Abstract
Objectives
The positron emission tomography (PET) could predict the prognosis of DLBCL patients, but the exact procedure on interim PET (iPET) to determine chemoresistant patients remains elusive.
Methods
We retrospectively analyzed 593 newly diagnosed DLBCL patients uniformly treated with R-CHOP regimen. Among them, 352 patients diagnosed from August 2010 to December 2016 were included in the test cohort and 241 patients diagnosed from January 2017 to December 2019 were included in the validation cohort. The iPET was evaluated with Deauville criteria and ΔSUVmax method. The reduction of maximal SUV between baseline and after 4 cycles of chemotherapy were defined as ΔSUVmax. The survival functions were depicted using the Kaplan–Meier method and compared with the log-rank test.
Results
Patients with iPET Deauville 4 had heterogeneous outcome and end of treatment complete response rates (eCRR). Combined Deauville with ΔSUVmax method, we proposed a modified-Deauville model: patients with Deauville 4 and ΔSUVmax > 70%, as well as those with Deauville 1–3, were reclassified into the modified-Deauville negative group, while patients with Deauville 4 and ΔSUVmax ≤ 70%, as well as those with Deauville 5, into the modified-Deauville positive group. In the test cohort, 3-year PFS, OS and eCRR of modified-Deauville negative group were 80.2%, 89.9% and 91.8%, significantly higher than those of positive group (12.5%, 27.3% and 29.2%, p ≤ .001). Similar results were found in the validation cohort, that 3-year PFS, OS and eCRR were 87.8%, 95.4%, 96.3% in modified-Deauville negative group, and 27.4%, 32.5%, 13.5% in positive group. Through modified-Deauville model, patients in iPET positive group had very low eCRR and were resistant to conventional chemotherapy.
Conclusions
The modified-Deauville model could better distinguish DLBCL patients with poor response to chemotherapy. Accordingly, these patients could be recognized early and provided with alternative therapeutic agents, which might improve the clinical outcome of refractory DLBCL patients.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma (NHL) and constitutes about 40% of all adult NHLs [Citation1]. Immunochemotherapy R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) has greatly improved the outcome of DLBCL patients, yet 30–40% of patients eventually become relapsed or refractory to R-CHOP treatment [Citation2]. Of note, about 15-20% of patients that are refractory to R-CHOP had a cross-resistance to other chemotherapeutic regimens with a dismal outcome, with a median survival of 6 months [Citation3]. Recently, chimeric antigen receptor (CAR)-T cell therapy has successfully rescued refractory DLBCL patients, achieving an overall response rate of 50–82% [Citation4]. Among these chemorefractory patients, those with prior regimens >4, had worse drug efficacy and more severe side effects with CAR-T therapy than the other patients [Citation5,Citation6]. Therefore, it is crucial to identify chemoresistant patients early during standard immunochemotherapy, who may benefit from alternative therapeutic strategies.
International prognostic index (IPI) is widely used in clinical practice to predict the prognosis of patients with DLBCL. More recently, positron emission tomography/computed tomography (PET/CT) has been recommended for DLBCL diagnosis, staging, and assessment of treatment efficacy. The standardized uptake value (SUV) is the ratio of the FDG concentration to the injected dose normalized to the patient body weight, accepted as a semi-quantitative index for tumor glucose metabolism. The most commonly used parameter is SUVmax, the maximal SUV value in the region of interest, and ΔSUVmax is defined as the reduction in SUVmax. Although the evaluation methods of interim PET (iPET), such as Deauville criteria and ΔSUVmax, were reported to be prognostic for DLBCL patients[Citation7], Deauville criteria is more easy-to-use for DLBCL assessment [Citation8]. Different from end-of-treatment PET, the benefit of iPET remains controversial [Citation9–13], particularly the cutoff point of Deauville criteria to define positive iPET and negative iPET. Several studies using Deauville 4–5 as positive [Citation12,Citation14], but others suggest only Deauville 5 can be considered positive [Citation15]. Of note, prospective trials have explored the efficacy of switching lymphoma patients to intensive treatment based on positive iPET (Deauville 4–5) but had negative results [Citation16], indicating that the patients resistant to R-CHOP regimen might also fail to other intensive regimens including autologous stem-cell transplantation (ASCT). Accumulating data show that those chemoresistant patients could benefit from other new therapeutic modalities, such as checkpoint inhibitors and CAR-T cell therapies. Thus, early recognition of resistant patients is crucial for application of new targeted therapeutic modalities in refractory DLBCL.
This study aims to explore the effectiveness of iPET evaluation to determine the chemo-resistant DLBCL patients, which may facilitate a clinical decision to optimize risk-adapted therapeutic strategies.
Patients and methods
Study population
We retrospectively studied 593 patients with newly diagnosed DLBCL from 2010 to 2019. From August 2010 to December 2016, 352 DLBCL patients were included in the test cohort. From January 2017 to December 2019, 241 DLBCL patients were included in the validation cohort. The pathological diagnosis was established according to the World Health Organization (WHO) classification [Citation8]. Germinal center B-cell (GCB) and non-GCB subtypes were defined through Hans algorithm. Patients with primary central nervous system lymphoma, primary mediastinal B-cell lymphoma or Richter’s transformation were excluded. All patients received the treatment immediately after diagnosis and were uniformly treated with 6 cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and predinisone)-21 regimen followed by 2 cycles of rituximab as first-line therapy. Radiotherapy was delivered when focal residual diseases remained at the end of treatment. If patients didn’t achieve response or had disease progression, and then they would receive second-line regimens, such as L-ICE ± R (lenalidomide, ifosfamide, carboplatin, etoposide ± rituximab), DAEPOCH ± R (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin ± rituximab), hyperCVAD ± R (cyclophosphamide, doxorubicin, vincristine, predinisone, methotrexate, cytosar ± rituximab), DHAP ± R (high dose cytosar, cisplatin, dexamethasone ± rituximab) and GDP ± V (gemcitabine, dexamethasone, cisplatin ± velcade).
iPET was performed after 4 cycles of R-CHOP treatment. Among the 593 patients enrolled in this study, 513 had iPET evaluation.
All the patients signed their informed consent. The study was performed in accordance with the Declaration of Helsinki and the protocol was approved by the Ethics Committee of Shanghai Rui Jin Hospital.
18-FDG PET/CT and analysis
Patients avoided strenuous exercise for 24 h and fasted for at least 6 h before the PET scan. Blood glucose level was routinely checked (< 8.0 mmol/L) before the exam. After injection of 5-6MBq of 18F-FDG (Fluoro-deoxyglucose) per kilogram of body weight, a whole-body acquisition was started after 60 min’ rest using CT-PET camera (GE, Discavery STE, NY, USA), as previously reported [Citation17].
All the PET/CT images were specifically reviewed for this study by two experienced nuclear medicine physicians. Images were reconstructed with or without attenuation correction, using the same workstation, and the entire tumor region was evaluated to ensure the SUVmax. iPET was evaluated by Deauville criteria and the percentages of SUVmax decreased from baseline PET to iPET (ΔSUVmax = [baseline SUVmax-iPET SUVmax]/baseline SUVmax × 100%) [Citation18,Citation19], with a cutoff value of 70% [Citation20]. Patients who had a major lymphoma lesion resection were excluded when referring to SUVmax. 18F-FDG uptake was evaluated between potential lesions and areas of physiological uptake as previously described [Citation8]. The results of PET were interpreted by two experienced nuclear medicine physicians, and discrepancies were resolved by discussion. In the case of PET evaluation performed at other hospital, the images were reconstructed using our workstation and re-interpreted by two experienced nuclear medicine physicians. For suspicious lesions, biopsies or core needle biopsies would be performed to confirm the pathological diagnosis.
Statistical analysis
Overall survival (OS) was measured from the start of treatment to the date of death or last follow-up. Progression-free survival (PFS) was calculated from the start of treatment to the date of relapse/progression or last follow-up. Kaplan–Meier curves were generated for the time-to-event outcomes according to different evaluation methods. Categorical variables were grouped based on clinical findings, the results were compared using the χ2 test or Fisher’s exact test. Survival curves were depicted using the Kaplan–Meier method and compared using the log-rank test. Multivariate analyses were performed using a Cox proportional hazards model. Positive predictive value (PPV) was defined as the proportion of patients who had true positive findings at the end of treatment to all positive findings in iPET [Citation21], and the proportion of patients who had true negative findings to all negative findings was nominated as negative predictive value (NPV). P-value was measured using χ2 test or Fisher’s exact test to describe the difference in PPV and NPV comparing modified-Deauville with different methods. All tests were 2-sided, and the significance level was set at 0.05. Analyses were performed in Statistical Package for the Social Sciences (SPSS) 23.0 software (SPSS Inc., Chicago, Illinois).
Results
Clinical characteristics and survival of patients
The clinical characteristics were similar between the test cohort of 352 patients and the validation cohort of 241 patients (). Among all, only 17 patients with focal residual diseases after chemotherapy had radiotherapy. The median follow-up was 41.9 months (0.5–123.8 months). Relapse or progression occurred in 168 patients at a median of 9.4 months after diagnosis, and 109 patients died at a median of 15.3 months. Relapsed and refractory patients received second-line regimens, such as L-ICE ± R, DAEPOCH ± R, hyperCVAD ± R, DHAP ± R, GDP ± V and 7 of them received ASCT after salvage therapy. For the 593 patients in this study, the 3-year PFS and 3-year OS were 72.3% and 81.6%, respectively.
Table 1. Patients characteristics.
Deauville standard and ΔSUVmax of iPET in test cohort
In the test cohort, iPET was performed on 287 patients, and 243 patients had Deauville score 1–3, 25 had score 4, and 19 had score 5. Patients with Deauville score 1–3 had better end-of-treatment complete response rate (eCRR, 92.2%) and outcome (3-year PFS, 81.5%; 3-year OS, 90.9%) than those with score 4 (eCRR, 72.0%, p = .001; 3-year PFS, 55.7%, p = .005; 3-year OS, 71.1%, p = .002) and those with score 5 (eCRR, 26.3%, p ≤ .001; 3-year PFS 10.5%, p ≤ .001; 3-year OS 26.3%, p ≤ .001, ). Meanwhile, iPET was reassessed in the same cohort of patients, using ΔSUVmax method. The cutoff value for negativity was ΔSUVmax greater than 70%18. The patients with positive iPET (ΔSUVmax ≤ 70%) had dismal outcome, as compared to those negative iPET with ΔSUVmax > 70% (eCRR, 46.2% vs 90.8%, p ≤ .001; 3-year PFS, 26.9% vs 76.9%, p ≤ .001; 3-year OS, 45.0% vs 88.1%, p ≤ .001, ).
Figure 1. Survival curves of patients in the test cohort according to Deauville criteria and ΔSUVmax method of iPET. (A and B) Progression-free survival (A) and overall survival (B) of patients classified by Deauville criteria. (C and D) Progression-free survival (C) and overall survival (D) of patients classified by ΔSUVmax method. (E and F) Progression-free survival (E) and overall survival (F) of patients classified by the combination of Deauville criteria and ΔSUVmax method.
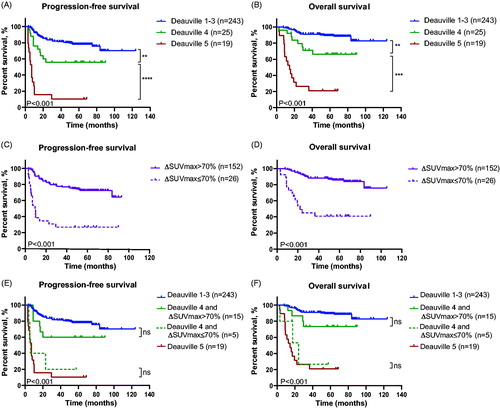
Among the 25 patients with Deauville score 4, five patients had surgery and the major tumor mass were resected before baseline PET, thus they were not included in ΔSUVmax assessment. Of note, patients with Deauville score 4 and ΔSUVmax > 70% (n = 15) had higher eCRR (86.7%) and better outcome (3-year PFS, 60.0%; 3-year OS, 73.3%) than those with ΔSUVmax ≤ 70% (n = 5) (eCRR 40.0%, p = .037; 3-year PFS, 20.0%, p = .044; 3-year OS, 26.7%, p = .046) (). These results indicated that patients with iPET Deauville score 4 were heterogeneous in responses to treatment and prognosis. Further comparison in patients with iPET Deauville score 1–3 and those with iPET Deauville score 4 but ΔSUVmax > 70% showed that 3-year PFS and 3-year OS had no statistical difference between these patients (PFS, 81.5% vs 60.0%, p = .082; OS, 90.9% vs 73.3%, p = .070). Similar results were observed in patients with iPET Deauville score 5 and those with iPET Deauville score 4 but ΔSUVmax ≤ 70% (PFS, 10.5% vs 20.0%, p = .576; OS, 26.3% vs 26.7%, p = .481).
Modified-Deauville model of iPET in test cohort
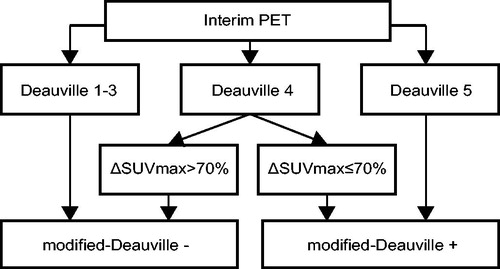
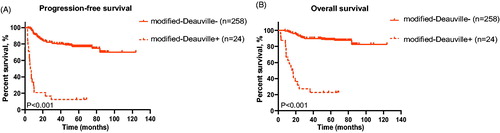
Thus, we proposed a modified-Deauville model: patients with iPET Deauville score 4 and ΔSUVmax > 70%, as well as those with Deauville score 1-3, were reclassified into the negative group of modified-Deauville model, while patients with iPET Deauville score 4 and ΔSUVmax ≤ 70%, as well as those with Deauville score 5, into the positive group of modified-Deauville model. The schematic graphs of modified-Deauville model of iPET were shown in . And 3-year PFS and 3-year OS of patients according to modified-Deauville classification were shown in (3-year PFS, negative vs positive; 80.2% vs 12.5%, p ≤ .001; 3-year OS, negative vs positive; 89.9% vs 27.3%, p ≤ .001. The multivariate Cox analysis revealed that patients in modified-Deauville negative group had better outcome than those in modified-Deauville positive group (PFS, HR, 0.103, 95%CI, 0.057–0.189, p ≤ .001; OS, HR, 0.095, 95%CI, 0.049–0.183, p ≤ .001; Table S1).
Figure 3. Survival curves of patients in the test cohort by modified-Deauville model. (A and B) Progression-free survival (A) and overall survival (B) of patients classified by the modified-Deauville model.
Among the patients with iPET modified-Deauville negative (Deauville score 1–3 and Deauville score 4 with ΔSUVmax > 70%), eCRR was 91.8%, (92.2% eCRR in iPET Deauville score 1–3 and 86.7% eCRR in Deauville score 4 with ΔSUVmax > 70%, p = .448). Among the patients of iPET modified-Deauville positive group (Deauville score 5 and Deauville score 4 with ΔSUVmax ≤ 70%), eCRR was 29.2%, (26.3% eCRR in iPET Deauville score 5 and 40.0% eCRR in Deauville score 4 with ΔSUVmax ≤ 70%, p = .384).
Chemoresistant patients identified by iPET modified-Deauville model
Interestingly, through modified-Deauville model of iPET, among 24 patients with positive results, 8 patients continued to receive R-CHOP regimen, 8 were switched to intensified regimens, such as L-ICE ± R, DAEPOCH ± R, hyperCVAD ± R, DHAP ± R, GDP ± V and 8 had radiotherapy after chemotherapy due to local residual disease. eCRR of patients with radiotherapy combined with chemotherapy was higher (62.5%) than those with chemotherapy alone (6.25%, p = .003). Among 16 patients without radiotherapy, regardless of chemotherapeutic regimens, only 1 patient achieved complete remission after 3 cycles of L-ICE + R, and the remaining 15 patients all experienced no response or disease progression after chemotherapy alone (.
Due to different mechanisms of action, radiotherapy could cure certain chemoresistant patients. Therefore, excluding patients who received radiotherapy, we further compared eCRR among iPET positive patients in term of these three methods. The results showed that patients with iPET positive using modified-Deauville model had a significantly lower eCRR (6.25%) than iPET positive using Deauville criteria (47.2%, p = .004) or ΔSUVmax method (45.0%, p = .010). Thus, the modified-Deauville model of iPET was more effective to determine patients resistant to chemotherapy than the other two methods.
Validation of the modified-Deauville model
In the validation cohort of 241 patients, the median follow-up was 23.9 months (0.5–45.6 months). Among them, 52 patients had a disease relapse or progression at a median of 7.5 months after diagnosis, and 30 patients died at a median of 11.9 months. 3-year PFS and 3-year OS of the patients were 76.5% and 85.2%, respectively.
Interim PET was performed on 226 patients. According to Deauville criteria, 183 patients had iPET Deauville score 1–3, 17 had score 4, and 26 had score 5. The eCRR and 3-year PFS, OS of patients with iPET Deauville score 1–3, score 4, and score 5 had remarkable difference (eCRR, 97.8%, 41.2% and 11.5%, p ≤ .001; PFS, 88.0%, 55.5%, and 22.6%, p ≤ .001; OS, 95.3%, 65.6%, and 26.5%, p ≤ .001) (). As for ΔSUVmax method, patients with iPET ΔSUVmax > 70% also had higher eCRR and longer survival time than those with iPET ΔSUVmax ≤ 70% (eCRR, 95.8% vs 33.3%, p ≤ .001; PFS, 86.6% vs 42.4%, p ≤ .001; OS, 94.7% vs 48.6%, p ≤ .001) ().
Figure 4. Responses of patients to R-CHOP regimen, intensified regimens and radiotherapy combined with chemotherapy in the test cohort according to the modified-Deauville model.
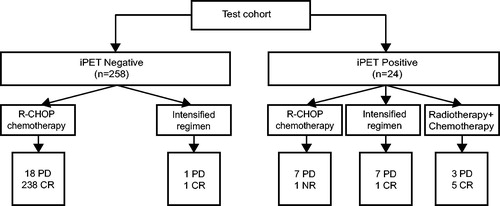
Figure 5. Survival curves of patients in validation cohort according to Deauville criteria and ΔSUVmax and modified-Deauville model of iPET. (A and B) Progression-free survival (A) and overall survival (B) of patients classified by Deauville criteria. (C and D) Progression-free survival (C) and overall survival (D) of patients classified by ΔSUVmax method. (E and F) Progression-free survival (E) and overall survival (F) of patients classified by the combination of Deauville criteria and ΔSUVmax method. (G and H) Progression-free survival (G) and overall survival (H) of patients classified by modified-Deauville model.
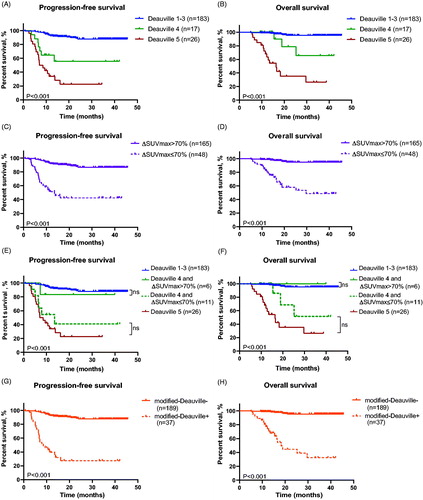
Similar as the test cohort, PFS and OS of patients with iPET Deauville score 1–3 showed no difference from those with Deauville score 4 and ΔSUVmax > 70%, and analogous results were found between patients with iPET Deauville score 4 but ΔSUVmax ≤ 70% and Deauville score 5 (). Therefore, 3-year PFS and 3-year OS rates of patients with negative iPET according to modified-Deauville model had better outcome than those with positive iPET (3-year PFS, 87.8% vs 27.4%, p ≤ .001; 3-year OS, 95.4% vs 32.5%, p ≤ .001) (), so as the eCRR (96.3% vs 13.5%, p ≤ .001). Multivariate analysis also supported that patients with negative modified-Deauville had better PFS (HR, 0.084; 95%CI, 0.042-0.167; p ≤ .001) and OS (HR, 0.034; 95%CI, 0.012-0.095; p ≤ .001) than positive modified-Deauville (Table S1).
Among patients with iPET Deauville 1-3, 97.8% achieved CR at the end of treatment. Thus, the eCRR (97.8%) had no significant difference with those of iPET Deauville 4 but ΔSUVmax > 70% (83.3%, p = .085), neither did patients with iPET Deauville 4 but ΔSUVmax ≤ 70% (eCRR, 18.2%) have remarkable difference with patients with iPET Deauville 5 (eCRR, 11.5%, p = .589).
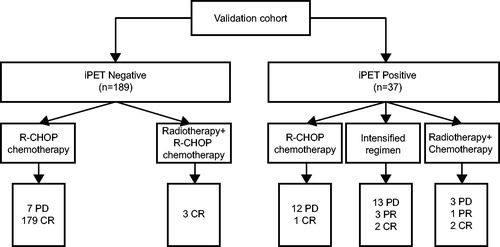
In the validation cohort, among 37 patients with iPET positive through modified-Deauville model, 13 patients received R-CHOP regimen and 18 patients had intensive regimens, while 6 patients had chemotherapy combined with radiotherapy for local residual disease. Among the 31 patients who had chemotherapy alone, only 3 achieved complete remission (9.7%), while 2 of 6 (33.3%) patients with chemotherapy combined with radiotherapy achieved complete remission (). Comparable with the test cohort, patients with radiotherapy had a trend of higher remission rate than those treated with chemotherapy alone.
Figure 6. Responses of patients to R-CHOP regimen, intensified regimens and radiotherapy combined with chemotherapy in the validation cohort according to the modified-Deauville model.
The PPV and NPV of complete remission at the end of treatment were assessed among 391 patients who were evaluable by all three methods: modified-Deauville, Deauville score and ΔSUVmax. The results were displayed in . Modified-Deauville model had significantly higher PPV (79.3%) than Deauville criteria (62.0%, p = .030) and ΔSUVmax methods (62.2%, p = .033). However, the NPV of modified-Deauville, Deauville score and ΔSUVmax methods were 93.7%, 94.2%, 93.4%, respectively (p = .904, ). All three methods had high NPV and no statistical significance was observed among them.
Table 2. Predictive value of three methods of iPET evaluation.
Discussion
DLBCL is a heterogeneous disease and R-CHOP regimen is recommended as the standard first-line treatment. Eventually, 12.7–15% of patients are primary refractory to R-CHOP and may have cross-resistance to other intensive chemotherapy [Citation16,Citation22–24]. How to recognize these patients at an early stage and assign them with new and more effective therapeutic strategies is critical. Due to the low positive predictive value of iPET, the function of iPET in DLBCL assessment remains controversial. To better review the literatures with iPET in DLBCL, we searched Pubmed using the terms ‘diffuse large B-cell lymphoma and interim PET’ for studies published in English from 2016 up to now, and 22 researches discussing iPET and outcome of DLBCL were found, summarized in Table S2. In these researches, most studies [Citation7, Citation15,Citation25–41] reported that iPET could predict prognosis of patients except three studies [Citation42–44], and most of them using different evaluation methods to calculate the survival time of patients, few of them made prediction of CR rate at the end of treatment [Citation34]. Here, we reported a new model combining the Deauville method with ΔSUVmax to demarcate patients into high eCRR (modified-Deauville negative) group and low eCRR (modified-Deauville positive) group, indicating the patients in positive modified-Deauville group of iPET were chemoresistance and for whom new therapeutic strategies are warranted.
Deauville criteria has been proven as a standard evaluation method of PET-CT [Citation8,Citation45]. According to the Lugano 2014 guideline, patients with a Deauville score of 1–3 are regarded as negative of disease, while those with a Deauville score of 4 and 5 are positive of disease. In our study, patients with iPET Deauville 1-3, 4 and 5 not only had different survival time, but also had different eCRR to R-CHOP regimen. Patients with iPET scores 1–3 had high eCRR, 92.2% in the test cohort and 97.8% in the validation cohort. These results confirmed the previous report that iPET has a high negative predictive value for patients [Citation7,Citation15,Citation25–41]. In our study, the patients with iPET Deauville score 4 could be further reclassified into ΔSUVmax > 70% and ΔSUVmax ≤ 70%, eCRR were 86.7% and 40% in test group, 83.3% and 18.2% in validation group, indicating patients with iPET Deauville score 4 were heterogeneous and presented different response to chemotherapy.
We then proposed a modified-Deauville model: patients with Deauville 4 and ΔSUVmax > 70%, as well as those with Deauville 1–3, were reclassified as the negative group of the modified-Deauville evaluation, while Deauville 4 and ΔSUVmax ≤ 70%, as well as those with Deauville 5, into the positive group of the modified-Deauville evaluation. In the test cohort, the eCRR and 3-year PFS, 3-year OS were 91.8%, 80.2%, 89.9% in iPET modified-Deauville negative patients and 29.2%, 12.5%, 27.3% in positive patients. Similar results were also found in our validation cohort, that higher eCRR (96.3%) and 3-year PFS (87.8%), 3-year OS (95.4%) in iPET modified-Deauville negative patients, compared to positive patients (eCRR, 13.5%; 3-year PFS, 27.4%; 3-year OS, 32.5%). Interestingly, in test cohort of patients received chemotherapy alone, comparing the three evaluation methods, Deauville criteria, ΔSUVmax and modified-Deauville, the eCRR of iPET modified-Deauville positive patients was dramatically lower (6.25%) than those of Deauville (47.2%, p = .004) and ΔSUVmax positive patients (45.0%, p = .010). Similar trend was also found in validation cohort. In consistent with these results, modified-Deauville model of iPET had higher PPV than Deauville and ΔSUVmax, suggesting that the modified-Deauville model of iPET evaluation had high efficiency to discriminate chemoresistant patients.
In terms of low eCRR of iPET positive patients with chemotherapy alone, regardless of R-CHOP regimen or intensified regimens, our results further confirmed the previous reports that intensive chemotherapy or even ASCT could not rescue patients with positive iPET [Citation13,Citation46,Citation47]. All these results indicated that patients with positive iPET were resistant to chemotherapy, alternative therapeutic strategies other than chemotherapy, such as radiotherapy or immunotherapy, might be employed to improve the prognosis of these patients. However, radiotherapy was only applicable for a limited group of patients with local lesions. Next-generation sequencing is another potential approach for choosing targeted therapeutic agents.
In conclusion, we proposed a modified-Deauville model by combining Deauville score and ΔSUVmax method for better prognostic ability and early recognition of chemoresistance patients, offering clinicians a convenient method for risk-adapt individualized therapeutic strategies in DLBCL.
Supplemental Material
Download MS Word (21.4 KB)Supplemental Material
Download MS Word (19.1 KB)Acknowledgments
We thank Dr. Peter Liao for English editing.
Disclosure statement
The authors declare no conflicts of interest.
Additional information
Funding
References
- Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood. 2010;116(12):2040–2045.
- Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28(27):4184–4190.
- Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800–1808.
- Schuster SJ, Svoboda J, Chong EA, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377(26):2545–2554.
- Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(1):31–42.
- Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544.
- Liu T, Chen L, Pan J, et al. Retrospective analysis of a new prognostic score for diffuse large B-cell lymphoma based on interim positron emission tomography-computed tomography. Acta Haematol. 2018;139(3):148–157.
- Cheson BD, Fisher RI, Barrington SF, United Kingdom National Cancer Research Institute, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068.
- Mamot C, Klingbiel D, Hitz F, et al. Final results of a prospective evaluation of the predictive value of interim positron emission tomography in patients with diffuse large B-cell lymphoma treated with R-CHOP-14 (SAKK 38/07). J Clin Oncol. 2015;33(23):2523–2529.
- Chow A, Phillips M, Siew T, et al. Prognostic nomogram for diffuse large B-cell lymphoma incorporating the International Prognostic Index with interim-positron emission tomography findings. Intern Med J. 2013;43(8):932–939.
- Gonzalez-Barca E, Canales M, Cortes M, et al. Predictive value of interim (1)(8)F-FDG-PET/CT for event-free survival in patients with diffuse large B-cell lymphoma homogenously treated in a phase II trial with six cycles of R-CHOP-14 plus pegfilgrastim as first-line treatment. Nucl Med Commun. 2013;34(10):946–952.
- Pregno P, Chiappella A, Bello M, et al. Interim 18-FDG-PET/CT failed to predict the outcome in diffuse large B-cell lymphoma patients treated at the diagnosis with rituximab-CHOP. Blood. 2012;119(9):2066–2073.
- Yoo C, Lee DH, Kim JE, et al. Limited role of interim PET/CT in patients with diffuse large B-cell lymphoma treated with R-CHOP. Ann Hematol. 2011;90(7):797–802.
- Barrington SF, Qian W, Somer EJ, et al. Concordance between four European centres of PET reporting criteria designed for use in multicentre trials in Hodgkin lymphoma. Eur J Nucl Med Mol Imaging. 2010;37(10):1824–1833.
- Kim J, Song YS, Lee JS, et al. Risk stratification of diffuse large B-cell lymphoma with interim PET-CT based on different cutoff Deauville scores. Leuk Lymphoma. 2018;59(2):340–347.
- Kasamon YL, Wahl RL, Ziessman HA, et al. Phase II study of risk-adapted therapy of newly diagnosed, aggressive non-Hodgkin lymphoma based on midtreatment FDG-PET scanning. Biol Blood Marrow Transplant. 2009;15(2):242–248.
- Gomez Leon N, Delgado-Bolton RC, Del Campo Del Val L, et al. Multicenter Comparison of Contrast-Enhanced FDG PET/CT and 64-Slice Multi-Detector-Row CT for Initial Staging and Response Evaluation at the End of Treatment in Patients With Lymphoma. Clin Nucl Med. 2017;42(8):595–602.
- Lin C, Itti E, Haioun C, et al. Early 18F-FDG PET for prediction of prognosis in patients with diffuse large B-cell lymphoma: SUV-based assessment versus visual analysis. J Nucl Med. 2007;48(10):1626–1632.
- Schoder H, Polley MC, Knopp MV, et al. Prognostic value of interim FDG-PET in diffuse large cell lymphoma: results from the CALGB 50303 Clinical Trial. Blood. 2020;135(25):2224–2234.
- Casasnovas RO, Ysebaert L, Thieblemont C, et al. FDG-PET-driven consolidation strategy in diffuse large B-cell lymphoma: final results of a randomized phase 2 study. Blood. 2017;130(11):1315–1326.
- Chantadisai M, Buschner G, Kronke M, et al. Positive predictive value and correct detection rate of (18)F-rhPSMA-7 PET in biochemically recurrent prostate cancer validated by composite reference standard. J Nucl Med. 2020. DOI:10.2967/jnumed.120.255661
- Arboe B, Olsen MH, Gorlov JS, et al. Treatment intensity and survival in patients with relapsed or refractory diffuse large B-cell lymphoma in Denmark: a real-life population-based study. Clin Epidemiol. 2019;11:207–216.
- Hitz F, Connors JM, Gascoyne RD, et al. Outcome of patients with primary refractory diffuse large B cell lymphoma after R-CHOP treatment. Ann Hematol. 2015;94(11):1839–1843.
- Sehn LH, Hardy ELG, Gill KK, et al. Phase 2 trial of interim PET scan-tailored therapy in patients with advanced stage diffuse large B-cell lymphoma (DLBCL) in British Columbia (BC). Conference Abstract]. Blood. 2014;124(21):392–392.
- Gyorke T, Carr R, Cerci JJ, et al. Combined visual and semi-quantitative evaluation improves outcome prediction by early mid-treatment (18)F-fluoro-deoxi-glucose positron emission tomography in diffuse large B-cell lymphoma. J Nucl Med. 2020;61(7):999–1005.
- Yim SK, Yhim HY, Han YH, et al. Early risk stratification for diffuse large B-cell lymphoma integrating interim Deauville score and International Prognostic Index. Ann Hematol. 2019;98(12):2739–2748.
- Schmitz C, Huttmann A, Muller SP, et al. Dynamic risk assessment based on positron emission tomography scanning in diffuse large B-cell lymphoma: post-hoc analysis from the PETAL trial. Eur J Cancer. 2020;124:25–36.
- Kitajima K, Okada M, Yoshihara K, et al. Predictive value of interim FDG-PET/CT findings in patients with diffuse large B-cell lymphoma treated with R-CHOP. Oncotarget. 2019;10(52):5403–5411.
- Nyilas R, Farkas B, Bicsko RR, et al. Interim PET/CT in diffuse large B-cell lymphoma may facilitate identification of good-prognosis patients among IPI-stratified patients. Int J Hematol. 2019;110(3):331–339.
- Li X, Sun X, Li J, et al. Interim PET/CT based on visual and semiquantitative analysis predicts survival in patients with diffuse large B-cell lymphoma. Cancer Med. 2019;8(11):5012–5022.
- Islam P, Goldstein J, Flowers CR. PET-derived tumor metrics predict DLBCL response and progression-free survival. Leuk Lymphoma. 2019;60(8):1965–1971.
- Toledano MN, Vera P, Tilly H, et al. Comparison of therapeutic evaluation criteria in FDG-PET/CT in patients with diffuse large-cell B-cell lymphoma: Prognostic impact of tumor/liver ratio. PLoS One. 2019;14(2):e0211649.
- Zhang YY, Song L, Zhao MX, et al. A better prediction of progression-free survival in diffuse large B-cell lymphoma by a prognostic model consisting of baseline TLG and %DeltaSUVmax. Cancer Med. 2019;8(11):5137–5147.
- Onate-Ocana LF, Cortes V, Castillo-Llanos R, et al. Metabolic tumor volume changes assessed by interval 18fluorodeoxyglucose positron emission tomography-computed tomography for the prediction of complete response and survival in patients with diffuse large B-cell lymphoma. Oncol Lett. 2018;16(2):1411–1418.
- Wang R, Xu B, Liu C, et al. Prognostic value of interim fluorodeoxyglucose and fluorothymidine PET/CT in diffuse large B-cell lymphoma. Br J Radiol. 2018;91(1091):20180240.
- Baratto L, Davidzon GA, Moghbel M, et al. Comparison between different PET and CT-based imaging interpretation criteria at interim imaging in patients with diffuse large B-cell lymphoma. Clin Nucl Med. 2018;43(1):1–8.
- Zhang Y, Fan Y, Ying Z, et al. Can the SUVmax-liver-based interpretation improve prognostic accuracy of interim and posttreatment (18)F-FDG PET/CT in patients with diffuse large B-cell lymphoma? Leuk Lymphoma. 2018;59(3):660–669.
- Del Puig Cozar-Santiago M, Garcia-Garzon JR, Moragas-Freixa M, et al. Optimisation of metabolic criteria in the prognostic assessment in patients with lymphoma. A multicentre study. Rev Esp Med Nucl Imagen Mol. 2017;36(5):304–311.
- Wu X, Bhattarai A, Korkola P, et al. The association between liver and tumor [(18)F]FDG uptake in patients with diffuse large B cell lymphoma during chemotherapy. Mol Imaging Biol. 2017;19(5):787–794.
- Han EJ, O JH, Yoon H, et al. FDG PET/CT response in diffuse large B-cell lymphoma: reader variability and association with clinical outcome. Medicine (Baltimore)). 2016;95(39):e4983.
- de Oliveira Costa R, Hallack Neto A, Siqueira S, et al. Interim fluorine-18 fluorodeoxyglucose PET-computed tomography and cell of origin by immunohistochemistry predicts progression-free and overall survival in diffuse large B-cell lymphoma patients in the rituximab era. Nucl Med Commun. 2016;37(10):1095–1101.
- Gupta N, Singh N. To evaluate prognostic significance of metabolic-derived tumour volume at staging 18-flurodeoxyglucose PET-CT scan and to compare it with standardized uptake value-based response evaluation on interim 18-flurodeoxyglucose PET-CT scan in patients of non-Hodgkin's lymphoma (diffuse large B-cell lymphoma). Nucl Med Commun. 2020;41(4):395–404.
- Cherng HJ, Sargent RL, Nasta SD, et al. Interim PET/CT result is not predictive of survival in patients with MYC-rearranged non-Burkitt aggressive B-cell lymphoma. Clin Lymphoma Myeloma Leuk. 2018;18(10):673–678.
- Hertzberg M, Gandhi MK, Trotman J, Australasian Leukaemia Lymphoma Group (ALLG), et al. Early treatment intensification with R-ICE and 90Y-ibritumomab tiuxetan (Zevalin)-BEAM stem cell transplantation in patients with high-risk diffuse large B-cell lymphoma patients and positive interim PET after 4 cycles of R-CHOP-14. Haematologica. 2017;102(2):356–363.
- Zelenetz AD, Gordon LI, Abramson JS, et al. NCCN guidelines insights: B-cell lymphomas, version 3.2019. J Natl Compr Canc Netw. 2019;17(6):650–661.
- Swinnen LJ, Li H, Quon A, et al. Response-adapted therapy for aggressive non-Hodgkin's lymphomas based on early [18F] FDG-PET scanning: ECOG-ACRIN Cancer Research Group study (E3404). Br J Haematol. 2015;170(1):56–65.
- Moskowitz CH, Schoder H, Teruya-Feldstein J, et al. Risk-adapted dose-dense immunochemotherapy determined by interim FDG-PET in Advanced-stage diffuse large B-Cell lymphoma. J Clin Oncol. 2010;28(11):1896–1903.