Figure 3. Renal cell carcinoma: HR for Overall Survival (OS), Progression-Free Survival (PFS) and Relapse-Free Survival (RFS) for each included meta-analysis as well as our findings.
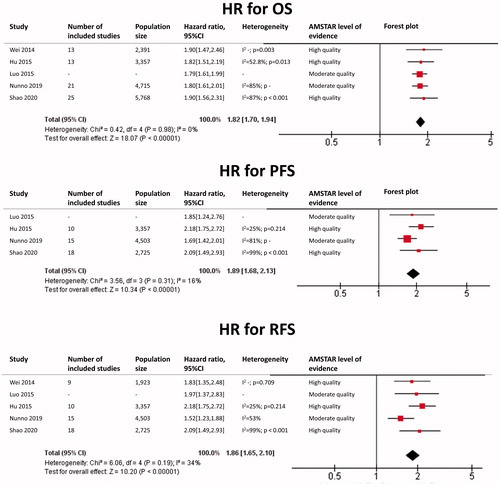
In subgroup analysis, HR for OS in the metastatic subgroup was 2.03 (95%CI: 1.79–2.31), with a very low heterogeneity (I2 = 0%). HR for PFS in the metastatic subgroup was 2.01 (95%CI: 1.57–2.57), with a very low heterogeneity (I2 = 0%). HR for OS in the non-metastatic subgroup was 1.62 (95%CI: 1.35–1.95), with a very low heterogeneity (I2 = 0%).
Bladder cancer
Concerning bladder cancer, most meta-analyses were conducted in the pre-cystectomy setting (muscle invasive bladder cancer, MIBC) while other few of them were conducted in the pre-TURBT setting (non-muscle invasive bladder cancer, NMIBC). Combined overall results were scant, therefore we have undergone our analysis while separating MIBC from NMIBC.
NLR in MIBC
The results of each meta-analysis as well as our findings are shown in . The summary HR for OS in MIBC was 1.32 (95%CI: 1.20–1.45, p < 0.0001), with a significant heterogeneity (I2 = 77%). No source of heterogeneity was detected. The summary HR for PFS in MIBC was 1.57 (95%CI: 1.23–2.00; p = 0.0002), with a significant heterogeneity (I2 = 83%). Heterogeneity was due to some of the results of Luo et al. [Citation29] and Zhang et al. [Citation28]. When excluding these results, HR for PFS in MIBC was 1.22 (95%CI: 1.16, 1.28), with a very low heterogeneity (I2 = 0%). The summary HR for RFS in MIBC was 1.56 (95%CI: 1.28–1.90; p < 0.0001), with a significant heterogeneity (I2 = 68%). Heterogeneity was due to some of the results of Wu et al. [Citation27]. When excluding these results, HR for RFS in MIBC was 1.65 (95%CI: 1.42–1.92; p < 0.0001), with a very low heterogeneity (I2 = 0%). Finally, the summary HR for CSS was 1.42 (95%CI: 1.28–1.57; p < 0.0001) with a significant heterogeneity (I2 = 73%). No source of heterogeneity was detected.
NLR in NMIBC
The summary HR for PFS pre-TURBT for NMIBC was 1.87 (95%CI: 1.55–2.25; p < 0.0001), with a very low heterogeneity (I2 = 0%). The summary HR for RFS pre-TURBT for NMIBC was 1.85 (95%CI: 1.43–2.40; p < 0.0001), with a significant heterogeneity (I2 = 59%). Heterogeneity was due to some of the results of Suh et al. [Citation21]. When excluding these results, HR for RFS was 1.61 (95%CI: 1.32–1.98, p < 0.0001), with a very low heterogeneity (I2 = 0%).
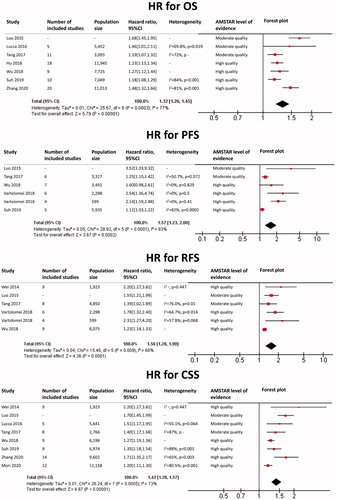
Upper tract urothelial carcinoma
The results of each meta-analysis as well as our findings are shown in . The summary HR for OS was 1.70 (95%CI: 1.53–1.90; p < 0.0001), with a very low heterogeneity (I2 = 0%). The summary HR for PFS was 1.92 (95%CI: 1.36–2.72; p = 0.0002), with a moderate heterogeneity (I2 = 53%). The summary HR for RFS was 1.58 (95%CI: 1.44–1.72; p < 0.0001), with a very low heterogeneity (I2 = 0%). The summary HR for CSS was 1.80 (95%CI: 1.64–1.98; p < 0.0001), with a very low heterogeneity (I2 = 0%).
Testicular and penile cancers
No published meta-analysis or systematic reviews were found for testicular and penile tumors.
Level of evidence
Level of evidence of each finding based on the GRADE criteria was as follows:
Strong for:
OS and RFS in prostate cancer
OS, PFS and RFS in renal cell carcinoma
PFS and RFS in NMIBC
OS, RFS and CSS in upper tract urothelial carcinoma
Highly suggestive for:
PFS in prostate cancer
OS, PFS, RFS and CSS in MIBC
PFS in upper tract urothelial carcinoma
Discussion
This umbrella review is the first to highlight and combine the highest level of evidence from systematic reviews and meta-analyses studying the prognostic role of NLR in urological tumors, in all disease settings (overall, pre-operative, pretreatment, etc.). Our review has shown, with strong or highly suggestive evidence, that an elevated NLR predicts worse OS, PFS and RFS in prostate cancer, worse OS, PFS and RFS in renal cell carcinoma, worse OS, PFS, RFS and CSS in MIBC, worse PFS and RFS in NMIBC, and worse OS, PFS, RFS and CSS in urothelial upper tract carcinoma.
Finding new biomarkers, in order to predict the clinical course of any cancer, is of supreme importance. Many elements define the usefulness of a biomarker: the degree of its correlation with the disease, the simplicity of testing and its interpretation, and its cost. Although several biomarkers have been recently suggested as predictors of urological cancers, the most well-studied biomarker remains NLR, which is both a simple and cheap test to use in clinical practice; once its correlation with disease prognosis is demonstrated, it becomes a useful prognosticator and biomarker to be utilized in clinical practice.
Although NLR constitues a prognosticator of different cancers including breast [Citation45], colon [Citation46], and esophagus [Citation47], it is not only associated to cancer prognosis. It is also associated with the prognosis of many other diseases such as cardiovascular diseases [Citation48], sepsis [Citation49], and even in traumatology [Citation50] and psychiatric diseases [Citation51]. The exact pathophysiological explanation remains unknown until now and cannot be well understood nor clearly explained.
However, what we already know is that an elevated NLR can be the result of (1) either an increased proportion of neutrophils, or (2) a decreased proportion of lymphocytes. We hereby therefore expose the possible reasons behind our findings.
On the one hand, neutrophils play a key role in the inflammatory response, and inflammation is a crucial element of the pathogenesis and progression of malignant tumors [Citation52,Citation53]. Moreover, neutrophils may indirectly change the tumor microenvironment to promote cancer metastasis [Citation54]. The acute phase reaction in cancer is driven by the excessively produced inflammatory cytokines [Citation55]. In addition, neoplasms produce cytokines (as IL-1, IL-6, IL-8 and TNF) and are, as well, infiltrated by inflammatory cells [Citation56]. Thus, early evaluation of inflammatory response in cancer patients reflects neoplastic activity and may bring major details about tumor profile and characteristics. As concrete example, Interleukin-8 (IL-8) has been linked with progression of many urological and non-urological tumors [Citation57]. This cytokine is known to be one of the first factors to activate neutrophils [Citation58]. To conclude, an elevated proportion of neutrophils (i.e., a consequently elevated NLR) means a higher neoplastic activity, therefore explaining worse survival outcomes.
On the other hand, lymphocytes play the key role in the lymphocyte mediated immunity and, therefore, are considered as the main anti-tumoral cells that mediate anti-tumor immune responses. In contrast to neutrophils, an increased infiltration of tumors by lymphocytes is known to have a beneficial effect on response to treatment and prognosis [Citation59], and lymphopenia in cancer patients compromises the lymphocyte-mediated anti-tumor response [Citation60].
These pathophysiological mechanisms could provide a sufficient material to include NLR in prognostic scores of urological malignancies to use it in clinical practice, but inflammatory biomarkers, one of which is NLR, present a lack of specificity due to a significant number of confounding factors which could potentially bias their concentrations, and which cannot be all taken into consideration in the subsequent analysis [Citation61]. Factors that could affect the NLR are known to be infective states [Citation62], acute physiologic stress (<6 h) [Citation9], other cancers, and other comorbidities abovementioned in many medical domains. It is even known that NLR could vary based on the hour of the day when blood is drawn [Citation63].
Our review is not devoid of limitations. First, the included meta-analyses were all based mostly on retrospective studies, due to the lack of prospective studies in the field. Future prospective studies should be done in order to confirm these findings. Second, the NLR cutoff was not evaluated in our review. One reason behind this is that most studies use this prognosticator as a continuous variable with no cutoff, and then assess the cutoff in a subgroup analysis. The other reason is that a significant heterogeneity still exists in the current literature regarding the value of this cutoff, which could vary from 2 to 5: the cutoff is usually chosen either based on the previously published studies or that achieving the highest level of statistical significance. Third, our study did not evaluate the prognostic role of NLR on pathological findings such as lympho-vascular invasion, lymph node invasion or tumor stage, and this is due to conflicting results in the literature with the lack of a significant number of meta-analyses evaluating these findings, in order to be eligible to an umbrella review. Fourth, our review did not evaluate the prognostic role of NLR in specific therapeutic regimens such as BCG bladder instillations in NMIBC, tyrosine kinase inhibitors and check point inhibitors in RCC or chemotherapy and next-generation hormonal treatments such as enzalutamide/abiraterone in advanced prostate cancer. This is also due to the lack of a significant number of meta-analyses evaluating these findings, in order to be eligible to an umbrella review. Finally, testicular and penile cancers were not evaluated since no meta-analyses exist until now regarding NLR in these urological tumors.
Conclusion
Our umbrella review confirms that NLR is a key prognostic factor in prostate, renal, and urothelial bladder and upper tract carcinomas and should be included in prognostic scores of these cancers. Prospective trials are awaited to define the role of NLR in the management of urologic neoplasms.
Supplemental Material
Download MS Word (64 KB)Disclosure statement
The authors report no conflict of interest.
Data availability statement
Data is available upon request from corresponding author.
References
- Schulz WA, Sørensen KD. Epigenetics of Urological Cancers. Int J Mol Sci. 2019;20:4775.
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899.
- Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res. 2011;71:2411–2416.
- Allin KH, Nordestgaard BG. Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit Rev Clin Lab Sci. 2011;48:155–170.
- Qi F, Xu Y, Zheng Y, et al. Pre-treatment Glasgow prognostic score and modified Glasgow prognostic score may be potential prognostic biomarkers in urological cancers: a systematic review and meta-analysis. Ann Transl Med. 2019;7:531.
- Wang J, Liu Y, Zhang N, et al. Prognostic role of pretreatment platelet to lymphocyte ratio in urologic cancer. Oncotarget. 2017;8:70874–70882.
- Faria SS, Fernandes PC, Silva MJB, et al. The neutrophil-to-lymphocyte ratio: a narrative review. Ecancermedicalscience. 2016;10:702.
- Cantiello F, Russo GI, Vartolomei MD, et al. Systemic inflammatory markers and oncologic outcomes in patients with high-risk non-muscle-invasive urothelial bladder cancer. Eur Urol Oncol. 2018;1:403–410.
- Zahorec R. Ratio of neutrophil to lymphocyte counts–rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102:5–14.
- Wei Y, Jiang Y-Z, Qian W-H. Prognostic role of NLR in urinary cancers: a meta-analysis. PloS One. 2014;9:e92079.
- Lu Y, Huang HH, Lau WKO. Evaluation of neutrophil-to-lymphocyte ratio as a prognostic indicator in a Singapore cohort of patients with clinically localized prostate cancer treated with prostatectomy. World J Urol. 2020;38:103–109.
- Aromataris E, Fernandez R, Godfrey CM, et al. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. 2015;13:132–140.
- Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
- Cupp MA, Cariolou M, Tzoulaki I, et al. Neutrophil to lymphocyte ratio and cancer prognosis: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020;18:360.
- Marchioni M, Cindolo L, Autorino R, et al. High neutrophil-to-lymphocyte ratio as prognostic factor in patients affected by upper tract urothelial cancer: a systematic review and meta-analysis. Clin Genitourin Cancer. 2017;15:343–349.e1.
- Mori K, Janisch F, Mostafaei H, et al. Prognostic value of preoperative blood-based biomarkers in upper tract urothelial carcinoma treated with nephroureterectomy: a systematic review and meta-analysis. Urol Oncol. 2020 May;38:315–333.
- Shao Y, Li W, Wang D, et al. Prognostic value of preoperative lymphocyte-related systemic inflammatory biomarkers in upper tract urothelial carcinoma patients treated with radical nephroureterectomy: a systematic review and meta-analysis. World J Surg Oncol. 2020;18:273.
- Vartolomei MD, Kimura S, Ferro M, et al. Is neutrophil-to-lymphocytes ratio a clinical relevant preoperative biomarker in upper tract urothelial carcinoma? A meta-analysis of 4385 patients. World J Urol. 2018;36:1019–1029.
- Li X, Ma X, Tang L, et al. Prognostic value of neutrophil-to-lymphocyte ratio in urothelial carcinoma of the upper urinary tract and bladder: a systematic review and meta-analysis. Oncotarget. 2017;8:62681–62692.
- Marchioni M, Primiceri G, Ingrosso M, et al. The clinical use of the neutrophil to lymphocyte ratio (NLR) in urothelial cancer: a systematic review. Clin Genitourin Cancer. 2016;14:473–484.
- Suh J, Jung JH, Jeong CW, et al. Clinical significance of pre-treated neutrophil-lymphocyte ratio in the management of urothelial carcinoma: a systemic review and meta-analysis. Front Oncol. 2019;9:1365.
- Hu G, Xu F, Zhong K, et al. The prognostic role of preoperative circulating neutrophil-lymphocyte ratio in primary bladder cancer patients undergoing radical cystectomy: a meta-analysis. World J Urol. 2019;37:1817–1825.
- Lucca I, Jichlinski P, Shariat SF, et al. The neutrophil-to-lymphocyte ratio as a prognostic factor for patients with urothelial carcinoma of the bladder following radical cystectomy: validation and meta-analysis. Eur Urol Focus. 2016;2:79–85.
- Mori K, Miura N, Mostafaei H, et al. Prognostic value of preoperative hematologic biomarkers in urothelial carcinoma of the bladder treated with radical cystectomy: a systematic review and meta-analysis. Int J Clin Oncol. 2020;25:1459–1474.
- Tang X, Du P, Yang Y. The clinical use of neutrophil-to-lymphocyte ratio in bladder cancer patients: a systematic review and meta-analysis. Int J Clin Oncol. 2017;22:817–825.
- Vartolomei MD, Porav-Hodade D, Ferro M, et al. Prognostic role of pretreatment neutrophil-to-lymphocyte ratio (NLR) in patients with non-muscle-invasive bladder cancer (NMIBC): A systematic review and meta-analysis. Urol Oncol. 2018;36:389–399.
- Wu S, Zhao X, Wang Y, et al. Pretreatment neutrophil-lymphocyte ratio as a predictor in bladder cancer and metastatic or unresectable urothelial carcinoma patients: a pooled analysis of comparative studies. Cell Physiol Biochem. 2018;46:1352–1364.
- Zhang L, Li L, Liu J, et al. Meta-analysis of multiple hematological biomarkers as prognostic predictors of survival in bladder cancer. Medicine (Baltimore). 2020;99:e20920.
- Luo Y, She D-L, Xiong H, et al. Pretreatment neutrophil to lymphocyte ratio as a prognostic predictor of urologic tumors: a systematic review and meta-analysis. Medicine (Baltimore). 2015;94:e1670.
- Hu K, Lou L, Ye J, et al. Prognostic role of the neutrophil-lymphocyte ratio in renal cell carcinoma: a meta-analysis. BMJ Open. 2015;5:e006404.
- Na N, Yao J, Cheng C, et al. Meta-analysis of the efficacy of the pretreatment neutrophil-to-lymphocyte ratio as a predictor of prognosis in renal carcinoma patients receiving tyrosine kinase inhibitors. Oncotarget. 2016;7:44039–44046.
- Nunno VD, Mollica V, Gatto L, et al. Prognostic impact of neutrophil-to-lymphocyte ratio in renal cell carcinoma: a systematic review and meta-analysis. Immunotherapy. 2019;11:631–643.
- Shao Y, Wu B, Jia W, et al. Prognostic value of pretreatment neutrophil-to-lymphocyte ratio in renal cell carcinoma: a systematic review and meta-analysis. BMC Urol. 2020;20:90.
- Cao J, Zhu X, Zhao X, et al. Neutrophil-to-lymphocyte ratio predicts psa response and prognosis in prostate cancer: a systematic review and meta-analysis. PloS One. 2016;11:e0158770.
- Gu X, Gao X, Li X, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in prostate cancer: evidence from 16,266 patients. Sci Rep. 2016;6:22089.
- Guan Y, Xiong H, Feng Y, et al. Revealing the prognostic landscape of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in metastatic castration-resistant prostate cancer patients treated with abiraterone or enzalutamide: a meta-analysis. Prostate Cancer Prostatic Dis. 2020;23:220–231.
- Guo J, Fang J, Huang X, et al. Prognostic role of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in prostate cancer: a meta-analysis of results from multivariate analysis. Int J Surg Lond Engl. 2018;60:216–223.
- Peng H, Luo X. Prognostic significance of elevated pretreatment systemic inflammatory markers for patients with prostate cancer: a meta-analysis. Cancer Cell Int. 2019;19:70.
- Tang L, Li X, Wang B, et al. Prognostic value of neutrophil-to-lymphocyte ratio in localized and advanced prostate cancer: a systematic review and meta-analysis. PloS One. 2016;11:e0153981.
- Wang Z, Peng S, Xie H, et al. Neutrophil-lymphocyte ratio is a predictor of prognosis in patients with castration-resistant prostate cancer: a meta-analysis. CMAR. 2018;10:3599–3610.
- Yin X, Xiao Y, Li F, et al. Prognostic role of neutrophil-to-lymphocyte ratio in prostate cancer: a systematic review and meta-analysis. Medicine (Baltimore). 2016;95:e2544.
- Nabavizadeh R, Bobrek K, Master VA. Risk stratification for bladder cancer: biomarkers of inflammation and immune activation. Urol Oncol. 2020;38:706–712.
- Boissier R, Campagna J, Branger N, et al. The prognostic value of the neutrophil-lymphocyte ratio in renal oncology: A review. Urol Oncol. 2017;35:135–141.
- Review Article Prognostic value of neutrophil to lymphocyte ratio in patients with bladder cancer: a meta-analysis. Available from: https://1library.net/document/q2n4482q-review-article-prognostic-neutrophil-lymphocyte-patients-bladder-analysis.html
- Ethier JL, Desautels D, Templeton A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. 2017;19:2.
- Mazaki J, Katsumata K, Kasahara K, et al. Neutrophil-to-lymphocyte ratio is a prognostic factor for colon cancer: a propensity score analysis. BMC Cancer. 2020;20:922.
- Arigami T, Okumura H, Matsumoto M, et al. Analysis of the fibrinogen and neutrophil-lymphocyte ratio in esophageal squamous cell carcinoma: a promising blood marker of tumor progression and prognosis. Medicine (Baltimore). 2015;94:e1702.
- Bhat T, Teli S, Rijal J, et al. Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev Cardiovasc Ther. 2013;11:55–59.
- Huang Z, Fu Z, Huang W, et al. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: A meta-analysis. Am J Emerg Med. 2020;38:641–647.
- Park JM. Neutrophil-to-lymphocyte ratio in trauma patients. J Trauma Acute Care Surg. 2017;82:225–226.
- Mazza MG, Lucchi S, Tringali AGM, et al. Neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in mood disorders: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2018;84:229–236.
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867.
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436–444.
- Shaul ME, Fridlender ZG. Tumour-associated neutrophils in patients with cancer. Nat Rev Clin Oncol. 2019;16:601–620.
- Esper DH, Harb WA. The cancer cachexia syndrome: a review of metabolic and clinical manifestations. Nutr Clin Pract. 2005;20:369–376.
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? The Lancet. 2001;357:539–545.
- Aggarwal BB, Gehlot P. Inflammation and cancer: how friendly is the relationship for cancer patients? Curr Opin Pharmacol. 2009;9:351–369.
- Zeilhofer HU, Schorr W. Role of interleukin-8 in neutrophil signaling. Curr Opin Hematol. 2000;7:178–182.
- Gooden MJM, de Bock GH, Leffers N, et al. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105:93–103.
- Wang B, Gu W, Wan F, et al. Prognostic significance of the dynamic changes of systemic inflammatory response in metastatic renal cell carcinoma. Int Braz j Urol. 2019;45:89–99.
- Albisinni S, Moussa I, Aoun F, et al. The impact of postoperative inflammatory biomarkers on oncologic outcomes of bladder cancer. Progres En Urol J Assoc Francaise Urol Soc Francaise Urol. 2019;29:270–281.
- Qu J, Yuan H-Y, Huang Y, et al. Evaluation of neutrophil-lymphocyte ratio in predicting bloodstream infection. Biomark Med. 2019;13:1255–1261.
- Abrahamsen JF, Smaaland R, Sandberg S, et al. Circadian variation in serum cortisol and circulating neutrophils are markers for circadian variation of bone marrow proliferation in cancer patients. Eur J Haematol. 1993;50:206–212.