Abstract
Purpose
To determine the survival and prognostic factors of esophageal squamous cell carcinoma (ESCC) patients undergoing radical (chemo)radiotherapy in the era of three-dimensional conformal radiotherapy (3DCRT) and intensity modulated radiotherapy (IMRT) in China.
Material and methods
The Jing-Jin-Ji Esophageal and Esophagogastric Cancer Radiotherapy Oncology Group (3JECROG) conducted the first nationwide survey of nine institutions. Detailed information was accumulated on 5185 patients with ESCC who received definitive 3DCRT/IMRT between 2002 and 2018. Relevant prognostic factors were evaluated to assess their influence on overall and progression-free survivals.
Results
After a median follow-up time of 47.0 (0.9–157.4) months, the 1-year, 2-year, 3-year and 5-year overall survival rates of the whole group were 69.8%, 46.6%, 37.9% and 30.1%. The 1-year, 2-year, 3-year, and 5-year progression-free survival rates were 54.1%, 36.6%, 30.5% and 24.9%. Multivariate analysis demonstrated that sex, clinical stage, treatment modality and radiation dose were prognostic factors for OS. The survival of patients who received concurrent chemoradiotherapy (CCRT) was better than that of patients who received radiotherapy alone or sequential chemoradiotherapy. Patients receiving adjuvant chemotherapy after CCRT had a better OS than patients receiving CCRT alone. Patients receiving higher radiation dose had a better OS than those patients receiving low-dose radiotherapy.
Conclusions
The survival of ESCC patients undergoing radical (chemo)radiotherapy was relatively satisfactory in the era of 3DCRTand IMRT. As the largest-scale multicenter research on esophageal cancer radiotherapy conducted in China, this study establishes national benchmarks and helps to provide references for subsequent related researches.
Introduction
Esophageal cancer is a malignant tumor with a high mortality rate. According to a recent global epidemiological survey, esophageal cancer is the fifth most common cause of cancer-related death, with a global age-adjusted annual mortality rate of 5.5 per 100,000 population [Citation1]. Specifically, China leads the world in both numbers of diagnoses and deaths. According to national epidemiological research conducted in 2015, esophageal cancer ranks third in the incidence of malignant tumors in China and the fourth in mortality [Citation2]. Unlike most Western countries, the pathological type of esophageal cancer in China is still dominated by squamous cell carcinoma (SCC), and patients with esophageal squamous cell carcinoma account for more than 90% of all esophageal cancer patients [Citation3,Citation4].
For the management of esophageal cancer, a large proportion of patients fail to meet the prerequisite of surgery due to various reasons, such as advanced age, advanced tumor stage, severe comorbidities, concerns about postoperative complications and so on. Therefore, non-surgical treatment plays an important role in the treatment of esophageal cancer patients. Over the past few decades, researchers from different countries have conducted a lot of nationwide surveys on the practice of esophageal cancer radiotherapy [Citation5–10]. However, previous reports rarely mentioned specific radiotherapy techniques, and a considerable portion of them was conducted in the era of two-dimensional radiotherapy (2DRT). In recent years, radiotherapy techniques have continuously developed, and the emergence of three-dimensional conformal radiotherapy (3DCRT) and intensity-modulated radiotherapy (IMRT) has greatly improved the accuracy of radiotherapy, but few researches have been conducted focusing on patients receiving these advanced radiotherapy techniques.
Based on this situation, Jing-Jin-Ji Esophageal and Esophagogastric Cancer Radiotherapy Oncology Group (3JECROG) was established in 2015 in Beijing, aiming to strengthen the cooperation of radiotherapy facilities nationwide and to establish national benchmarks for the radiotherapy treatment of esophageal cancer. 3JECROG now contains more than twenty medical facilities with both large-scale academic institutions in first-line cities and small nonacademic hospitals in county-level cities, covering more than ten provinces/municipalities across the country. Since its establishment, 3JGCROG has conducted several nationwide surveys and built the largest database with more than 5000 cases of esophageal cancer patients treated with radiotherapy in China. In this research, we will present the general characteristics, treatment details and survival results of the investigated patients and discuss several existing controversial issues in the treatment of esophageal cancer, in the hope of reviewing the full picture of esophageal cancer patients receiving advanced radiation techniques in China.
Material and methods
Study population
This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) cohort reporting guideline. The data of this study was collected with the same template from nine institutions from seven different provinces. The following data were included: (1) patient and tumor baseline characteristics, including patients’ age, sex, tumor location and tumor stage; (2) radiation technique, total radiation dose and fractionation; (3) regimens of concurrent chemotherapy, and induction or consolidation chemotherapy; (4) survival and recurrent status and their last documentation time (recurrence were determined by both clinical examination and symptoms; overall survival considering deaths due to any causes). A specialized person was responsible for collecting and merging the datasheets filled by each institution. The datasheet would be returned to the corresponding center for revision if any entries were missing.
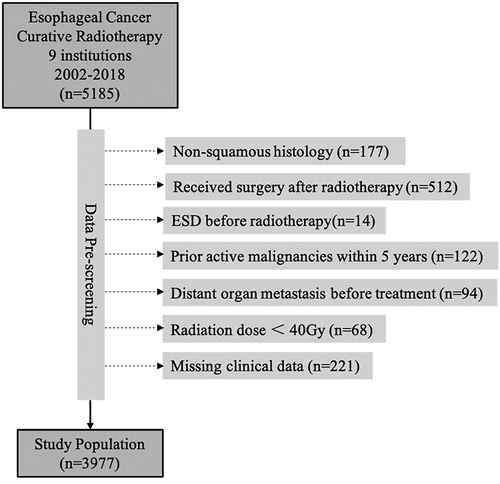
This study includes patients with esophageal squamous cell carcinoma (ESCC) underdoing non-surgical treatment from 2002 to 2018 at 3JECROG. A total of 5185 cases were collected. Patients were excluded from this study if they had (1) histological types other than squamous cell carcinoma (e.g., adenocarcinoma, small cell carcinoma, and so on; n = 177); (2) received surgery afterwards (n = 512); (3) undergone endoscopic submucosal resection (ESD) before radiotherapy (n = 14); (4) active malignancies (other than curable non-melanoma skin cancer or in situ cervical cancer) within the past 5 years (n = 122); (5) stage IVB disease (only patients with distant organ metastasis were excluded, stage IVA/B patients with distant lymph node metastasis were not excluded) (n = 94); (6) radiation dose < 40 Gy (n = 68); (7) missing clinical data (n = 221). The final cohort comprised 3977 patients ().
Treatment
All patients received CT simulation and their radiotherapy plans were developed and evaluated based on CT images. The majority of patients received static IMRT (65.4%), and some patients received volumetric-modulated arc therapy (VMAT) (11.5%) and 3DCRT (23.0%). In terms of radiation dose, the median radiation dose of the whole group is 60 Gy (range: 40–76 Gy). Most patients receive conventionally fractionated radiotherapy, with a single dose of 1.8–2.0 Gy/f/d, 5 f/w. A small number of patients (6.8%) received simultaneous integrated boost IMRT (SIB-IMRT), with the boost dose 2.1–2.4 Gy (median 2.14 Gy). For patients not receiving SIB-IMRT, a re-simulation was often performed after 40–50 Gy and the target volumes would be shrunk to high-risk areas (usually primary tumor and metastatic lymph nodes plus relevant margins). To make the total radiotherapy dose between SIB and conventionally-fractionated patients comparable, we converted the total dose into an equivalent dose in 2 Gy/fraction (EQD2) using an α/β of 10. The delineation of target volumes may have slight variations depending on different radiation oncologists, but the typical target delineations of upper, middle and lower tumors could be seen in our previous study [Citation11]. In terms of chemotherapy, for patients receiving concurrent chemoradiotherapy (CCRT, n = 1808), the most commonly-used chemotherapeutic regimens in order were taxel + platinum (n = 788, 43.6%), fluorouracil + platinum (n = 392, 21.7%) and single platinum agent (n = 118, 6.5%). Patients who did not receive concurrent chemotherapy but received induction chemotherapy (IC) or adjuvant chemotherapy (AC) (or both) were defined as patients who received sequential chemoradiotherapy (sCRT). The most commonly-used sCRT regimens in order were taxel + platinum (n = 102, 35.8%) and fluorouracil + platinum (n = 44, 15.4%). For more detailed chemotherapeutic regimens, please refer to Supplementary Table 1.
Statistical analysis
Descriptive statistics, including frequencies and percentages for categorical variables, and median and standard deviation for quantitative variables, were computed to summarize patient characteristics for the overall patient cohort. Survival estimates were calculated from the first day of radiotherapy using the Kaplan–Meier estimator, and differences were evaluated by the log-rank test. The overall survival (OS) time was defined as the time interval between the start of radiotherapy and death for any cause. Progression free survival (PFS) time was defined as the time interval between the start of radiotherapy and progression at any site or death for any cause. Treatment failures were classified as local-regional recurrence (LRR) or distant metastases (DM). Cumulative LRR and DM incidence estimates were computed using the Aalen-Johansen method. LRR was defined as any recurrence at the primary site or in regional lymph nodes. DM included hematogenous metastasis and non-regional lymph node metastasis. A Cox proportional hazards model was used for multivariate analyses of the effect of covariates on OS and PFS. The significance level was set to 5%. SPSS software version 26.0 was used for all calculations.
Results
In total, 3977 patients with ESCC treated by non-surgical approach were included from nine institutions. shows baseline characteristics of all patients enrolled in the analysis. The median age is 64 years old, with the oldest patient 90 and youngest 29. 72.9% patients were male, and nearly half (47.8%) patients had tumor located in the middle thorax. The majority of patients in this study are from northern China (including provinces and municipalities such as Beijing, Tianjin and Hebei), and a small number are from eastern and central China. The 6th edition of UICC/AJCC (International Union Against Cancer/American Joint Committee on Cancer) TNM classification system was adopted to determine the clinical disease stage of enrolled patients [Citation12]. Most patients had advanced disease at their initial diagnosis, with more than half (55.0%) patients had stage III disease while more than 20% (23.3%) patients had stage IVA or IVB disease when diagnosed. All patients diagnosed as stage IV patients were patients with non-regional lymph node metastasis (cervical, supraclavicular, celiac LNM, etc.).
Table 1. Patients, tumor and treatments characteristics.
Most patients (81.0%) received EQD2 ≥ 60 Gy, and a small number of patients received EQD2 of 50–60 Gy (14.1%) and <50 Gy (4.9%). About half of the patients (45.5%) received concurrent chemoradiotherapy (CCRT), and a small number of patients (7.2%) received sequential chemoradiotherapy (sCRT). Among patients receiving CCRT, a small number of patients (2.2%) received IC, and 17.5% patients received AC.
The median follow-up periods for surviving patients was 47.0 months (ranged from 0.9 to 157.4 months). presents the OS and PFS rates of the entire group. The median OS and PFS in the entire cohort were 21.8 months (95% CI: 20.7–22.9 months) and 14.0 months (95% CI: 13.2–14.8 months), respectively. The 1-year, 2-year, 3-year, and 5-year OS rates were 69.8% (95% CI: 68.4–71.2%), 46.6% (95% CI: 45.0–48.2%), 37.9% (95% CI: 36.3–39.5%), and 30.1% (95% CI: 28.5–31.7%), respectively. The 1-year, 2-year, 3-year, and 5-year PFS rates were 54.1% (95% CI: 52.5–55.7%), 36.6% (95% CI: 35.0–38.2%), 30.5% (95% CI: 28.9–32.1%), and 24.9% (95% CI: 23.3%-26.5%), respectively.
Figure 2. (A) Kaplan–Meier curves of overall survival (OS) and progression-free survival (PFS) of the whole cohort (B) Cumulative incidences of locoregional recurrence (LRR) and distant metastasis (DM) adjusted for the competing risk of death of the whole cohort.
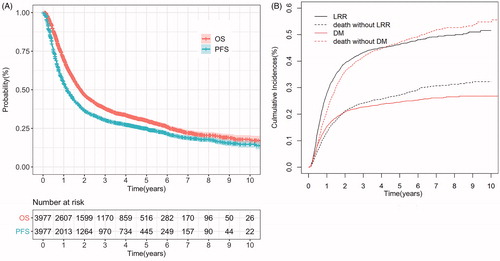
Among all 3977 patients, 3781 patients had complete records of DM status, and 3799 patients had complete records of LRR status. Up to the last follow-up time, 2224 (55.9%) patients in the whole group experienced treatment failures, of which 1357 (34.1%) patients had LRR, 586 (14.7%) patients had DM, and 281 (7.1%) patients had both. It can be seen that locoregional failure is still the dominant cause of treatment failure in ESCC patients receiving radical radiotherapy. shows the cumulative rates of LRR and DM of the whole group. The 1-year, 2-year, 3-year, and 5-year competing risk-adjusted cumulative rates of LRR of the entire group were 27.9%, 39.2%, 43.2%, and 46.3%, respectively; of DM were 15.3%, 20.9%, 22.7%, and 24.5%, respectively. The median time to LRR was 9.0 months, and the median time to DM was 8.2 months.
The efficacy of the 6th edition of UICC/AJCC TNM staging system in distinguishing the prognosis of ESCC patients was tested in . The median OS time of stage I, IIA, IIB, III, IVA and IVB patients were 65.1 (95% CI 46.5–83.6 months), 49.4 (95% CI 36.4–58.4 months), 31.5 (95% CI 21.5–41.4 months), 20.4 (95% CI 19.1–21.6 months), 17.2 (95% CI 15.0–19.3 months), and 16.6 months (95% CI 14.7–18.5 months), respectively. No difference of OS was observed between clinical stage III and IVA stage (p = 0.21) or between stage IVA and stage IVB patients (p = 0.12).
Figure 3. Kaplan–Meier curves of overall survival (OS) by (A) 6th edition of UICC/AJCC clinical stage (B) treatment modality (C) treatment modalities within the concurrent chemoradiotherapy (CCRT) group and (D) radiotherapy dose. RT: radiotherapy; CCRT: concurrent chemoradiotherapy; sCRT: sequential chemoradiotherapy; IC: induction chemotherapy; AC: adjuvant chemotherapy.
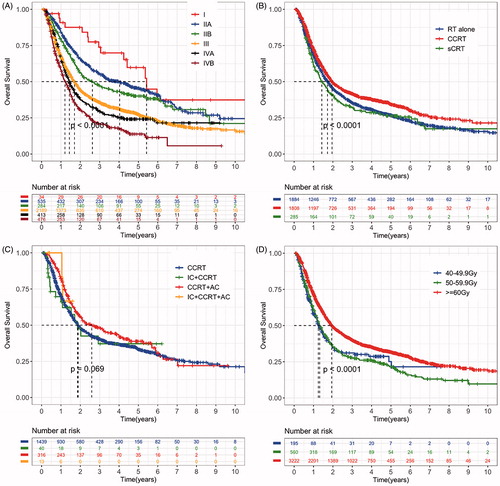
We further explored the effects of treatment modality and radiation dose on patients’ prognosis. shows actuarial survival curves by treatment modality. Survival difference was detected between CCRT vs. radiotherapy (RT) alone group (p < 0.001) and CCRT vs. sCRT group (p < 0.001). No difference was detected in OS between RT alone group and sCRT group (p = 0.18). The median OS of RT alone, CCRT and sCRT group were 20.7 months, 23.5 and 17.6 months, respectively. We also compared the OS of patients in each subgroup within the CCRT group (). Survival difference was detected between CCRT alone vs. CCRT + AC group (p = 0.011). No difference was detected in OS between CCRT alone group vs. IC + CCRT group (p = 0.07) or CCRT alone group vs. IC + CCRT + AC group (p = 0.47). The median OS of CCRT alone, IC + CCRT and CCRT + AC groups were 22.7, 22.2 and 31.1 months, respectively. shows the survival of patients receiving different doses of radiotherapy. Survival difference was detected between ≥60 Gy group vs. 40–49.9 Gy (p < 0.001) and ≥60 Gy group vs. 50–59.9 Gy group (p < 0.001). No difference in OS was detected between the 40–49.9 Gy group and the 50–59.9 Gy group (p = 0.93). The median OS time of ≥ 60 Gy, 50–59.9 Gy and 40–49.9 Gy group were 23.3, 15.8 and 15.0 months, respectively. Further subgroup analysis showed differences in OS between different dose groups regardless of the location of the tumor (upper: p = 0.017; middle: p = 0.009; lower: p < 0.001) (Supplementary Figure 1).
represents the results of multivariable Cox proportional hazards analysis assessing factors associated with OS and PFS. The results confirmed the influence of treatment modality and radiotherapy dose on the prognosis. CCRT group showed a survival advantage over RT alone group (OS: HR = 0.75, 95% CI: 0.68–0.82; PFS: HR = 0.78, 95% CI: 0.71–0.86), and the EQD2 ≥ 60 Gy group showed a survival advantage over the < 50 Gy group (OS: HR = 0.77, 95% CI: 0.63–0.94; PFS: HR = 0.81, 95% CI: 0.68–0.98). In addition, gender and UICC/AJCC 6th clinical stage are also important factors affecting prognosis, while radiotherapy technique, year of diagnosis, institution location and age (<70 y vs. ≥70 y) do not show obvious impact on OS or PFS.
Table 2. Results of multivariate COX analysis for progression-free survival and overall survival.
Discussion
Non-surgical intervention plays an important role in the treatment of esophageal cancer. According to a large observational analysis of 11,112 patients with resectable esophageal cancer from National Cancer Data Base (NCDB), more than 70% of patients received non-surgical treatment [Citation13]. Most esophageal cancer patients in China had advanced disease at the initial diagnosis. According to statistics, only 20–30% of patients are operable at the time of initial diagnosis. Therefore, radiotherapy-based non-surgical treatment is particularly important among Chinese esophageal cancer patients. However, in the past few decades, there have been few studies on patients undergoing non-surgical treatment of esophageal cancer in China. Most large-scale researches published were single-institutional analysis, which are relatively outdated and the radiation techniques used were mainly 2DRT [Citation14]. Therefore, 3JECROG, a multi-institutional radiation study group was built in Beijing in 2015. Since its establishment, 3JECROG has launched several multi-institutional prospective clinical trials nationwide [Citation15–17] and conducted multiple multi-center retrospective investigations [Citation18]. With its support, this study includes the largest published sample of non-surgical ESCC patients in mainland China so far.
This study adopted the 6th edition of UICC/AJCC staging manual, which is the most commonly used staging system in mainland China for non-surgical patients. Results showed that this version of staging manual had a relatively satisfactory predictive value for non-surgical ESCC patients. In the whole group, with the increase of the clinical stage, the 5-year OS rates decreased from 61.0% to 22.8%, and the difference between the groups was statistically significant (p < 0.001). However, when pairwise comparisons were performed for each stage, no differences were found between the OS of stage III patients and stage IVA patients (p = 0.21), or between stage IVA patients and stage IVB patients (p = 0.12). This result suggests the modification of supraclavicular/celiac lymph nodes from non-regional to regional may allow for better stratification of overall survival for ESCC patients, which correlates to the findings from several previous studies [Citation19,Citation20].
Regarding management, this study indicates that linear accelerators have become very common in mainland China. Most centers have been equipped with linear accelerators capable of delivering static IMRT. The 5-year OS and PFS rates of the entire group are 30.1% and 24.9%, which is equivalent to the survival results of most contemporaneous large-scale observational studies [Citation10,Citation13,Citation21]. The overall concurrent chemotherapy rate, however, is relatively low compared to other large-scale retrospective studies of the same era [Citation10]. This situation may be due to the following reasons: advanced age, poor performance status, poor economic status, patient rejection, etc. Although the rate of chemotherapy is low, the survival results of patients in the RT alone group are satisfactory, with a 5-year OS of 26.2%, which is higher than previous reports in the era of 2DRT [Citation14,Citation22,Citation23]. This may be related to the advancement of radiotherapy and imaging techniques in recent years. These results indicate that for patients who cannot tolerate CCRT, radiation monotherapy can still bring promising survival results. The addition of concurrent chemotherapy has further improved 5-year OS to 33%, a result better than that of RT alone and sCRT group, indicating that for ESCC patients who can tolerate concurrent chemotherapy, CCRT is still the optimal treatment option in the era of 3DCRT/IMRT. The value of AC after CCRT is controversial in esophageal cancer. So far, no large-scale prospective clinical trials have been performed to confirm the efficacy of AC following CCRT in patients with esophageal cancer, and the published retrospective studies have different conclusions [Citation24,Citation25]. In our analysis, patients receiving AC after CCRT showed superior survival results than those receiving CCRT alone (median OS: 22.7 vs. 31.1 months, p = 0.011). Although this conclusion needs to be confirmed by further prospective studies, we believe that AC could be recommended for patients with good general conditions after CCRT.
In terms of radiotherapy dose, in this study, most centers used a radical radiotherapy dose of about 60 Gy, a commonly used radical dose in most Asian countries nowadays. This dose differs from the current dose recommended by most guidelines (50.4 Gy). 50.4 Gy became the standard dose for definitive chemoradiotherapy after Radiation Therapy Oncology Group (RTOG) 85–01 [Citation22] and RTOG 94–05 [Citation26], two prospective clinical trials conducted in the era of 2DRT. However, recent studies have proved that locoregional control is poor with this dose [Citation27]. Many large-sample retrospective studies have also suggested that higher radiation doses may provide better local control as well as survival benefit for patients with advanced esophageal cancer [Citation28–30], especially in ESCC. In univariate analysis of this study, patients receiving an EQD2 ≥ 60 Gy had better OS compared to that of patients receiving an EQD2 of 40–49.9 Gy or 50–59.9 Gy. This finding was confirmed in the multivariate analysis. These results support the idea that patients with ESCC may indeed need a higher definitive radiation dose. Higher radiation dose may improve survival by improving local control rate or delaying the progression of local disease. Nevertheless, higher radiation dose will inevitably bring higher treatment toxicities. For some patients, pathological complete regression (pCR) was achieved after an irradiation dose as low as 40–50 Gy [Citation31]. Therefore, we believe that if the patients’ sensitivity to (chemo) radiotherapy could be properly predicted in the future, it would be beneficial to give stratified radiation doses according to their sensitivity.
As a multi-center retrospective study, our research has several limitations. For instance, although our participating members contain both large-scale academic institutions and small nonacademic facilities, the former one plays a dominant role in this study. Moreover, due to the relatively long time span and large number of centers involved, the cases collected in this study may have inconsistencies in the terms of treatment details (e.g., chemotherapeutic regimens, fractionation strategies, etc.). Besides, due to the retrospective nature of this study, we can only include limited items for analysis, and there is a lack of information about several detailed variables (i.e., performance status, nutritional status, comorbidities, treatment-related side effects, quality of life, etc.), which affects our further investigation on these factors in this study.
Conclusions
In conclusion, this is the first large-scale multi-center retrospective survey focused on ESCC radiotherapy conducted in China in the era of 3DCRT/IMRT. Results show relatively promising survival results compared to contemporaneous large-scale researches. For patients who can tolerate concurrent chemotherapy, CCRT is still the optimal option for patients underwent non-surgical procedure, and patients receiving AC after CCRT had better survival than patients receiving CCRT alone. In terms of radiation dose, patients receiving higher radiation dose (EQD2 ≧ 60 Gy) had a better OS than that of patients receiving lower doses. As a cooperative oncology group in the country with largest number of esophageal cancer cases, 3JECROG will pay further attention to the development of treatment for esophageal cancer and start a series of prospective and retrospective studies accordingly.
Supplemental Material
Download MS Word (25.1 KB)Supplemental Material
Download MS Word (1.5 MB)Supplemental Material
Download TIFF Image (1.5 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–1953.
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132.
- Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet. 2013;381(9864):400–412.
- Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64(3):381–387.
- Coia LR, Minsky BD, John MJ, et al. The evaluation and treatment of patients receiving radiation therapy for carcinoma of the esophagus. Cancer. 1999;85(12):2499–2505.
- Tanisada K, Teshima T, Ikeda H, et al. A preliminary outcome analysis of the patterns of care study in Japan for esophageal cancer patients with special reference to age: non surgery group. Int J Radiat Oncol. 2000;46(5):1223–1233.
- Coia LR, Minsky BD, Berkey BA, et al. Outcome of patients receiving radiation for cancer of the esophagus: results of the 1992-1994 patterns of care study. J Clin Oncol. 2000;18(3):455–455.
- Suntharalingam M, Moughan J, Coia LR, et al. Outcome results of the 1996-1999 patterns of care survey of the national practice for patients receiving radiation therapy for carcinoma of the esophagus. J Clin Oncol. 2005;23(10):2325–2331.
- Nishimura Y, Koike R, Ogawa K, et al. Clinical practice and outcome of radiotherapy for esophageal cancer between 1999 and 2003: the Japanese Radiation Oncology Study Group (JROSG) Survey. Int J Clin Oncol. 2012;17(1):48–54.
- Tachimori Y, Ozawa S, Numasaki H, et al. Comprehensive registry of esophageal cancer in Japan, 2012. Esophagus. 2019;16(3):221–245.
- Li C, Ni W, Wang X, et al. A phase I/II radiation dose escalation trial using simultaneous integrated boost technique with elective nodal irradiation and concurrent chemotherapy for unresectable esophageal Cancer. Radiat Oncol. 2019;14(1):48.
- Greene FL, Page DL, Fleming ID, et al. AJCC cancer staging manual. 6th ed. New York: Springer; 2002.
- Naik KB, Liu Y, Goodman M, et al. Concurrent chemoradiotherapy with or without surgery for patients with resectable esophageal cancer: an analysis of the National Cancer Data Base. Cancer. 2017;123(18):3476–3485.
- De-Ren S. Ten-year follow-up of esophageal cancer treated by radical radiation therapy: analysis of 869 patients. Int J Radiat Oncol. 1989;16(2):329–334.
- Li C, Wang X, Wang X, et al. A multicenter phase III study comparing Simultaneous Integrated Boost (SIB) radiotherapy concurrent and consolidated with S-1 versus SIB alone in elderly patients with esophageal and esophagogastric cancer – the 3JECROG P-01 study protocol. BMC Cancer. 2019;19(1):397.
- Gao L, Wang X, Han W, et al. A multicenter prospective phase III clinical randomized study of simultaneous integrated boost intensity-modulated radiotherapy with or without concurrent chemotherapy in patients with esophageal cancer: 3JECROG P-02 study protocol. BMC Cancer. 2020;20(1):901.
- Wang X, Ge X, Wang X, et al. S-1–based chemoradiotherapy followed by consolidation chemotherapy with S-1 in elderly patients with esophageal squamous cell carcinoma: a multicenter phase II. Trial. Front Oncol. 2020;10:1–11.
- Chen M, Liu X, Han C, et al. Does chemoradiotherapy benefit elderly patients with esophageal squamous cell cancer? A propensity-score matched analysis on multicenter data (3JECROG R-03A). BMC Cancer. 2020;20(1):36.
- Yamasaki M, Miyata H, Miyazaki Y, et al. Evaluation of the nodal status in the 7th edition of the UICC-TNM classification for esophageal squamous cell carcinoma: proposed modifications for improved survival stratification. Ann Surg Oncol. 2014;21(9):2850–2856.
- Nomura M, Shitara K, Kodaira T, et al. Prognostic impact of the 6th and 7th American Joint Committee on Cancer TNM staging systems on esophageal cancer patients treated with chemoradiotherapy. Int J Radiat Oncol. 2012;82(2):946–952.
- Jingu K, Umezawa R, Matsushita H, et al. Chemoradiotherapy for T4 and/or M1 lymph node esophageal cancer: experience since 2000 at a high-volume center in Japan. Int J Clin Oncol. 2016;21(2):276–282.
- Herskovic A, Martz K, Al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326(24):1593–1598.
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281(17):1623–1627.
- Chen M, Shen M, Lin Y, et al. Adjuvant chemotherapy does not benefit patients with esophageal squamous cell carcinoma treated with definitive chemoradiotherapy. Radiat Oncol. 2018;13(1):150.
- Koh HK, Park Y, Koo T, et al. Adjuvant chemotherapy and dose escalation in definitive concurrent chemoradiotherapy for esophageal squamous cell carcinoma. Anticancer Res. 2020;40(3):1771–1778.
- Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20(5):1167–1174.
- Welsh J, Settle SH, Amini A, et al. Failure patterns in patients with esophageal cancer treated with definitive chemoradiation. Cancer. 2012;118(10):2632–2640.
- Chang C-L, Tsai H-C, Lin W-C, et al. Dose escalation intensity-modulated radiotherapy–based concurrent chemoradiotherapy is effective for advanced-stage thoracic esophageal squamous cell carcinoma. Radiother Oncol. 2017;125(1):73–79.
- Chen C-Y, Li C-C, Chien C-R. Does higher radiation dose lead to better outcome for non-operated localized esophageal squamous cell carcinoma patients who received concurrent chemoradiotherapy? A population based propensity-score matched analysis. Radiother Oncol. 2016;120(1):136–139.
- Song T, Liang X, Fang M, et al. High-dose versus conventional-dose irradiation in cisplatin-based definitive concurrent chemoradiotherapy for esophageal cancer: a systematic review and pooled analysis. Exp Rev Anticancer Ther. 2015;15(10):1157–1169.
- Rice TW, Chen L-Q, Hofstetter WL, et al. Worldwide Esophageal Cancer Collaboration: pathologic staging data. Dis Esophagus. 2016;29(7):724–733.