Abstract
Background
The impact of lifestyle changes on cancer risk is yet to be elucidated. We investigated this issue in the Swedish Women's Lifestyle and Health Cohort Study.
Material and methods
We measured changes by comparing two questionnaires, filled in 1991/92 and 2003. We followed women for cancer from 2003 until 2012. We used Cox regression models to assess the effect of changes in smoking, alcohol consumption, body mass index (BMI), physical activity and a lifestyle score on the risk of lifestyle-related cancer. One point was added to the lifestyle score for each of these: non-smoking, alcohol consumption ≤12 grams/day, BMI <25 kg/m2 and high level of physical activity.
Results
We included 29,930 women. From 1991/92 to 2003, median age changed from 40.0 to 51.7 years, alcohol consumption from 2.5 to 4.7 grams/day, BMI from 22.7 to 24.5 kg/m2, proportion of current smokers from 31.0 to 20.6% and women reporting high physical activity from 27.2 to 37.0%. Women who quit smoking had lower risk of smoking-related cancers compared to women who continued (hazard ratio (HR) 0.74, 95% confidence interval (CI) 0.55–1.00). Women who reduced their weight by more than 5%, compared to women with stable weight, had lower risk of breast cancer (HR 0.49, 95% CI 0.31–0.78). Among women with score of 0–2 in 1992/93, those who improved to 3-4 had lower risk of lifestyle-related cancers compared to women who did not (HR 0.81, 95% CI 0.66–0.99).
Conclusions
Healthy lifestyle changes, particularly smoking cessation and weight reduction, were associated with a decreased risk of cancer.
Background
A healthy lifestyle is associated with a longer life expectancy [Citation1–3] and more life years without chronic diseases [Citation4] compared to an unhealthy lifestyle. Favourable factors include non-smoking, adequate physical activity, normal body weight, diet rich in vegetables, fruit and whole grains, and no or low alcohol consumption [Citation1–4]. It is well-known that lifestyle factors are associated with the risk of cancer [Citation5,Citation6], while less is known about the impact of changes in these factors on the risk of cancer.
Smoking cessation has a well-established effect on reducing the risk of lung cancer [Citation7]. There is evidence that increasing physical activity level during adult life reduces cancer mortality [Citation8] and that improving the cardiorespiratory physical fitness reduces cancer risk and mortality [Citation9]. When it comes to breast cancer, body fatness is associated only with the risk only in postmenopausal women [Citation6]. Weight gain is an established risk factor for postmenopausal breast cancer, and there is highly suggestive evidence that it increases the risk of endometrial cancer [Citation10] and suggestive evidence for increasing the risk of colorectal cancer [Citation11]. On the other hand, reduction in body weight has been reported to lower the risk of breast cancer [Citation12] and endometrial cancer [Citation13]. There is evidence that increasing alcohol consumption increases the risk of breast cancer [Citation14], while cessation of alcohol intake in adult age reduces the risk of head and neck [Citation15,Citation16] and oesophageal cancers [Citation17]. The effect of changes in alcohol consumption on other cancer types is unclear, possibly because of the required long-term follow-up [Citation17,Citation18]. The effect of dietary improvements on the risk of several cancer types is uncertain, with verified evidence only for ovarian cancer [Citation19]. However, a randomised study showed that a successful smoking cessation and dietary intervention reduced the long-term risk of lifestyle-related cancers in middle-aged men at high risk for cancer [Citation20].
Further evidence is required to establish the effect of lifestyle changes on cancer risk and to support lifestyle recommendations and interventions in primary health care. In the Swedish Women's Lifestyle and Health Cohort Study, women reported on their health behaviour twice, with an interval of 12 years. In this study, we aimed at exploring if changes in smoking habits, alcohol consumption, body weight, physical activity and a lifestyle score combining these four factors, had an effect on their cancer risk.
Materials and methods
Participants
The Swedish Women’s Lifestyle and Health cohort and data collection have been described in detail previously [Citation21]. Briefly, 96,000 women aged 30–49 years were randomly selected among residents in the Uppsala Health Care Region in Sweden during 1991 and 1992. They were invited by mail to fill in a baseline questionnaire and 49,259 (51.3%) returned it. In 2003 a follow-up questionnaire was sent to the 47,290 women still alive and residing in Sweden, and 34,402 (72.7%) returned it. After the exclusion of 1484 (4.3%) women with a previous diagnosis of invasive cancer, and 2,988 (8.7%) women with missing information on changes in any of the four lifestyle factors of interest described below, we finally included 29,930 women. The date on which they filled the follow-up questionnaire in 2003 represents the baseline of our study. The study was approved by the Swedish Data Inspection Board and the regional Ethical Committee at Uppsala University, Sweden.
Exposure
We selected four lifestyle factors of interest: smoking status, alcohol consumption, body mass index (BMI, kg/m2) and physical activity. Smoking was assessed by questions on current smoking status and the number of cigarettes smoked daily during different periods preceding the time at filling in the questionnaire. The questions on alcohol consumption consisted of the number of units of each beverage type (beer, wine and spirits) consumed per week or month which was re-calculated into daily intake in grams. BMI was assessed by questions on current body weight in kilograms and height in centimetres. Physical activity was assessed by asking the participant to state their activity level according to a visual scale from 1 (very low) to 5 (very high) in the 1991/92 questionnaire and from 1 (very low) to 10 (very high) in the 2003 questionnaire [Citation21]. To measure changes in each of those factors, we compared the information reported by each woman in the two questionnaires, categorising each lifestyle factor according to adherence vs. non-adherence to health guidelines. For smoking, we compared the smoking status: smoker vs. non-smoker. For alcohol consumption, we compared the grams consumed per day (g/d): <12 g/d vs. ≥12 g/d. For weight, we compared the BMI: <25 vs. ≥25 kg/m2. We also analysed weight trends as weight gain/lost/maintenance, using ±5% change from 1991/92 to 2003 as cut-off values, as a change of this size is considered as clinically relevant [Citation22]. The middle of the scale for physical activity was marked with the word “normal”. It was not possible to precisely categorise physical activity according to adherence to health guidelines. We therefore first dichotomised the scale into high physical activity level (i.e., more than normal) and low to medium physical activity level (less than normal or normal) and then compared the resulting variable between the two questionnaires.
We calculated a lifestyle score, ranging from 0 to 4 points, based on the four factors of interest. A point was added to the score for each of these conditions: non-smoking, alcohol consumption ≤12 g/d, BMI <25 kg/m2 and high physical activity. Lifestyle was coded as bad, or unfavourable, if the score was two or less, good, or favourable, if the score was three or four.
Outcome
Women were followed for incident invasive cancer from the date of the questionnaire at 2003 until the end of 2012. Cancer cases were retrieved through linkage with the Swedish Cancer Registry, which by law contains information on essentially all malignant cancers diagnosed in Sweden since 1958. In case of multiple cancers, only the first was analysed. We investigated five lifestyle-related cancer subgroups, defined using the International Classification of Diseases, Revision 7 (ICD7):
Smoking-related cancers: buccal cavity and pharynx (codes 140–141, 143–148, salivary gland excluded), larynx (161) bronchus, trachea and lung (162), oesophagus (150), stomach (151), colorectum (153–154), biliary passages and liver (155), pancreas (157), kidney (180), bladder and other urinary organs (181), cervix uteri (171);
Alcohol-related cancers: buccal cavity and pharynx (codes 140–141, 143–148, salivary gland excluded), larynx (161), oesophagus (150), colorectum (153–154), biliary passages and liver (155), breast (170);
BMI-related cancers: oesophagus (150), colorectum (153–154), pancreas (157), breast (170), corpus uteri (172), kidney (180), thyroid gland (194);
Physical activity-related cancers: colorectum (153–154), breast (170);
Lifestyle-related cancers: combination of all cancers listed above.
We defined those five cancer groups based on indications from the International agency for research on cancer [Citation23] and the European prospective investigation into cancer and nutrition cohort study [Citation5]. With regard to single cancer types, we analysed only breast cancer, as it was the only one with a sufficient number of cases.
Statistical methods
Women were followed from the date of the questionnaire at 2003 until diagnosis of invasive cancer, emigration, death or 31 December 012, whichever occurred first. The data was analysed in 2020. We used Cox regression models, with attained age as the time scale, to assess the effect of changes in lifestyle factors and lifestyle score on the risk of lifestyle-related cancer. Proportionality was assessed with Schoenfeld residuals. We reported hazard ratios (HR) with 95% Wald type confidence intervals (CI). To evaluate the effect of lifestyle changes on cancer risk, we divided the population in groups based on the information at 1991/92 and the 2003. The models included either all four lifestyle factors simultaneously or the lifestyle score, and were adjusted for educational level (categorical, ≤10/11–12/13–14 and >14 years) reported at the questionnaire at 1991/92 and menopausal status (pre/post) reported at the questionnaire at 2003. We also evaluated the potential confounding effect of three conditions, reported at the questionnaire at 2003: history of hypertension, history of increased levels of cholesterol or triglyceride and current diagnosis of diabetes. Since the average age at menopause in Sweden is 50 years [Citation24], we recoded any missing menopausal status as premenopause for women up to 50 years, and postmenopause for those older than 50. Among women who had an unfavourable lifestyle (score 0–2) in 1992/93, a subgroup analysis by age was performed, according to the median age in 2003. Interaction between age (≤median/>median in the questionnaire at 2003) and each of the lifestyle changes (constant unfavourable/improved to favourable) was evaluated entering an interaction term in the Cox model. Curves for the cumulative incidence of lifestyle-related cancers were estimated in a competing risk framework, where cancers unrelated to lifestyle were treated as competing events.
For lifestyle-related, alcohol-related, BMI-related and physical activity-related cancers, we performed an additional analysis where we censored women with breast cancer at cancer diagnosis. All analyses were based on women with complete information on changes in the four lifestyle factors of interest. Complete case analysis was shown to be appropriate in this cohort [Citation25]. In addition, we performed a sensitivity analysis where we included women with incomplete information and the missing values were included as a separate category. All statistical analyses were carried out using the SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA), except for the cumulative incidence curves that were calculated using the cuminc function in R software version 3.3.3 (www.r-project.org). All tests of statistical hypotheses were done on the two-sided 5% level of significance.
Results
We included 29,930 women with complete information on lifestyle changes. In 2003, median age was 51.7 years, median years of schooling was 12 years, and 17,582 (58.7%) women were postmenopausal (); women with a favourable lifestyle (score of 3–4) were younger and more educated than women with an unfavourable lifestyle (score 0–2).
Table 1. Women’s characteristics at start of follow-up (2003), total and by lifestyle score.
From 1991/92 to 2003 median alcohol consumption increased from 2.5 to 4.7 g/d, and BMI from 22.7 to 24.5 kg/m2. The proportion of current smokers decreased from 31.0 to 20.6% (). The proportion of women drinking ≤12 g/d changed from 95.8 to 85.2%, women with BMI < 25 kg/m2 from 75.7 to 55.3% and women reporting high physical activity from 27.2 to 37.0%.
Table 2. Changes in lifestyle factors from baseline questionnaire (1991/92) to follow-up questionnaire (2003).
During a median follow-up of 9.5 years, we observed 1205 of any lifestyle-related, 413 smoking-related, 885 alcohol-related, 1018 BMI-related, 849 physical activity-related and 685 breast cancers (). Among women with a lifestyle score of 0–2 in 1991/92, those who improved their lifestyle score to 3 or 4 had a lower risk of lifestyle-related cancers compared to women who did not (HR 0.81; 95% CI 0.66–0.99; proportionality assumption test p-value 0.530; ). The association was statistically significant in women aged 52–61 years in 2003 (HR 0.77; 95% CI 0.60–0.99) but not in those aged 40–51 years (HR 0.89; 95% CI 0.64–1.24; P for interaction 0.575). Among women with a favourable lifestyle in 1991/92, those who worsened their lifestyle had a similar risk of lifestyle-related cancers compared to women who did not (HR 0.98; 95% CI 0.83–1.16).
Figure 1. Lifestyle changes and cumulative incidence of lifestyle-related cancers, stratified by lifestyle at 1991/92 questionnaire. (A) Women who had a good lifestyle (score 3 or 4) at 1991/92 questionnaire. (B) Women who had a bad lifestyle (score 0, 1, or 2) at 1991/92 questionnaire. HR: hazard ratio, adjusted for age, education and menopausal status at the follow-up questionnaire; CI: confidence interval.
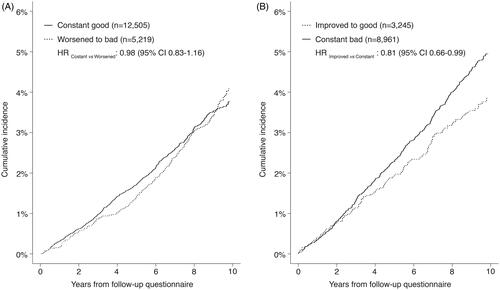
Table 3. Association between lifestyle changes and lifestyle-related cancers.
When analysing the single factors, women who quit smoking had a lower risk of smoking-related cancers compared to women who continued (HR 0.74, 95% CI 0.55–1.00; ). Women who decreased their BMI from ≥25 to <25 kg/m2 had a non- statistically significant reduction in the risk of BMI-related cancer (HR 0.64, 95% CI 0.37–1.10), but a statistically significant reduction in the risk of all lifestyle-related cancers combined (HR 0.59; 95% CI 0.35–0.99), physical activity-related cancers (HR 0.47, 95% CI 0.23–0.95) and breast cancer (HR 0.36, 95% CI 0.15–0.88). When we analysed weight trends, women who reduced their weight by more than 5%, compared to women with stable weight (i.e., with changes within 5%), had a reduced risk of BMI-related cancers (HR 0.65; 95% CI 0.46–0.91) as well as lifestyle-related cancers (HR 0.75; 95% CI 0.56–1.00), alcohol-related cancers (HR 0.53; 95% CI 0.36–0.78), physical activity-related cancers (HR 0.51, 95% CI 0.34-0.78) and breast cancer (HR 0.49, 95% CI 0.31–0.78). When we censored breast cancer cases instead of counting them as events, we no longer observed a protective effect of weight loss on the risk of lifestyle-related cancers (n = 520; HR 1.05; 95% CI 0.87–1.27), alcohol-related cancers (n = 200; HR 0.94; 95%CI 0.70–1.27), BMI-related cancers (n = 333; HR 1.12; 95% CI 0.88–1.41) and physical activity-related cancers (n = 164; HR 1.03; 95% CI 0.74–1.43). No statistically significant association were observed when we used ±10% instead of ±5% to define weight trends (data not shown). Changes in alcohol consumption and physical activity were not associated with cancer risk. For all the single-factor models, proportionality of hazards was not violated.
In a sensitivity analysis, we included women with incomplete information on lifestyle changes, and results did not change substantially. The only notable change was that the association between smoking cessation and the risk of smoking-related cancer was fully statistically significant (HR 0.72, 95% CI 0.54–0.96). Finally, adjusting the multivariable analyses for history of hypertension, history of increased levels of cholesterol or triglyceride and current diagnosis of diabetes did not change the results.
Discussion
In this large cohort of women in their 30–50s, we observed that women who improved their lifestyle in a 12-year interval lowered their subsequent risk of lifestyle-related cancers compared to those who did not. In particular, the risk of smoking-related cancers was reduced in women who quit smoking compared to women who continued smoking, and the risk of breast cancer was reduced in women who lost weight compared to women who did not. On the other side we did not observe an increased risk of lifestyle-related cancers in women who changed to a more unfavourable lifestyle.
Smoking cessation has been shown to reduce the risk of not only lung cancer [Citation7,Citation26], but also other cancer types such as oesophageal squamous cell carcinoma [Citation27] pancreatic cancer [Citation28,Citation29], head and neck and gastric cancer [Citation30], colorectal cancer [Citation31], and bladder cancer [Citation32]. While smoking cessation rapidly reduces the risk of lung cancer, it is estimates that it reduces the risk of pancreatic and head and neck cancers only after 10 years, and the risk of colorectal, gastric and bladder cancers only after 20 years [Citation30–32]. While smoking increases the risk of breast cancer, cessation is not established to reduce its risk significantly [Citation30,Citation33]. We could not observe any effect of smoking cessation on breast cancer risk, while a beneficial effect was visible for the group of smoking-related cancers.
Reduction in body weight in adult age has been found associated with a decreased risk of endometrial [Citation13] and breast cancer [Citation34]. We found that weight loss was associated with a reduction of risk of BMI-related, alcohol-related, physical activity-related as well as all lifestyle-related cancers. However, these associations were driven by the effect of weight loss on breast cancer risk. This might be due to a real lack of effect on cancers other than breast cancer, but also due to a statistical power issue, as the majority of cancer cases in this population are breast cancer. Substantial weight gain may double the risk of ER + PR + breast cancer, while it has less effect on ER-PR- breast cancer [Citation12,Citation35]. It has also been reported that weight gain might increase the risk of postmenopausal breast cancer and postmenopausal endometrial cancer in those who do not use hormone therapy, and postmenopausal ovarian cancer in non- and low-users of hormone therapy [Citation36]. CRC risk is increased by adult weight gain [Citation11,Citation36,Citation37] while there is no evidence that adult weight loss reduces the risk [Citation11]. In the present study, we did not observe an increase in risk of cancer groups or breast cancer with weight gain, and numbers were too small to analyse single cancer types other than breast cancer.
There is evidence that increase in alcohol intake in adult age increases the risk of postmenopausal breast cancer [Citation14], while cessation of alcohol consumption in adult age may reduce the risk of head and neck cancers after a minimum of 20 years cessation time [Citation15,Citation16] and oesophageal cancer after a minimum of 16 years cessation time [Citation38]. It is still unclear whether alcohol cessation may reduce the risk of liver and stomach cancers [Citation17,Citation18]. In a previous study carried out in the present population, alcohol consumption was found to be not associated with mortality of alcohol-related or other cancers [Citation39]. In this study, we did not find any association between changes in alcohol consumption and risk of any lifestyle related cancers. This may be explained by the low average alcohol consumption and modest 12-year changes in this population.
The literature on the effect of increasing in physical activity level in adult age on cancer risk is restricted to one study in Norwegian men, which showed a beneficial effect on cancer incidence [Citation9], and a study in a large cohort of UK men and women, which showed a beneficial effect on cancer mortality [Citation8]. In the present study we did not observe any association between change in physical activity level and cancer risk. One possible explanation is that the assessment of physical activity at baseline and follow-up questionnaires was too simplistic and this led to a non-optimal assessment of changes in physical activity levels.
Our earlier study with a very long-term follow-up time of Norwegian men suggested that beneficial changes in lifestyle have an effect on reduced cancer risk only after 18 years of follow-up [Citation20]. The follow-up time in the present cohort of Swedish women may have been too short to show a full effect. We found that the worsening of lifestyle, from favourable to unfavourable, was not associated with an increased risk of cancer. According to the above cited literature, this is unlikely. A longer follow-up period is probably needed to observe an effect of unhealthy changes. We are unaware of other studies examining changes in a score combining several modifiable risk factors, and cancer risk.
This follow-up study in the Swedish Women's Lifestyle and Health cohort is one of the few existing studies on the effect of multifactorial lifestyle changes on cancer risk. The strength of this study is the large population-based recruitment of women and a high number of lifestyle-related cancers during the follow-up, which allowed the combination of cancers in groups based on different risk factors. Data on incident cancers was virtually complete, and a set of sensitivity analyses confirmed the robustness of our findings.
Limitations of the study include that our results might be affected by the healthy volunteer bias, as non-respondents of follow-up questionnaire reported higher prevalence of smoking, fewer years of education, and less physical activity at baseline [Citation21]. The follow-up period was relatively short, and the majority of women did not reach the age at which cancer risk significantly increases. The lifestyle data was self-reported, which covers high probability for response bias. The data was not suitable to be fitted into standardised and validated lifestyle scores which are known to predict cancer risk. Some of the lifestyle changes were relatively rare, i.e., only 3% of the non-smoking women in 1991/2 started to smoke, and 8% of the overweight or obese women lost weight during the follow-up. Also, assessment of change in physical activity had limitations due to different scales used in 1991/92 and 2003 questionnaires. Our data did not allow any detailed adjustment for potential time varying confounding, e.g., changes in comorbid condition. Finally, measurement of changes in lifestyle using only two time-points does not capture the complexity of the real lifestyle changes, such as cyclical changes.
Conclusion
In conclusion, this study suggests that women in their 30–50s can improve their lifestyle to lower their risk of cancer. In particular, our results suggest that smoking cessation might lower the risk of smoking-related cancers and reduction in weight might lower the risk of breast cancer, which is the most common cancer in women. Our study supplies important evidence in support of recommendations of lifestyle changes and interventions in primary health care.
Author contributions
EB and PB searched the literature, initiated the study and drafted the manuscript. EB and SS conducted the analyses. EW and SS initiated and organised the study cohort. All authors reviewed the manuscript for important intellectual content and approved the final version to be published.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Behrens G, Fischer B, Kohler S, et al. Healthy lifestyle behaviors and decreased risk of mortality in a large prospective study of U.S. women and men. Eur J Epidemiol. 2013;28(5):361–372.
- Kvaavik E, Batty GD, Ursin G, et al. Influence of individual and combined health behaviors on total and cause-specific mortality in men and women: the United Kingdom health and lifestyle survey. Arch Intern Med. 2010;170(8):711–718.
- Loef M, Walach H. The combined effects of healthy lifestyle behaviors on all cause mortality: a systematic review and meta-analysis. Prev Med. 2012;55:163–170.
- Nyberg ST, Singh-Manoux A, Pentti J, et al. Association of healthy lifestyle with years lived without major chronic diseases. JAMA Intern Med. 2020;180(5):760.
- McKenzie F, Biessy C, Ferrari P, et al. Healthy lifestyle and risk of cancer in the European prospective investigation into cancer and nutrition cohort study. Medicine. 2016;95:e2850.
- World Cancer Research Fund International. WCRF: diet, nutrition, physical activity and cancer: a global perspective. The third expert report; 2018. https://www.wcrf.org/dietandcancer/cancers.
- Peto R, Darby S, Deo H, et al. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ. 2000;321:323–329.
- Mok A, Khaw KT, Luben R, et al. Physical activity trajectories and mortality: population based cohort study. BMJ. 2019;365:l2323.
- Robsahm TE, Heir T, Sandvik L, et al. Changes in midlife fitness, body mass index, and smoking influence cancer incidence and mortality: a prospective cohort study in men. Cancer Med. 2019;8:4875–4882.
- Raglan O, Kalliala I, Markozannes G, et al. Risk factors for endometrial cancer: an umbrella review of the literature. Int J Cancer. 2019;145:1719–1730.
- Karahalios A, English DR, Simpson JA. Weight change and risk of colorectal cancer: a systematic review and meta-analysis. Am J Epidemiol. 2015;181:832–845.
- Chan DSM, Abar L, Cariolou M, et al. World Cancer Research Fund International: continuous update project-systematic literature review and meta-analysis of observational cohort studies on physical activity, sedentary behavior, adiposity, and weight change and breast cancer risk. Cancer Causes Control. 2019;30:1183–1200.
- Zhang X, Rhoades J, Caan BJ, et al. Intentional weight loss, weight cycling, and endometrial cancer risk: a systematic review and meta-analysis. Int J Gynecol Cancer. 2019;29:1361–1371.
- Dam MK, Hvidtfeldt UA, Tjonneland A, et al. Five year change in alcohol intake and risk of breast cancer and coronary heart disease among postmenopausal women: prospective cohort study. BMJ. 2016;353:i2314.
- Ahmad Kiadaliri A, Jarl J, Gavriilidis G, et al. Alcohol drinking cessation and the risk of laryngeal and pharyngeal cancers: a systematic review and meta-analysis. PLoS One. 2013;8:e58158.
- Marron M, Boffetta P, Zhang ZF, et al. Cessation of alcohol drinking, tobacco smoking and the reversal of head and neck cancer risk. Int J Epidemiol. 2010;39:182–196.
- Heckley GA, Jarl J, Asamoah BO, et al. How the risk of liver cancer changes after alcohol cessation: a review and meta-analysis of the current literature. BMC Cancer. 2011;11:446.
- Jarl J, Heckley G, Brummer J, et al. Time characteristics of the effect of alcohol cessation on the risk of stomach cancer–a meta-analysis. BMC Public Health. 2013;13:600.
- Prentice RL, Thomson CA, Caan B, et al. Low-fat dietary pattern and cancer incidence in the women's health initiative dietary modification randomized controlled trial. J Natl Cancer Inst. 2007;99:1534–1543.
- Botteri E, de Lange T, Tonstad S, et al. Exploring the effect of a lifestyle intervention on cancer risk: 43-year follow-up of the randomized Oslo diet and antismoking study. J Intern Med. 2018;284:282–291.
- Roswall N, Sandin S, Adami HO, et al. Cohort profile: The Swedish women's lifestyle and health cohort. Int J Epidemiol. 2017;46:e8.
- Yumuk V, Tsigos C, Fried M, et al. European guidelines for obesity management in adults. Obes Facts. 2015;8:402–424.
- Humans IWGotEoCRt. Personal habits and indoor combustions. Volume 100 E. A Review of Human Carcinogens. IARC Monogr Eval Carcinog Risks Hum. 2012;100:1–538.
- Weiderpass E, Baron JA, Adami HO, et al. Low-potency oestrogen and risk of endometrial cancer: a case-control study. Lancet. 1999;353:1824–1828.
- El Reda D, Strom P, Sandin S, et al. Determinants of long-term weight change among middle-aged Swedish women. Obesity. 2017;25:476–485.
- Rose G, Colwell L. Randomised controlled trial of anti-smoking advice: final (20 year) results. J Epidemiol Community Health. 1992;46:75–77.
- Wang QL, Xie SH, Li WT, et al. Smoking cessation and risk of esophageal cancer by histological type: systematic review and meta-analysis. J Natl Cancer Inst. 2017;109(12):djx115.
- Lugo A, Peveri G, Bosetti C, et al. Strong excess risk of pancreatic cancer for low frequency and duration of cigarette smoking: a comprehensive review and meta-analysis. Eur J Cancer. 2018;104:117–126.
- Zou L, Zhong R, Shen N, et al. Non-linear dose-response relationship between cigarette smoking and pancreatic cancer risk: evidence from a meta-analysis of 42 observational studies. Eur J Cancer. 2014;50:193–203.
- Ordonez-Mena JM, Schottker B, Mons U, et al. Quantification of the smoking-associated cancer risk with rate advancement periods: meta-analysis of individual participant data from cohorts of the CHANCES consortium. BMC Med. 2016;14:62.
- Botteri E, Borroni E, Sloan E, et al. Smoking and colorectal cancer risk, overall and by molecular subtypes: a meta-analysis. Am J Gastroenterol.2020;115(12):1940–1949.
- van Osch FH, Jochems SH, van Schooten FJ, et al. Quantified relations between exposure to tobacco smoking and bladder cancer risk: a meta-analysis of 89 observational studies. Int J Epidemiol. 2016;45:857–870.
- Gaudet MM, Gapstur SM, Sun J, et al. Active smoking and breast cancer risk: original cohort data and meta-analysis. J Natl Cancer Inst. 2013;105:515–525.
- Hardefeldt PJ, Penninkilampi R, Edirimanne S, et al. Physical activity and weight loss reduce the risk of breast cancer: a meta-analysis of 139 prospective and retrospective studies. Clin Breast Cancer. 2018;18(4):e601–e612.
- Vrieling A, Buck K, Kaaks R, et al. Adult weight gain in relation to breast cancer risk by estrogen and progesterone receptor status: a meta-analysis. Breast Cancer Res Treat. 2010;123(3):641–649.
- Keum N, Greenwood DC, Lee DH, et al. Adult weight gain and adiposity-related cancers: a dose-response meta-analysis of prospective observational studies. J Natl Cancer Inst. 2015;107(2):djv088.
- Schlesinger S, Lieb W, Koch M, et al. Body weight gain and risk of colorectal cancer: a systematic review and meta-analysis of observational studies. Obes Rev. 2015;16:607–619.
- Jarl J, Gerdtham UG. Time pattern of reduction in risk of oesophageal cancer following alcohol cessation–a meta-analysis. Addiction. 2012;107:1234–1243.
- Licaj I, Sandin S, Skeie G, et al. Alcohol consumption over time and mortality in the Swedish women's lifestyle and health cohort. BMJ Open. 2016;6:e012862.
