Abstract
Background and aim: Few studies have focused on the symptoms of loco-regional morbidity in shoulders, arms, and breasts related to oncoplastic breast surgery (OPS). This study aimed to determine if a difference exists in the prevalence or variety of subjective symptoms of shoulder, arm, and breast morbidity in patients undergoing OPS compared with patients receiving conventional breast conserving surgery (C-BCS). Cosmetic result and body image were included as secondary endpoints.
Methods: This prospective follow-up study with 18 months of questionnaire-based follow-up included women with breast cancer or ductal carcinoma in situ. They were divided into two groups – C-BCS or OPS – depending on type of surgery performed. Furthermore, patient, disease, and treatment characteristics were recorded.
Results: Among 334 completers, 229 (69%) received C-BCS and 105 (31%) received OPS. Participants were comparable regarding age, comorbidity, BMI, re-excision rate (15–16%), and axillary surgery. As for tumor characteristics, a more advanced disease stage was shown in the OPS than in the C-BCS group with larger tumor and lumpectomy size, more multifocality, and the corresponding following systemic adjuvant therapy.
The questionnaire revealed that the two groups were comparable with no significant differences in frequency or variety of symptoms of shoulder and arm morbidity. Overall, participants were highly satisfied with the cosmetic results in both groups and no significant inter-group differences were observed.
Conclusion: In patients with larger tumors, breast conserving surgery utilizing oncoplastic techniques yields results regarding subjective shoulder, arm, and breast morbidity as well as cosmetic outcome comparable with those of C-BCS performed on smaller tumors.
Trial registration: ClinicalTrials.gov, registration number: NCT02159274 (2014).
Subjective symptoms of shoulder, arm, and breast morbidity are comparable when oncoplastic breast surgery is compared to conventional breast conserving surgery.
The variety of symptoms of shoulder and arm morbidity following oncoplastic surgery does not differ from symptoms following conventional breast conserving surgery.
The cosmetic outcome following oncoplastic breast surgery is comparable to breast conserving surgery without oncoplastic techniques.
HIGHLIGHTS
Introduction
The introduction of oncoplastic surgery allows more breast cancer (BC) patients to be treated by breast conserving surgery (BCS) [Citation1]. In Denmark, the national guidelines of the Danish Breast Cancer Cooperative Group (DBCG) classify oncoplastic breast surgery (OPS) as BCS combined with techniques of plastic surgery to recreate the shape of the breast. The DBCG divides OPS into three groups: Volume displacement OPS – Re-arrangement of nearby glandular breast tissue to fill in the lumpectomy cavity and reshape the breast, often combined with a repositioning of the nipple-areola complex (NAC); Volume reduction OPS – A technique countering loss of breast tissue caused by lumpectomy through a breast reduction procedure; Volume replacement OPS – Application of loco-regional perforator or latissimus dorsi flaps to fill in the lumpectomy cavity (www.dbcg.dk).
OPS allows satisfying oncological and cosmetic results to be achieved even in large and multifocal tumors. Most prior studies have focused on oncological safety in OPS, and report re-resection rate, local recurrence rate, disease-free survival, and overall survival by comparing with conventional breast conserving surgery (C-BCS) [Citation2–7].
A meta-analysis investigating whether the risk of early postoperative complications is higher in OPS than in C-BCS was unable to confirm any difference between the two approaches even where OPS involved extensive remodeling [Citation2]. Other studies found OPS complication rates in the 9–38% range [Citation4,Citation8–11]. A Danish and a Turkish study examined the risk of delaying the onset of adjuvant chemotherapy in relation to OPS, but found that the period was comparable to those of C-BCS and mastectomy [Citation12,Citation13]. Furthermore, a Dutch study found that OPS delayed adjuvant radiation therapy (RT) in 8.2% of patients [Citation11].
In appropriately selected patients, C-BCS can be performed with a satisfying cosmetic result. Selection for C-BCS and the various OPS techniques depends on tumor size, tumor localization, breast size, and breast density [Citation14–17]. A review assessed the cosmetic result following OPS to be excellent in the majority of participants and studies [Citation18].
Comparatively more extensive OPS might invite the assumption that risk of shoulder, arm, and breast morbidity is increased following OPS. A retrospective study included patient-reported outcome (PRO) by the BREAST-Q among large-breasted BC patients treated with bilateral reduction mammoplasty compared with large-breasted women with unilateral C-BCS, finding no significant differences in physical well-being between the two groups [Citation19]. A possible benefit of less extensive radiation therapy (RT) to a reduced breast might have favorable outcomes [Citation20,Citation21]. Thus, a recently published PRO study indicated superior health-related quality of life outcomes following OPS compared with C-BCS [Citation22]. We are unaware of any studies focusing on the risk of specific shoulder and arm late effects caused by OPS. The present prospective follow-up study therefore aimed to establish any difference in the prevalence and variety of symptoms in patients undergoing OPS compared with patients receiving C-BCS. The cosmetic result and body image were included as secondary endpoints.
Methods and materials
Study design
In this prospective non-inferiority study with 18-month follow-up, the prevalence of shoulder, arm, and breast morbidity was recorded using a questionnaire and compared for BCS with or without oncoplastic techniques. Patients completed the questionnaire preoperatively and again 18 months after surgery. The study was planned and conducted according to the EQUATOR guidelines (Supplementary 1) [Citation23].
Patient cohort
From May 2014 to April 2017, women eligible for BCS at the three breast surgical units in the Central Denmark Region (Randers, Viborg, and Aarhus) were asked to participate. Inclusion criteria were a diagnosis of early BC or ductal carcinoma in situ (DCIS), age 18–75 years, and no prior surgery in the breasts, shoulders, or arms. Exclusion criteria were secondary mastectomy, disease relapse, secondary cosmetic breast surgery, or a re-operation changing the primary grouping (see later). Oral and written consent was obtained.
Based on type of surgery received, patients were divided into two groups; C-BCS – Conventional breast conserving surgery and OPS – Oncoplastic breast surgery.
The decision as to type of surgery was made by the patient, guided by the breast surgeon based on the breast size, breast shape, and tumor size and location. OPS was performed with the aim of obtaining an enhanced cosmetic outcome, reducing breast volume, or avoiding mastectomy in small breasts. No randomization was performed.
Endpoints
The primary endpoint was shoulder and arm morbidity, based on functional impairments as reduced hand and arm function, and individual symptoms regarding shoulder mobility, pain, sensibility, and arm swelling. Secondary endpoints were cosmetic result, and body image.
Subjective evaluation
The questionnaire, adapted from a prior study on morbidity in relation to sentinel lymph-node biopsy and axillary lymph-node dissection (ALND) [Citation24], comprised questions about subjective symptoms of shoulder, arm, and breast morbidity. Participants were asked to elaborate on the frequency of symptoms, and to assess its severity by a ‘bother score’ (BS) (1 = no problem, 2 = minor problem, 3 = moderate problem, 4 = major problem). Questions about pain were supplemented by a visual analog scale (VAS), grading 1–10. Most questions were dichotomous (Yes/No); others were dichotomized during data analysis.
We added questions regarding functional impairment, inspired by Gärtner et al. [Citation25], and questions about the cosmetic result, body image, and clothing habits.
To enlighten the cosmetic result, participants were asked questions about scar appearance, breast shape, and breast symmetry. Each question was elaborated via a ‘satisfaction score’ (SS) grading the patient’s satisfaction with the cosmetic result (0 = excellent, 1 = good, 2 = fair, 3 = poor). Questions about body image were also asked and some were elaborated by the SS.
Ethics, data collection, analysis, and statistics
The study was approved by The Local Research Ethics Committee of the Central Denmark Region and the Danish Data Protection Agency. Oral and written information was given to the participants before obtaining their written consent. Study data were collected and managed using REDCap (Research Electronic Data Capture tools) hosted at Aarhus University, Denmark [Citation26]. Data were analyzed using Stata/IC 13 (StataCorp LLC). Most results were presented as prevalence and prevalence proportion with 95% confidence intervals (CIs). Dichotomous data were compared between groups by Pearson’s chi-squared test and Fisher’s exact test. The significance level was set to p < .05 (two-sided tests). The 95% CIs were also used to describe any non-inferiority of OPS.
The primary objective was to establish the equivalence or at least non-inferiority of OPS compared with C-BCS with respect to shoulder, arm, and breast morbidity. The symptoms of functional impairment were reported with the hypothesis of equivalence; while the other symptoms of shoulder, arm, and breast morbidity were elaborated by superiority analysis. Based on the results from Husted Madsen et al., 25% of the patients were expected to have a reduced performance status of the shoulder and arm (in this study referred to as reduced ‘hand and arm function’) following their BC surgery [Citation24]. We here intended to include as many patients as needed to detect a more than 15% deviation from this, i.e., less than 10% or more than 40% with a reduced performance status. The number of patients needed in each group under these conditions was 165 (SigmaPlot version 11.0; Comparing Proportions Using the z-Test). Study recruitment was not as successful as expected although we extended the inclusion period. We therefore conducted an underpowered study, as only 63% (104/165) of the intended cohort in the OPS group completed the study. In the C-BCS group, recruitment was sufficient.
Results
Study population, tumor, and treatment characteristics
Of 403 women meeting the inclusion criteria and accepting study inclusion (), 135 (33%) were treated with OPS and 268 (67%) with C-BCS. In all, 69 (17%) participants were excluded. Exclusion was caused mainly by secondary mastectomy (n = 32) due to insufficient radicality. Others were excluded because they received cosmetic breast surgery during follow-up (mean 18.1 months) or experienced disease relapse, and some withdrew their consent for participation. Finally, 229 (85%) participants completed follow-up in the C-BCS group and 105 (78%) in the OPS group. The final distribution of participants by surgery group was 69% C-BCS and 31% OPS.
Figure 1. Flow diagram of the study population. The upper half of the figure is based on data obtained from the DBCG and illustrates patients diagnosed with early breast cancer or DCIS during the inclusion period in the Central Denmark Region. The lower part of the figure illustrates the participants who met the inclusion criteria and accepted study participation.a Among the 1,807 patients treated with BCS, 199 had DCIS. No details of OPS/C-BCS were available for these 199 patients. N: number; BC: breast cancer; DCIS: ductal carcinoma in situ; BCS: breast conserving surgery; C-BCS: conventional breast conserving surgery; NACT: neoadjuvant chemotherapy; OPS: oncoplastic breast surgery; DBCG: Danish Breast Cancer Cooperative Group.
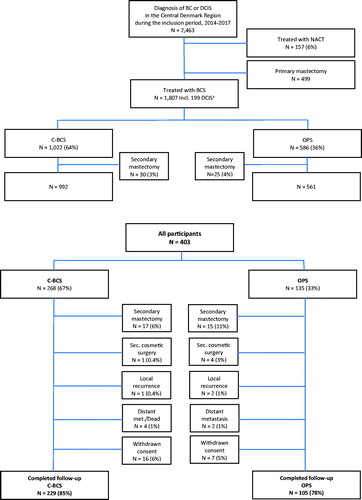
BCS procedures (Supplementary 2) performed in the OPS group were distributed as follows: 51% NAC repositioning, 26% roundblock mastopexia, 19% therapeutic reduction, and 4% intercostal artery perforator (ICAP) replacement flap. Experiencing only 4 participants (4%) were operated with the ICAP-procedure, it was decided to remove these participants from the further management of results. The results including these 4 participants are available as Supplementary material (Supplementary 4, 5, 6, 7). At the time of surgery, participants had a median age of 61 (range 31–75) years, and the Charlson Comorbidity Index (CCI) was low (CCI = 0–1) for 95% (). Pathological findings of lumpectomy size, tumor size, multifocality, node positivity (32% versus 20%, p = .017), and tumor characteristics (estrogen and HER2-receptor status) differed significantly between groups with somewhat more advanced disease being observed in the OPS group. Subsequent systemic adjuvant therapy with chemotherapy and HER2-targeted treatment was therefore also considerably more frequent in the OPS group. No significant difference in re-excision rate (15–16%), extent of the axillary surgery, or RT was detected between the groups.
Table 1. Patient, disease, and treatment characteristics at the time of surgery.
Symptoms of shoulder, arm, and breast morbidity
The results obtained from the questionnaire regarding functional impairment and symptoms of shoulder, arm and breast morbidity are shown in and . At baseline, no significant difference in occurrence of symptoms was observed between the two treatment groups (Supplementary 3).
Table 2. Symptoms of functional impairment 18 months after BCS.
Table 3. Symptoms of shoulder, arm, and breast morbidity 18 months after BCS.
Symptoms of functional impairment were evaluated post-operatively (). No significant differences in symptom prevalence were noted between the two groups; still, most of the impairments were more frequent in the C-BCS group. The prevalence proportion of participants with reduced hand and arm function was 17% and 21% in the C-BCS and the OPS-group, respectively – leading to a risk difference of −4%. Comparing the risk of functional impairment when C-BCS and OPS is performed, none of the risk differences nor the 95% CIs exceeded ±15%; which was declared as the maximum acceptable difference to suggest equivalence. The most prominent impairments were carrying heavy bags (28 vs. 27%), reaching above the head (23 vs. 22%), and changed habits of household chores (21 vs. 20%).
At the 18-month postoperative follow-up, the frequency of symptoms was equal in both groups (). The only symptom recording a significant difference (p = .046) was pain in the shoulder, which was more frequent in the C-BCS group post-operatively (34% versus 23% in the OPS group). Pain in the shoulder was more frequent among those who had ALND. When judged by the BS, pain in the shoulder also seemed to be a larger problem (BS = 3–4) in the C-BCS than in the OPS group; although pain intensity was the same (VAS = 3).
All the remaining symptoms including paresthesia or swelling of the arm were reported with only minor and non-significant differences in occurrences between the two groups, pre-operatively as well as post-operatively. Pain in the breast was the most frequent postoperative symptom; 62% in the C-BCS group and 66% in the OPS group. For the majority of participants, pain was not a problem or only a minor problem (BS = 1–2).
An increase in the frequency of all symptoms was observed when comparing answers from before the operation to answers provided 18 months postoperatively. One of the most obvious changes was seen for decreased shoulder mobility with a pre-operative prevalence of 3% in the OPS group and 6% in the C-BCS-group (Supplementary 3). Post-operatively, these values increased to 25% and 34%, respectively ().
Cosmetic result and body image
Overall, the cosmetic results were highly satisfying (SS = 0–1) for 91–98% in both groups (). The only significant difference (p = .031) between the C-BCS and the OPS group was found for scar appearance. A relatively high percentage of women in both groups judged their look undressed as well as dressed as ‘Good’ (SS = 1), and some participants remarked that their judgment of their undressed appearance was unrelated to their BC surgery or treatment. The percentage of women who had unchanged dressing habits and unchanged social life was high in both groups (88–100%). In total, we found only few (0–7%) women who judged one or more of the cosmetic aspects as ‘Fair’ or ‘Poor’.
Table 4. Cosmetic result and body image 18 months after BCS.
Discussion
Women treated for BC are at risk of developing shoulder and arm morbidity. Our study indicates that no significant differences exist in PRO-data for C-BCS versus OPS although OPS patients have a larger tumor size and more advanced disease. In both groups, results were comparable regarding symptoms of shoulder, arm, and breast morbidity; and equivalence was established regarding the risk of functional impairments. An overall satisfaction with the cosmetic result was high.
Tumor characteristics and subsequent adjuvant systemic therapy differed between groups. Tumor size and lumpectomy size were significantly larger in the OPS group and the OPS group counted more node-positive patients. A larger proportion of OPS than C-BCS patients received chemotherapy and HER2-targeted treatment. RT did not differ between groups. Unfortunately, breast volume data were not available to the authors. Calculation of the tumor-to-breast volume ratio would have assisted comparison with other studies and could be used to guide future selection of surgery candidates for the various OPS techniques. In a large Danish cohort study (N = 58,331) comparing BCS to mastectomy in relation to survival, patients undergoing BCS had smaller tumors (15 mm), whereas patients undergoing mastectomy had larger tumors (median 23 mm) [Citation27]. The median tumor sizes in the C-BCS group was 13 mm; in the OPS group, 18 mm. Thus, we suppose that a large proportion of the patients in the OPS group would have been treated by mastectomy if oncoplastic surgery had not been an option [Citation14,Citation28]. Despite the larger tumor and lumpectomy size, we managed to perform BCS and achieve results comparable to those achieved for C-BCS regarding morbidity and cosmesis.
The 33% OPS prevalence reported here mimics the distribution of all BCS in the Central Denmark Region in the period () and approaches the distribution in Denmark in the period of 2012–2018, where 27.5% were OPS [Citation29]. The presented prevalence is high compared with a multicenter study from the US, where use of OPS increased from 4% to 9% in the 2005–2016 period [Citation30]. In the present study, the majority of the OPS procedures were volume displacement OPS, e.g., ‘NAC repositioning’ (51%), ‘roundblock mastopexia’ (26%), and volume reduction OPS (19%). Only 4% had an ICAP procedure; thus, we can attribute no significance to the results on volume replacement OPS and these four participants were removed from the results. Removing these four participants did not affect the main results, nor conclusion. Others have suggested a possible bias caused by the potential selection of candidates to OPS favoring younger and healthier patients [Citation12]. This seemed not to be the case in our study where only minimal and non-significant differences were seen for age, BMI, and CCI. The equally comparable preoperative prevalence of symptoms also supports this assumption. Since the present study was conducted, surgeons overall have gained more experience in choosing the right patient for the right procedure [Citation14,Citation15].
Our re-excision rate was equal in both groups (15–16%) and considering the women who were excluded from the study due to a secondary mastectomy, re-excision rate was even higher. Our re-operation rate was comparable to previous results from Denmark. A newly published study from Denmark, found a modest decrease in re-excision rate when OPS was compared to C-BCS (14.4% vs. 15.6%, respectively) [Citation29]. In the 2000–2009 period, 17% of all Danish patients in the DBCG cohort of patients initially having BCS underwent re-excisions or converted to mastectomy [Citation31]. The definition of adequate resection margins was lowered in Denmark after the 11th St. Gallen International Expert Consensus Meeting in 2009 [Citation32]. In the present study, women with DCIS were also included. Comparable results were also reported in the small Danish study by Langhans et al., who found an overall re-excision rate of 18%; more frequent in DCIS (37%) than in BC (13%). The review by De La Cruz et al. only considered T1 and T2 BC patients and included studies with diverging definitions of insufficient margin (ranging from 10 mm to ‘tumor on ink’) in OPS. They reported a much lower re-excision rate of 6% [Citation18]. In the present study, the re-excision or mastectomy rate was almost equal in the two groups. A lower rate was expected in the OPS group as these techniques allow for larger resections, but this expectation was not confirmed [Citation7,Citation33]. We therefore believe that OPS was typically done to facilitate BCS in patients who would otherwise be recommended a mastectomy and not in order to achieve larger margins as such.
The questionnaire reveled comparable results in the two groups. To some extent it seemed that the prevalence of symptoms of shoulder and arm morbidity were marginally higher in the C-BCS group. Our hypothesis was that the rather widespread OPS procedure would cause more trauma in the breast and the surroundings, leading to a higher risk of shoulder, arm, and breast morbidity. This seems not to be the case. Others have described that lumpectomy combined with bilateral breast reduction may be associated with enhanced physical wellbeing in large-breasted woman [Citation19]. Few studies on quality of life in relation to OPS are available [Citation34–36].
Some of the predictors of a poor cosmetic outcome after BCS have been found to be tumor multifocality, large tumor size, small breast size, inferior, medial, and central tumor localization [Citation16,Citation35,Citation37,Citation38]. One of these studies comparing C-BCS with OPS reported no significant difference in cosmetic outcome regarding these factors. In the Finnish study by Ojala, patients who had C-BCS reported a more satisfying cosmetic result than patients who had OPS [Citation16]. A Danish long-term follow-up study by Lyngholm et al. used some of the questions also used in the present study and revealed a good or excellent cosmetic result in 88% of participants following BCS. Notably, in their study, poor agreement was reported between patient and clinician assessments [Citation39]. Patients were more satisfied with the cosmetic outcome than the clinicians were.
Most oncoplastic procedures require a longer and more visible scar, but in the present survey the appearance of the scar influenced the subjective judgment to a minor degree only. In our study, the participants in both groups were generally satisfied with their scar appearance (SS = 0).
For comparison with other studies, we could have employed the BREAST-Q or a similar disease-specific validated questionnaire [Citation40]. Still, several questions used in the present study are comparable to those of validated questionnaires and, to the best of our knowledge, no validated questionnaires have the BC-relevant focus on shoulder and arm morbidity adopted in our questionnaire. A newly developed and validated screening tool regarding surgery-specific neuropathic pain has been described after completion of the present study [Citation41].
A comparison with mastectomy patients would have been relevant too, as many patients in the OPS group were candidates to BCS only because OPS was an option. Mastectomy has been reported to be associated with a higher risk of shoulder and arm morbidity than BCS [Citation42]. At present, OPS and mastectomy have not been compared extensively. Kelsall et al. compared OPS to mastectomy combined with immediate reconstruction and identified the benefit of better psychosocial and self-rated satisfaction with breast appearance following OPS [Citation43]. Morbidity related to axillary surgery was expected to be equally distributed across the OPS and the C-BCS group () [Citation24,Citation44].
Our study would have benefited from a longer follow-up period, and we cannot exclude that the rate of late morbidity may increase over time [Citation45–47]. However, given our findings, we see no reason to believe that a widening of the differences between the groups would have occurred over time. Most morbidity appearing late after treatment is caused by adjuvant RT [Citation38], which should affect the two groups equally.
Some of the above-mentioned limitations could have been minimized by performing a randomized clinical trial (RCT), but that was not considered a viable solution. Therefore, confounding by indication and selection bias should be kept in mind when interpreting the results.
If the study cohort had been larger, it would also have been interesting to compare the different OPS performed. In Denmark, we have accelerated Breast Cancer Patient Pathways, and the intended time from diagnosis to treatment is less than 13 days. Some otherwise eligible participants declined participation because of the rather short timespan from diagnosis to operation because they were too distressed to even consider participation after the diagnosis had been made. Patients asked for participation were also encouraged to participate in a sub-study with objective evaluation of shoulder, arm, and breast morbidity. Undoubtedly, some declined participation because of the total amount of information on the day when their diagnosis was given and due to the expected time consumption for study participation. Unfortunately, despite prolonging the planned inclusion period from 18 to 36 months, we failed to recruit more participants.
Conclusion
In patients with larger tumors, BCS utilizing oncoplastic techniques yields results regarding shoulder, arm, and breast morbidity as well as cosmetic outcome that are comparable to those of C-BCS performed on smaller tumors, when evaluated subjectively by the patients.
Ethical approval
This study was approved by The Regional Research Ethics Committee for the Central Denmark Region: 1-16-02-669-14 (2014) and 1-10-72-335-13 (2013).
| Abbreviations | ||
| ALND | = | Axillary lymph node dissection |
| BC | = | Breast cancer |
| BCS | = | Breast conserving surgery |
| BMI | = | Body Mass Index |
| BS | = | Bother score |
| C-BCS | = | Conventional breast conserving surgery |
| CCI | = | Charlson Comorbidity Index |
| CI | = | Confidence interval |
| DBCG | = | Danish Breast Cancer Cooperative Group |
| DCIS | = | Ductal carcinoma in situ |
| ICAP | = | Intercostal artery perforator |
| NAC | = | Nipple-areola complex |
| NACT | = | Neoadjuvant chemotherapy |
| OPS | = | Oncoplastic breast conserving surgery |
| PRO | = | Patient-reported outcome |
| RT | = | Radiation therapy |
| SS | = | Satisfaction score |
| VAS | = | Visual analog score |
ionc_a_1900907_sm1042.zip
Download Zip (136 KB)Acknowledgements
The authors take this opportunity to express their gratitude to Professor Emeritus, PhD, Michael Vaeth – Aarhus University for his statistical assistance. Appreciation goes to the staff at the breast clinics in Randers, Viborg, and Aarhus; the medical students assisting our research; and – first and foremost – the study participants.
Disclosure statement
The authors have no conflicts of interest to report. The authors alone are responsible for the contents and writing of the paper.
Additional information
Funding
References
- Clough KB, Benyahi D, Nos C, et al. Oncoplastic surgery: pushing the limits of breast-conserving surgery. Breast J. 2015;21(2):140–146.
- Losken A, Dugal CS, Styblo TM, et al. A meta-analysis comparing breast conservation therapy alone to the oncoplastic technique. Ann Plast Surg. 2014;72(2):145–149.
- Asgeirsson KS, Rasheed T, McCulley SJ, et al. Oncological and cosmetic outcomes of oncoplastic breast conserving surgery. Eur J Surg Oncol. 2005;31(8):817–823.
- Clough KB, van la Parra RFD, Thygesen HH, et al. Long-term results after oncoplastic surgery for breast cancer: a 10-year follow-up. Ann Surg. 2018;268(1):165–171.
- De Lorenzi F, Hubner G, Rotmensz N, et al. Oncological results of oncoplastic breast-conserving surgery: Long term follow-up of a large series at a single institution. Eur. J. Surg Oncol. 2016;42(1):71–77.
- Chakravorty A, Shrestha AK, Sanmugalingam N, et al. How safe is oncoplastic breast conservation: comparative analysis with standard breast conserving surgery. Eur J Surg Oncol. 2012;38:395–398.
- Chen J-Y, Huang Y-J, Zhang L-L, et al. Comparison of oncoplastic breast-conserving surgery and breast-conserving surgery alone: a meta-analysis. J Breast Cancer. 2018;21(3):321–329.
- Peled AW, Sbitany H, Foster RD, et al. Oncoplastic mammoplasty as a strategy for reducing reconstructive complications associated with postmastectomy radiation therapy. Breast J. 2014;20(3):302–307.
- Kronowitz SJ, Feledy JA, Hunt KK, et al. Determining the optimal approach to breast reconstruction after partial mastectomy. Plast Reconstr Surg. 2006;117(1):1–11.
- Munhoz AM, Montag E, Arruda EG, et al. Critical analysis of reduction mammaplasty techniques in combination with conservative breast surgery for early breast cancer treatment. Plast Reconstr Surg. 2006;117(4):1091–1103.
- Hillberg NS, Meesters-Caberg MAJ, Beugels J, et al. Delay of adjuvant radiotherapy due to postoperative complications after oncoplastic breast conserving surgery. Breast. 2018;39:110–116.
- Klit A, Tvedskov TF, Kroman N, et al. Oncoplastic breast surgery does not delay the onset of adjuvant chemotherapy: a population-based study oncoplastic breast surgery does not delay the onset of adjuvant chemotherapy: a population-based study. Acta Oncol. 2017;56(5):719–723.
- Dogan L, Gulcelik MA, Karaman N, et al. Oncoplastic surgery in surgical treatment of breast cancer: Is the timing of adjuvant treatment affected? Clin Breast Cancer. 2013;13(3):202–205.
- Macmillan RD, McCulley SJ. Oncoplastic breast surgery: what, when and for whom? Curr Breast Cancer Rep. 2016;8(2):112–117.
- Clough KB, Ihrai T, Oden S, et al. Oncoplastic surgery for breast cancer based on tumour location and a quadrant-per-quadrant atlas. Br J Surg. 2012;99(10):1389–1395.
- Ojala K, Meretoja TJ, Leidenius MHK. Aesthetic and functional outcome after breast conserving surgery – comparison between conventional and oncoplastic resection. Eur J Surg Oncol. 2017;43(4):658–664.
- Pukancsik D, Kelemen P, Újhelyi M, et al. Objective decision making between conventional and oncoplastic breast-conserving surgery or mastectomy: an aesthetic and functional prospective cohort study. Eur J Surg Oncol. 2017;43(2):303–310.
- De La Cruz L, Blankenship SA, Chatterjee A, et al. Outcomes after oncoplastic breast-conserving surgery in breast cancer patients: a systematic literature review. Oncology. 2016;23:3247–3258.
- Di Micco R, O'Connell RL, Barry PA, et al. Standard wide local excision or bilateral reduction mammoplasty in large-breasted women with small tumours: surgical and patient-reported outcomes. Eur J Surg Oncol. 2017;43(4):636–641.
- Goldsmith C, Haviland J, Tsang Y, FAST Trialists' Group, et al. Large breast size as a risk factor for late adverse effects of breast radiotherapy: Is residual dose inhomogeneity, despite 3D treatment planning and delivery, the main explanation? Radiother Oncol. 2011;100(2):236–240.
- Moody AM, Mayles WP, Bliss JM, et al. The influence of breast size on late radiation effects and association with radiotherapy dose inhomogeneity. Radiother Oncol. 1994;33(2):106–112.
- Rose M, Svensson H, Handler J, et al. Patient-reported outcome after oncoplastic breast surgery compared with conventional breast-conserving surgery in breast cancer. Breast Cancer Res Treat. 2020;180(1):247–256.
- von Elm E, Altman DG, Egger M, STROBE Initiative, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457.
- Husted Madsen A, Haugaard K, Soerensen J, et al. Arm morbidity following sentinel lymph node biopsy or axillary lymph node dissection: a study from the Danish Breast Cancer Cooperative Group. Breast. 2008;17(2):138–147.
- Gärtner R, Jensen M-B, Kronborg L, et al. Self-reported arm-lymphedema and functional impairment after breast cancer treatment-a nationwide study of prevalence and associated factors. Breast. 2010;19(6):506–515.
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381.
- Christiansen P, Carstensen SL, Ejlertsen B, et al. Breast conserving surgery versus mastectomy: overall and relative survival-a population based study by the Danish Breast Cancer Cooperative Group (DBCG). Acta Oncol. 2018;57(1):19–25.
- Silverstein MJ, Savalia N, Khan S, et al. Extreme oncoplasty: breast conservation for patients who need mastectomy. Breast J. 2015;21(1):52–59.
- Heeg E, Jensen MB, Hölmich LR, et al. Rates of re-excision and conversion to mastectomy after breast-conserving surgery with or without oncoplastic surgery: a nationwide population-based study. Br J Surg. 2020;107(13):1762–1772.
- Jonczyk MM, Jean J, Graham R, et al. Surgical trends in breast cancer: a rise in novel operative treatment options over a 12 year analysis HHS Public Access. Breast Cancer Res Treat. 2019;173(2):267–274.
- Bodilsen A, Bjerre K, Offersen BV, et al. The influence of repeat surgery and residual disease on recurrence after breast-conserving surgery: a Danish Breast Cancer Cooperative Group Study. Ann Surg Oncol. 2015;22(S3):476–485.
- Goldhirsch A, Ingle JN, Gelber RD, Panel members, et al. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast cancer 2009. Ann Oncol. 2009;20(8):1319–1329.
- Bali R, Kankam HKN, Borkar N, et al. Wide local excision versus oncoplastic breast surgery: differences in surgical outcome for an assumed margin (0, 1, or 2 mm) distance. Clin Breast Cancer. 2018;18(5):e1053–e1057.
- Aristokleous I, Saddiq M. Quality of life after oncoplastic breast-conserving surgery: a systematic review. ANZ J Surg. 2019;89(6):639–646.
- Veiga DF, Veiga-Filho J, Ribeiro LM, et al. Quality-of-life and self-esteem outcomes after oncoplastic breast-conserving surgery. Plast Reconstr Surg. 2010;125:811–817.
- Haloua MH, Krekel NMA, Winters HAH, et al. A systematic review of oncoplastic breast-conserving surgery: current weaknesses and future prospects. Ann Surg. 2013;257(4):609–620.
- Santos G, Urban C, Edelweiss MI, et al. Long-term comparison of aesthetical outcomes after oncoplastic surgery and lumpectomy in breast cancer patients. Ann Surg Oncol. 2015;22(8):2500–2508.
- Vrieling C, Collette L, Fourquet A, et al. The influence of patient, tumor and treatment factors on the cosmetic results after breast-conserving therapy in the EORTC “'boost vs. no boost' trial. EORTC Radiotherapy and Breast Cancer Cooperative Groups”. Radiother Oncol. 2000;55(3):219–232.
- Lyngholm CD, Christiansen PM, Damsgaard TE, et al. Long-term follow-up of late morbidity, cosmetic outcome and body image after breast conserving therapy. A study from the Danish Breast Cancer Cooperative Group (DBCG). Acta Oncol. 2013;52(2):259–269.
- Cano SJ, Klassen AF, Scott AM, et al. The BREAST-Q: further validation in independent clinical samples. Plast Reconstr Surg. 2012;129(2):293–302.
- Mejdahl MK, Christoffersens KB, Andersen KG. Development and validation of a screening tool for surgery-specific neuropathic pain: neuropathic pain scale for postsurgical patients. Pain Physician. 2019;22(2):E81–E90.
- Lauridsen MC, Overgaard M, Overgaard J, et al. Shoulder disability and late symptoms following surgery for early breast cancer. Acta Oncol. 2008;47(4):569–575.
- Kelsall JE, McCulley SJ, Brock L, et al. Comparing oncoplastic breast conserving surgery with mastectomy and immediate breast reconstruction: case-matched patient reported outcomes. J Plast Reconstr Aesthetic Surg. 2017;70(10):1377–1385.
- Sackey H, Magnuson A, Sandelin K, et al. Arm lymphoedema after axillary surgery in women with invasive breast cancer. Br J Surg. 2014;101(4):390–397.
- Hauerslev KR, Madsen AH, Overgaard J, et al. Long-term follow-up on shoulder and arm morbidity in patients treated for early breast cancer. Acta Oncol. 2020;59(7):851–858.
- Armer JM, Stewart BR. Post-breast cancer lymphedema: incidence increases from 12 to 30 to 60 months. Lymphology. 2010;43(3):118–127.
- Sagen A, Kåresen R, Sandvik L, et al. Changes in arm morbidities and health-related quality of life after breast cancer surgery - a five-year follow-up study. Acta Oncol. 2009;48(8):1111–1118.
