Abstract
Background and purpose
The subventricular zone (SVZ) is an important niche for neural stem cells but probably also for brain tumor propagating cells, including the glioblastoma stem cell. The SVZ may become a target for radiation therapy in glioblastoma patients. However, reports studying the effect of irradiation of the SVZ on glioblastoma patient survival show conflicting results. We studied the correlation between incidental SVZ radiation dose and survival in a cohort of isocitrate dehydrogenase-wildtype (IDHwt) glioblastoma patients with inclusion of important survival prognosticators.
Patients and methods
In this retrospective analysis, only adult patients with supratentorial IDHwt glioblastoma were included who were treated with temozolomide-based chemoradiotherapy after surgery. The SVZ was contoured on the radiotherapy planning imaging. Cox proportional regression overall survival (OS) analysis was used to study the correlation between SVZ dose and survival. Age, Karnofsky Performance Score, extent of resection and O6-methylguanine-methyl-DNA-transferase gene promoter (MGMTp) methylation were used as covariates in multivariate analysis.
Results
In total, 137 patients were included. Median OS was 13.3 months. The MGMTp methylation was present in 40% of cases. Ipsilateral SVZ (iSVZ) mean dose was 44.4 Gy and 27.2 Gy for the contralateral SVZ (cSVZ). Univariate survival analysis showed an inverse relationship between cSVZ mean dose and OS (HR 1.029 (1.003–1.057); p= .032). However, there was no correlation between cSVZ mean dose and OS in multivariate analysis. iSVZ dose did not correlate with survival.
Conclusion
In this cohort of 137 IDHwt glioblastoma patients, iSVZ did not correlate with OS. Higher cSVZ dose was inversely correlated with OS in univariate survival analysis but lost its significance in multivariate analysis, including MGMTp-methylation. Hence, the correlation between SVZ radiation and glioblastoma patient survival remains unclear. Carefully designed prospective studies are needed to provide unequivocal results on this controversial topic.
Introduction
Circumscribing the lateral walls of the lateral ventricles, the subventricular zone (SVZ) is the largest reservoir of neural stem cells (NSCs) in the adult human brain [Citation1,Citation2]. In humans, the SVZ contains a unique ribbon of astrocytes which are capable of self-renewal but also of differentiation into neural cell types and glial cells. The cancer stem cell (CSC) theory states that oncological diseases are hierarchically organized. Oncogenic mutations in stem cells lead to CSCs. These CSCs share several characteristics with normal stem cells, such as multipotency, low self-renewal rate, and a strictly regulated balance between proliferation and cell death, in which the local microenvironment of the stem cell niche has a crucial role [Citation3]. Adapted to brain tumors, the CSC theory implies the existence of brain tumor propagating cells (BTPCs). In 2019, Lee et al. presented direct genetic evidence from both glioblastoma patients and mouse models that BTPCs develop from SVZ cells [Citation4]. BTPCs divide and differentiate into proliferating glioblastoma cells which build up the bulk of the glioblastoma tumor mass. If the BTPCs are not eradicated, the tumor will inevitably return. This theory explains the high tumor recurrence rate in the vast majority of glioblastoma patients, despite maximum safe resection, radiation therapy, and concurrent temozolomide (TMZ)-based chemotherapy. Hence, targeting BTPCs or their microenvironment seems a logical next step in glioblastoma treatment [Citation5]. Since BTPCs likely reside in the SVZ, this brain region may become a radiotherapeutic target in glioblastoma patients given that radiation therapy is already standard-of-care in glioblastoma treatment [Citation6]. The past decade, several articles were published on the correlation between SVZ radiation and glioblastoma patient survival [Citation7–20]. Three consecutive reviews of the available literature were unable to draw definitive conclusions from these reports [Citation21–23]. Some of the major criticisms on these studies are the lack of inclusion of molecular biological factors in survival analysis, especially isocitrate dehydrogenase (IDH) mutation and O6-methylguanine-DNA-methyltransferase gene promoter (MGMTp) methylation; relatively small sample sizes with subgroup analysis, limiting statistical power; and inclusion of other high-grade gliomas, next to glioblastoma, combined with lack of uniform first-tier treatment of patients at diagnosis [Citation22]. Hence, reports studying the impact of SVZ radiation on survival of IDH-wildtype (IDHwt) glioblastoma patients with survival correction for MGMTp-methylation status are sparse. We wanted to evaluate the effect of incidental radiation to the SVZ on survival in a larger cohort of IDHwt glioblastoma patients who were uniformly treated with TMZ-based chemoradiotherapy after surgery [Citation24]. MGMTp-methylation status was included in survival analysis.
Patients and methods
Patient selection
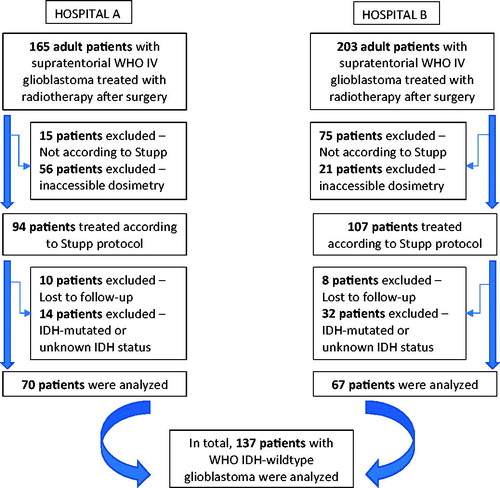
Adult patients (18 years and older) with supratentorial IDHwt glioblastoma were included in this retrospective study only if they completed the full radiotherapy course with concomitant TMZ chemotherapy after surgery. If patients did not complete six cycles of adjuvant TMZ, they were still eligible for inclusion. All patients were treated between 2003 and 2014 in two Flemish hospitals. Patients were excluded if the so-called Stupp protocol was not applied; if patients were lost to follow-up; or if radiotherapy planning data could not be retrieved or restored () [Citation24]. Demographic parameters were collected from the patient charts or electronic medical records. Date of death was cross-checked with the Belgian Cancer Registry. This study was approved by the Ethics Committee of both participating hospitals (Belgian Registration number B670201730765; UZG 2016/1594; AZD 17004). The need for informed consent was waived because of the retrospective study design and because the study did not involve any risk to the patients. The STROBE guideline was used as a guide to write the manuscript.
Imaging, surgery, and molecular biological factors
The presence of SVZ contact at diagnosis was evaluated on post-contrast T1 magnetic resonance (MR) imaging. All MR images were acquired on 1.5 T or 3 T MR imaging systems (Siemens, Erlangen, Germany). If the contrast-enhancing part of the tumor abutted the ventricle, the tumor was classified as SVZ contacting glioblastoma. Surgery was dichotomized as biopsy vs. resection. Resection was further categorized as gross total resection (GTR) if there was no residual contrast-enhancement on postoperative imaging but as subtotal resection (STR) if there was. The neuropathologist selected an appropriate tissue specimen from the tumor database. Histological diagnosis was reconfirmed according to the 2016 World Health Organization (WHO) criteria. The presence of IDH1 or 2-mutation in exon 4 was analyzed using next-generation sequencing [Citation25]. MGMTp-methylation was detected using semi-quantitative methylation-specific polymerase chain reaction (qMSP) as previously reported [Citation26]. Finally, none of the patients were treated with tumor treating fields.
Radiotherapy: contouring and dosimetry
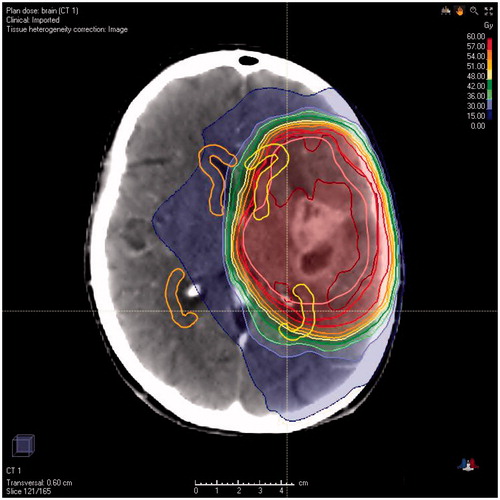
All patients were radiated using 3D conformal external beam therapy or intensity modulated radiotherapy. The radiation oncologist defined the GBM target volumes at the time of treatment planning. Gross tumor volume (GTV) was defined on planning computed tomography (CT) after co-registration with contrast-enhanced T1-weighted MR imaging. In general, a margin of 1–2 cm was used to generate the clinical target volume (CTV), taking into account anatomical borders and the FLAIR signal intensity. The planning target volume (PTV) was obtained by adding an additional 3–5 mm margin to the CTV. A median dose of 60 Gy in 30 fractions of 2 Gy was prescribed to the PTV. The SVZ was not contoured at the time of treatment and thus not intentionally irradiated. Ipsilateral and contralateral SVZs (cSVZs) were manually contoured on planning CT imaging at the time of this study. The SVZ was defined as a 5 mm zone surrounding the full extent of the lateral wall of the lateral ventricles and of the temporal horns (), as anatomically and histologically described [Citation2]. If the contrast-enhancing part of the tumor invaded the SVZ, then this region of the SVZ was not included in the contouring. The ipsilateral SVZ (iSVZ) was defined as the SVZ on the same side as the tumor. In case of multifocal or midline tumors, the iSVZ was defined as the side where the bulk of the tumor was located. Dose–volume histograms were calculated. Mean and quartile doses were obtained for the iSVZ and cSVZ.
Statistical analysis
Progression-free survival (PFS) was defined as the time between the date of first surgery and the date of radiologic evidence of disease recurrence or progression or the date of change in treatment plan due to clinical disease progression, or patient death if this occurred before there was radiologic evidence of disease progression. Overall survival (OS) was calculated as the time between the date of the first surgery and date of death. Patients who were alive at the time of the analysis were censored for OS. Patients without disease progression were censored for PFS at the time of the last registered follow-up visit. The median survival times were calculated using the Kaplan–Meier estimates. Prognostic factors for PFS and OS were studied using univariate Cox proportional hazards analysis, including the mean doses of iSVZ and cSVZ. If SVZ dose showed a significant correlation with survival in univariate analysis, multivariate Cox proportional hazard models were subsequentially fitted adjusted for the following prognostic factors: age, Karnofsky Performance Score (KPS), extent of resection (biopsy vs. resection), and MGMTp-methylation (). Although SVZ contact at diagnosis is described as a negative and independent prognosticator for glioblastoma patient survival [Citation27,Citation28], it was not included in multivariate survival analysis because of collinearity. Numerical variables were not dichotomized in Cox regression models [Citation29]. For categorical variables, graphical methods were applied to check the proportional hazards assumption while for numerical variables the assumption was verified by means of introduction of a time-dependent co-variable in the model. All analyses were performed with SPSS (v26, IBM, Armonk, NY, USA). P values less than .05 were considered significant for two-sided testing.
Results
The study included 137 IDHwt glioblastoma patients of whom 62.8% were males (). The average patient age was 62.2 years. In 69.3% of cases, the tumor abutted the ventricle at diagnosis. Patients underwent surgical resection in 74.4% of cases but in 25.6% only a biopsy was performed. MGMTp-methylation was present in 54 patients (39.4%). Mean incidental radiation doses to the SVZ were 44.4 Gy and 27.2 Gy for the iSVZ and cSVZ, respectively ().
Table 1. Demographical, radiological, molecular and treatment characteristics of 137 IDH-wildtype glioblastoma patients.
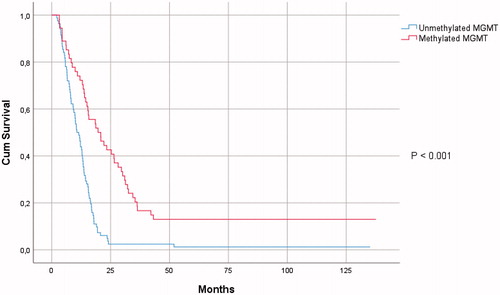
Eight patients were alive at the time of the database closure and were censored for OS. Median survival rates were 13.3 months for OS and 6.4 months for PFS. The following parameters proved statistically significant prognostic factors for both OS and PFS in univariate survival analysis: KPS; extent of resection; MGMTp-methylation (); and SVZ contact at diagnosis (). Higher mean cSVZ dose was correlated with worse OS (HR 1.029; 95% CI 1.003–1.057; p = .032) in univariate survival analysis. Using a multivariate Cox regression survival model, adjusting survival for age, KPS, extent of resection and MGMTp-methylation, cSVZ mean dose did not show a statistically significant correlation with OS (). iSVZ mean dose was not correlated with survival (). Age, extent of resection, and MGMTp-methylation proved independent prognosticators for OS in multivariate survival analysis ().
Table 2. Univariate survival analysis.
Table 3. Multivariate overall survival analysis model for contralateral subventricular zone mean dose.
Discussion
The results from univariate survival analysis in this study of IDHwt glioblastoma patients show that incidental radiation dose to the cSVZ has negative impact on OS although the hazard ratio is very small (1.029). However, after adjustment of survival for age, KPS, extent of resection and MGMTp-methylation, cSVZ mean dose lost its significance. iSVZ mean dose did not correlate with survival.
Molecular biological factors play a key role in glioblastoma patient survival. MGMTp-methylation is a well-known epigenetic phenomenon in glioblastoma, silencing the MGMT gene and consequentially rendering the tumor cells more vulnerable to the alkylating effects of chemotherapeutics [Citation26]. The presence or absence of the IDH-mutation is an essential molecular marker for all gliomas and since the publication of the 2016 WHO Classification of Central Nervous Tumors, IDHwt and IDH-mutated glioblastoma are to be distinguished [Citation30]. In most studies on SVZ radiation in glioblastoma, however, molecular markers are missing. IDH-mutation was only considered in two previous reports [Citation17,Citation18]. The IDH-mutation status was missing in more than 90% of cases in the publication by Achari et al., but MGMT-methylation could be determined in 97% of cases [Citation17]. The second report, by Muracciole et al., presented complete data for MGMT-methylation in an IDHwt glioblastoma patient population, similar to the cohort presented in this study [Citation18]. Although the patient cohorts in the publications by Achari et al. and Muracciole et al. were relatively small (61 and 50 patients, respectively), both reports concluded that higher SVZ radiation dose had a negative impact on glioblastoma patient survival. Together with the 2014 publication by Elicin et al., ours is the fourth article that shows that increasing SVZ dose may have a negative impact on glioblastoma patient survival [Citation12]. The deleterious effect may be explained by the known side-effects of brain radiation, most importantly neurocognitive toxicity and lymphopenia [Citation31]. Of course, also TMZ may cause moderate to severe lymphopenia. Furthermore, evidence shows the high vulnerability of NSCs to even low doses of radiation although a better long-term recovery for NSCs in the SVZ is assumed [Citation32]. Hence, higher radiation doses to the SVZ may result in definitive destruction of the majority of NSCs and subsequentially in severely diminished restorative capacities of the brain. Contrary, the results from many previous publications, that a beneficial effect on survival of higher SVZ dose is present, are possibly biased by the lack of inclusion of the aforementioned molecular markers in survival analysis [Citation7–11,Citation13–16,Citation22].
Another important consideration is the fact that there is at present no consensus in the literature on a cutoff value for SVZ radiation nor on contouring the SVZ [Citation21–23]. Most publications use a high-dose cutoff because of the evidence that BTPCs are very likely to be radioresistant, possibly aided by the hypoxic microenvironment that is also present in the normal SVZ [Citation33–35]. But even this ‘high-dose’ cutoff varies in the reports from 43 Gy to 59 Gy [Citation22]. We chose not to categorize the SVZ mean doses but to include the doses as continuous variables in regression analyses, contrary to most other reports, in order to maximize statistical power and to avoid the bias of small subgroup analyses [Citation29]. The lack of consensus on the methodology of contouring the SVZ is illustrated by the significant variability of published shapes and volumes of the SVZ [Citation22]. Interestingly, a recent report showed the occurrence of significant SVZ volume shifts also during treatment, impacting dosimetric results [Citation36]. Indeed, the published mean SVZ dose also varies widely across the literature [Citation22]. We chose to perform the contouring of the SVZ according to the published cytoarchitectonic findings, comprising the full extent of the lateral ventricular system and including the temporal horns [Citation1,Citation2,Citation22]. This approach explains why the SVZ volumes in this study are higher than in most previous publications and the SVZ mean doses are lower.
Contact of the contrast-enhancing part of the tumor with the SVZ is likely an independent negative prognostic factor for glioblastoma patient survival [Citation27,Citation28]. In this patient cohort, SVZ contact was present in almost 70% of cases and proved a significant prognostic factor for both PFS and OS (). Given the fact that traditional radiotherapy treatment protocols for glioblastoma have a 2–3 cm CTV margin, it logically follows that the iSVZ but also the cSVZ will receive by default higher radiation doses in the presence of SVZ contacting glioblastoma. Hence, SVZ contact could not be included in multivariate survival analysis because of collinearity as was the case in previous studies [Citation10]. Moreover, we agree with Nourallah et al. that it is nearly impossible to distinguish between the prognostic effect of SVZ contact and a possible survival impact of SVZ radiation in a retrospective study [Citation22]. This effect is present in every published study, including the current one.
Furthermore, the SVZ may be not the only niche of BTPCs in case of glioblastoma. Evidence suggests the existence of several niches for BTPCs and Aderetti et al. recently proposed the concept of the 'hypoxic peri-arteriolar glioma stem cell niche' [Citation37]. Moreover, the concepts of the SVZ niche and the peri-arteriolar niche are complementary, rather than mutually exclusive. It is likely that BTPCs migrate from one niche to another, given the complex interplay of signaling pathways and microtubes that is progressively unraveled. But the existence of other BTPCs niches outside the SVZ, may explain why SVZ irradiation yields no therapeutic benefit in case of glioblastoma.
This study is prone to several similar shortcomings as were previous reports. Most importantly, the retrospective study design is subject to selection bias. Corticosteroid use was not studied and treatment for tumor recurrence was not standardized nor included in the study. The contouring of the SVZ could only be performed on planning CT and not on CT-MR fusion images. We could not overcome the problem of collinearity between SVZ contact and SVZ radiation dose. But we think the weaknesses of the study are proportionate to its strengths. Molecular biological factors were analyzed using sensitive and reliable methods [Citation25,Citation26] and MGMTp-methylation was included in multivariate survival analysis. The cohort of IDHwt glioblastoma patients is relatively large, especially when compared with the only other report studying the impact of SVZ radiation in IDHwt glioblastoma patients [Citation18]. Finally, all patients received uniform first-tier treatment after surgery.
Conclusion
In this retrospective analysis of a cohort of uniformly treated IDHwt glioblastoma patients, higher cSVZ mean dose negatively impacted OS in univariate survival analysis, albeit with a very small hazard ratio, but lost its significance after adjustment of survival for age, KPS, extent of resection, and MGMTp-methylation. iSVZ mean dose did not correlate with survival.
Taken together, the results from SVZ radiation studies in glioblastoma are controversial at best. Adapting radiotherapeutic plans of IDHwt glioblastoma patients at this point in time to intentionally include or exclude the SVZ in the irradiated volume cannot be recommended. Unfortunately, one prospective trial (NCT02039778) studying the impact of deliberately radiating the SVZ in glioblastoma patients was terminated early because of poor patient accrual. Hopefully, the only ongoing prospective study (NCT02177578) of intentionally radiating the SVZ to 60 Gy in glioblastoma patients, will provide unequivocal results.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the ethics committee of each hospital (Belgian registration number B670201730765; UZG 2016/1594; AZD 17004).
Acknowledgements
The authors gratefully acknowledge the help of Prof. em. Dr. M. Mareel with the study. We are in debt to the radiophysicists of AZ Delta and Ghent University Hospital for their help in retrieving and restoring the dosimetric data. We would like to specifically acknowledge the efforts made by F. Vanhoutte, PhD, H. Vanhauwaert, T. Vercauteren, and W. De Gersem, PhD.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Research data are available on request.
Additional information
Funding
References
- Sanai N, Tramontin AD, Quiñones-Hinojosa A, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427(6976):740–744.
- Quiñones-Hinojosa A, Sanai N, Soriano-Navarro M, et al. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 2006;494(3):415–434.
- Gilbertson RJ, Rich JN. Making a tumour's bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7(10):733–736.
- Lee JH, Lee JE, Kahng JY, et al. Human glioblastoma arises from subventricular zone cells with low-level driver mutations. Nature. 2018;560(7717):243–247.
- Altmann C, Keller S, Schmidt MHH. The role of SVZ stem cells in glioblastoma. Cancers. 2019;11(4):448.
- Gzell C, Back M, Wheeler H, et al. Radiotherapy in glioblastoma: the past, the present and the future. Clin Oncol (R Coll Radiol). 2017;29(1):15–25.
- Evers P, Lee PP, DeMarco J, et al. Irradiation of the potential cancer stem cell niches in the adult brain improves progression-free survival of patients with malignant glioma. BMC Cancer. 2010;10(1):384.
- Marsh JC, Wendt JA, Herskovic AM, et al. High-grade glioma relationship to the neural stem cell compartment: a retrospective review of 104 cases. Int J Radiat Oncol Biol Phys. 2012;82(2):e159–e165.
- Gupta T, Nair V, Paul SN, et al. Can irradiation of potential cancer stem-cell niche in the subventricular zone influence survival in patients with newly diagnosed glioblastoma? J Neurooncol. 2012;109(1):195–203.
- Chen L, Guerrero-Cazares H, Ye X, et al. Increased subventricular zone radiation dose correlates with survival in glioblastoma patients after gross total resection. Int J Radiat Oncol Biol Phys. 2013;86(4):616–622.
- Lee P, Eppinga W, Lagerwaard F, et al. Evaluation of high ipsilateral subventricular zone radiation therapy dose in glioblastoma: a pooled analysis. Int J Radiat Oncol Biol Phys. 2013;86(4):609–615.
- Elicin O, Inac E, Uzel EK, et al. Relationship between survival and increased radiation dose to subventricular zone in glioblastoma is controversial. J Neurooncol. 2014;118(2):413–419.
- Malik M, Akram KS, Joseph D, et al. Prospective study of irradiation of potential stem cell niches in glioblastoma. Int J Radiat Oncol Biol Phys. 2015;93(3):S111.
- Adeberg S, Harrabi SB, Bougatf N, et al. Do increased doses to stem-cell niches during radiation therapy improve glioblastoma survival? Stem Cells Int. 2016;2016:8793462.
- Kusumawidjaja G, Gan PZH, Ong WS, et al. Dose-escalated intensity-modulated radiotherapy and irradiation of subventricular zones in relation to tumor control outcomes of patients with glioblastoma multiforme. Onco Targets Ther. 2016;9:1115–1122.
- Foro Arnalot P, Pera O, Rodriguez N, et al. Influence of incidental radiation dose in the subventricular zone on survival in patients with glioblastoma multiforme treated with surgery, radiotherapy, and temozolomide. Clin Transl Oncol. 2017;19(10):1225–1227.
- Achari R, Arunsingh M, Badgami RK, et al. High-dose neural stem cell radiation may not improve survival in glioblastoma. Clin Oncol (R Coll Radiol). 2017;29(6):335–343.
- Muracciole X, El-Amine W, Tabouret E, et al. Negative survival impact of high radiation doses to neural stem cells niches in an IDH-wild-type glioblastoma population. Front Oncol. 2018;8:803–807.
- Murchison SC, Wiksyk B, Gossman S, et al. Subventricular zone radiation dose and outcome for glioblastoma treated between 2006 and 2012. Cureus. 2018;10(11):e3618.
- Valiyaveettil D, Malik M, Syed K, et al. Prospective study to assess the survival outcomes of planned irradiation of ipsilateral subventricular and periventricular zones in glioblastoma. Ecancermedicalscience. 2020;14:1021–1011.
- Smith AW, Mehta MP, Wernicke AG. Neural stem cells, the subventricular zone and radiotherapy: implications for treating glioblastoma. J Neurooncol. 2016;128(2):207–216.
- Nourallah B, Digpal R, Jena R, et al. Irradiating the subventricular zone in glioblastoma patients: is there a case for a clinical trial? Clin Oncol (R Coll Radiol). 2017;29(1):26–33.
- Şuşman S, Leucuţa D-C, Kacso G, et al. High dose vs low dose irradiation of the subventricular zone in patients with glioblastoma—a systematic review and meta-analysis. Cancer Manag Res. 2019;11:6741–6753.
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466.
- Ji MS, Eldred BSC, Liu R, et al. Targeted next-generation sequencing of 565 neuro-oncology patients at UCLA: a single-institution experience. Neurooncol Adv. 2020;2(1):7–11.
- Pinson H, Hallaert G, Van der Meulen J, et al. Weak MGMT gene promoter methylation confers a clinically significant survival benefit in patients with newly diagnosed glioblastoma: a retrospective cohort study. J Neurooncol. 2020;146(1):55–62.
- Mistry AM, Kelly PD, Gallant J-N, et al. Comparative analysis of subventricular zone glioblastoma contact and ventricular entry during resection in predicting dissemination, hydrocephalus, and survival. Neurosurgery. 2019;85(5):E924–E932.
- Hallaert G, Pinson H, Van den Broecke C, et al. Subventricular zone contacting glioblastoma: tumor size, molecular biological factors and patient survival. Acta Oncol. 2020;94(7):1–6.
- Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25(1):127–141.
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820.
- Huang J, DeWees TA, Badiyan SN, et al. Clinical and dosimetric predictors of acute severe lymphopenia during radiation therapy and concurrent temozolomide for high-grade glioma. Int J Radiat Oncol Biol Phys. 2015;92(5):1000–1007.
- Hellström NAK, Björk-Eriksson T, Blomgren K, et al. Differential recovery of neural stem cells in the subventricular zone and dentate gyrus after ionizing radiation. Stem Cells. 2009;27(3):634–641.
- Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760.
- Jamal M, Rath BH, Tsang PS, et al. The brain microenvironment preferentially enhances the radioresistance of CD133(+) glioblastoma stem-like cells. Neoplasia. 2012;14(2):150–158.
- Prager BC, Bhargava S, Mahadev V, et al. Glioblastoma stem cells: driving resilience through chaos. Trends Cancer. 2020;6(3):223–235.
- Darázs B, Ruskó L, Végváry Z, et al. Subventricular zone volumetric and dosimetric changes during postoperative brain tumor irradiation and its impact on overall survival. Phys Med. 2019;68:35–40.
- Aderetti DA, Hira VV, Molenaar RJ, et al. The hypoxic peri-arteriolar glioma stem cell niche, an integrated concept of five types of niches in human glioblastoma. Biochim Biophys Acta Rev Cancer. 2018;1869(2):346–354.