Abstract
Aim/objectives
This study aimed to systematically review the literature on the impact of delay in diagnosis and treatment of oral cavity cancer.
Methods
PubMed and Embase were systematically searched for articles reporting impact of delay in diagnosis and treatment on cancer-stage and survival of oral cavity cancer. Studies comprising at least ten patients, and published since the year 2000, were included.
Results
Sixteen studies (n = 45,001, range: 62–18,677 per study, 83% men), from Australia, Asia, Europe, North America and South America, met the inclusion criteria. Eleven studies (n = 1,460) examined delay in diagnosis, while five studies (n = 43,541) reported delay in treatment. Eight of the eleven studies, examining delay in diagnosis (n = 1,220), analyzed the correlation between delay in diagnosis and tumor stage at diagnosis. Three studies found a significant correlation between patient delay and advanced stage at diagnosis (p < 0.05), whereas three other studies did not. The studies reporting a significant correlation were from Asian countries, whereas the three studies that did not find a correlation were from other continents. Studies reporting on professional delay and total diagnostic delay, generally, did not find a significant correlation with advanced cancer at diagnosis. Time to treatment (TTI), defined as time from diagnosis to treatment, was found significantly correlated with survival in three studies (p < 0.01, p < 0.001, p < 0.05), and nonsignificant in two studies.
Conclusion
A significant correlation between patient delay and advanced stage cancer was reported in Asian studies only, while professional delay and total diagnostic delay were generally found to be non-correlated with advanced stage cancer at diagnosis. TTI was in some studies reported to be correlated with poorer outcome, while other studies did not report a correlation. One study presented that there was no clear advantage in overall survival (OS) for patients treated within 30 days, compared to patients treated between 30 and 44 days.
Introduction
During the past decades, the incidence of oral cancer has been increasing in Western Europe, including Denmark, while the incidence has decreased in USA and Canada [Citation1–3]. Above 90% of these tumors are squamous cell carcinomas [Citation1]. The risk of developing oral cancer is associated with tobacco smoking, alcohol consumption, betel quid chewing and age [Citation2]. Moreover, multiple studies report men having considerably higher incidence of oral cavity cancer than women [Citation1,Citation4].
Delay in diagnosis is often divided into patient delay/primary delay and professional delay/secondary delay. Patient delay is often defined as the time from the onset of symptoms to the patient’s first professional visit, whereas professional delay is usually defined as the time from the first professional visit to the final diagnosis, however variations of these definitions occur [Citation5,Citation6]. A prolonged delay in diagnosis can thus be caused by the patient and/or by the professional service establishing the diagnosis. Delay in treatment is typically defined as the time from the date of diagnosis to the date when curative therapy is commenced, and is often referred to as time to treatment initiation (TTI) [Citation5,Citation7].
Traditionally, cancer stage at diagnosis is determinant in prognosis and in selection of treatment modalities [Citation1]. Delay in diagnosis and prolonged TTI has been reported to be associated with advanced stage at diagnosis as well as poor survival outcome in head and neck cancer [Citation5,Citation7,Citation8]. Longer TTI is often caused by comorbidity as well as more complex treatment planning, as demanded by advanced stage oral cancer [Citation9,Citation10]. However, only few studies have investigated delay in diagnosis and TTI specifically for oral cavity cancer patients.
The aim of this study is to systematically review the literature on the impact of delay in diagnosis and treatment of oral cavity cancer.
Methods and materials
In January 2021, one author (BBL) systematically searched PubMed and Embase for articles published in English regarding oral cavity cancer and impact of delay in diagnosis as well as impact of the time from diagnosis to treatment. The review was conducted according to PRISMA guidelines [Citation11]. PubMed and Embase were searched using the following keywords: oral, cavity, mouth, cancer, carcinoma, squamous cell carcinoma, tumor, malignancy, neoplasms, and cancer of mouth combined with delay, treatment delay, waiting time and time to treatment initiation. The Mesh Terms carcinoma, neoplasm, and mouth neoplasms were used in the PubMed search as well. See Supplementary for the detailed search strategy.
Studies with a minimum of ten patients reporting data on the impact of delay in diagnosis and/or treatment initiation of primary oral cavity cancer were included. Studies reporting on head and neck cancers in general, were only included if they presented data on oral cavity cancer. Systematic reviews, meta-analyses, conference abstracts as well as studies not reporting data specifically on oral cavity cancer were excluded. Studies published before the year 2000 were also excluded.
Results
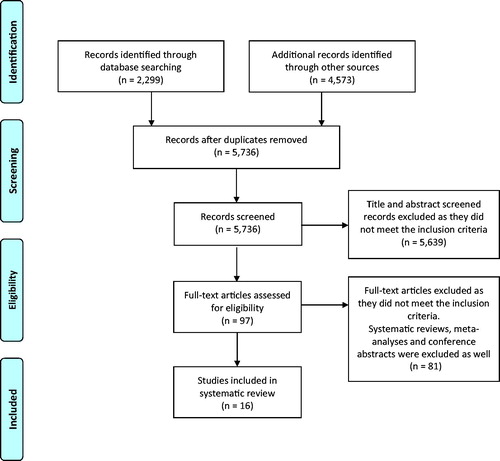
The PubMed and Embase search generated a total of 5,736 articles after removal of duplicates. Sixteen articles met the inclusion criteria, as shown in [Citation12–27].
Overall, the studies reported data on 45,001 oral cavity cancer patients (range: 62–18,677 patients per study) representing fourteen countries: Finland (n = 62), Spain (n = 63 and n = 88), China (n = 77), India (n = 79), Australia (n = 100), Iran (n = 100), Thailand (n = 161), Brazil (n = 180), England (n = 245), Poland (n = 305), Italy (n = 462), Denmark (n = 862), the USA (n = 4,868 and n = 18,672) and Taiwan (n = 18,677). The majority of patients were men (weighted average = 83%, ), and the mean age at diagnosis ranged from 48.8 to 63.0 years.
Table 1. Study characteristics: inclusion period, patient age and definition.
Delay in diagnosis
Eleven of the studies (n = 1,460) reported impact of delay in diagnosis of oral cavity cancer. Eight of them (n = 1,220) examined correlation between delay in diagnosis and tumor stage at diagnosis [Citation12,Citation14,Citation15,Citation19–21,Citation24,Citation25], while three studies (n = 240) examined impact on overall survival (OS) [Citation16,Citation26,Citation27].
Among the studies investigating diagnostic delay and cancer stage, six reported on patient delay and four on professional delay. The definition of patient delay was similar in all six of the studies reporting on this: the time from the onset of symptoms to the first professional visit [Citation12,Citation19–21,Citation24,Citation25]. Three of these studies, from Thailand, India and Iran, found that patient delay was significantly correlated with advanced stage at diagnosis (Kerdpon et al., p = 0.02; Kumar et al., p = 0.0121; Sargeran et al., p < 0.05), whereas three other studies, from Brazil, Finland and Poland, presented a nonsignificant correlation (Abdo et al., p = 0.24; Rutkowska et al., p > 0.05; Teppo et al., p-value not specified) [Citation12,Citation20,Citation21,Citation24,Citation25]. Regarding professional delay, the included studies investigating this had two different definitions: (A) the time from the first professional visit to the final diagnosis [Citation24,Citation25]; and (B) the time from the first professional visit to the first consultation with a treating specialist [Citation20,Citation21]. Three of the studies (n = 302) reported that professional delay did not significantly correlate with advanced stage at diagnosis (Kumar, p = 0.57; Kerdpon et al. and Teppo et al., p-values not specified) [Citation20,Citation21,Citation25]. Only an Iranian study, by Sargeran et al. (n = 100), suggested that advanced stage at diagnosis was determinant of professional delay (i.e., more than one month; p < 0.05) [Citation24].
Four studies, from Australia, England, Spain and Thailand (n = 594), reported that the total time of diagnostic delay (i.e., the sum of patient and professional delay) did not significantly correlate with tumor stage/advanced cancer, while the Iranian study by Sargeran et al. (n = 100) reported that total diagnostic delay had significant correlation with an advanced stage (Sargeran et al., p < 0.01, delay >4 months) [Citation14–16,Citation20,Citation24].
Furthermore, three studies (n = 240) investigated the relationship between total diagnostic delay and OS [Citation16,Citation26,Citation27]. A study from China by Tong et al. found that diagnostic delay of more than two months had a negative impact on OS (Tong, p = 0.03), whereas a study from Australia by Thomas et al., as well as a study from Spain by Seoane et al., did not find any significant association between OS and diagnostic delay (Thomas et al.: cut off point for delay = 85 days, p = 0.48; Seoane et al.: cut off point for delay = 1.5 months, p = 0.74) [Citation16,Citation26,Citation27].
Time to treatment initiation
The impact of TTI was examined in five of the included studies (n = 43,541) [Citation13,Citation17,Citation18,Citation22,Citation23]. Four studies categorized TTI in different interval-groups to examine the correlation with mortality [Citation13,Citation18,Citation22,Citation23].
The Italian study by Polesel et al. examined time from diagnosis to treatment initiation and its correlation with OS. The study found that correlation between prolonged TTI and OS was statistically significant. This study, along with a study from Taiwan by Liao et al. (n = 19,139), found that OS significantly decreased with increased TTI (Liao, p < 0.001; Polesel et al., p < 0.01) [Citation22,Citation23]. It is noteworthy that the Italian study found that there was no clear advantage in OS for patients treated within 30 days, compared to patients treated between 30 and 44 days [Citation23]. The US study by Rygalski et al. (n = 18,672) also reported a significant correlation for TTI of 61 to 90 days, and longer than 90 days, compared with 0–30 days. The study moreover found that HRs were further reduced for TTI after adjustment for additional covariates [Citation18]. Contrary to this, the US study by Fujiwara et al. (n = 4,868) reported a nonsignificant trend toward decreased OS with increased TTI (p = 0.055) [Citation13]. In this study, patients were considered delayed when the definitive surgery was performed later than 45 days after diagnosis was established. The Danish study by Jensen et al. (n = 862) also reported the effect of TTI as non-statistically significant when adjusting for age, sex, smoking, UICC stage, tumor sublocation, and CCI in multivariate analyses [Citation17]. Moreover, two of the studies (n = 23,545) reported that age had an impact on TTI for oral cavity cancer patients; Fujiwara et al. reported that oral cavity cancer patients >60 years were significantly more likely to have a longer TTI, and Liao et al. found that patients with a TTI shorter than 20 days had the lowest age at diagnosis [Citation13,Citation22].
Discussion
By classifying diagnostic delay according to patient delay and professional delay, different aspects can be examined through categorization and comparison. Three of the included studies (n = 340) reported that patient delay correlated with advanced stage cancer, whereas three other studies found no such correlation. Notably, all three studies reporting the correlation were from Asia (Thailand, India and Iran), whereas the other studies were not (n = 547, ) [Citation12,Citation19–21,Citation24,Citation25]. It is currently known that buccal cancer is more common among Asian populations due to betel quid/tobacco chewing habits [Citation2]. The study from Thailand suggests that a health campaign should be implemented, thus increasing the population’s awareness of early signs or symptoms of oral cancer and the limitation of traditional herbal medication as a treatment option [Citation20]. Multiple studies, moreover, present that dental visits have a positive impact on the detection of early-stage oral cancer. The studies report that tumors discovered accidentally tend to be smaller, compared to those found by patients themselves [Citation15,Citation28]. In a study from Iran, Sargeran et al. found that a determinant of patient delay is the patient not having visited a clinician within the past 12 months [Citation24]. This relates to a socioeconomic and healthcare aspect, as it prevents some patients from seeking health care when symptoms first appear [Citation26,Citation28]. Moreover, multiple studies report that educating the population should be prioritized [Citation14,Citation25,Citation28,Citation29]. An English study by Scott et al. reported that some patients did not consider their symptoms to be indicative of cancer. Instead, they were being interpreted as minor oral conditions such as an ulcer, trauma from dentures or a burn from hot food or drink [Citation30]. Even with free health care, as in England, some patients in the study did not find their symptoms severe enough to consult a health care professional. This indicates that there could be value in educating people regarding disease presentation and the importance of visiting a health care professional as soon as oral symptoms develop.
Table 2. Impact of delay in diagnosis and treatment initiation – results study by study.
Three of the included studies found a correlation between patient delay and advanced stage cancer [Citation20,Citation21,Citation24], however the studies reporting on professional delay [Citation20,Citation21,Citation25] generally did not find such correlation. Only one study from Iran (n = 100) found a correlation between professional delay and advanced stage cancer. This study defined professional delay as the time from the first professional visit to the final diagnosis [Citation24]. This is opposed to the two other studies, which defined professional delay as the time from first professional visit to the first consultation with the treating specialist [Citation20,Citation21]. The different definitions among the included studies make it difficult to compare the research results one to one, and this might explain the differences found in the significance of correlations. Moreover, it is inevitable that the data collection in the included studies vary. Four of the studies reporting on patient and professional delay adapted an approach of using a structured questionnaire [Citation15,Citation20,Citation21,Citation24]. On the other hand, the study from Finland and the study from Poland, searched data from patients’ medical records (Teppo et al. included dental records as well) [Citation19,Citation25]. The date of the onset of cancer symptoms was derived from these unbiased records, which were prepared at the time of the initial visit [Citation25]. Multiple studies investigated the correlation between tumor aggressiveness and delay by dividing the patients in two groups: one group of early stage patients and one group of late stage patients. The early stage included UICC stage I and II while the late stage included UICC stage III and IV [Citation12,Citation14–16,Citation19,Citation20,Citation24,Citation25,Citation27].
The combination of patient and professional delay represents the total diagnostic delay [Citation20]. One of the included studies (n = 100) found a correlation between total diagnostic delay and advanced stage cancer, while three of the included studies (n = 494) did not [Citation14,Citation15,Citation20,Citation24]. One study explains this by suggesting that the tumor might be ‘silent’ and the time of onset of symptoms may not correlate well with the true onset of disease [Citation15]. To detect oral cavity cancer in early stages, Seoane et al. proposed that the focus should be on screening programs, thus detecting disease during asymptomatic phases [Citation27]. Screening programs have already been implemented in countries such as Denmark where dentists are obligated to check the patients’ mucosa and forward patients with mouth ulcers to a head and neck department [Citation31,Citation32]. General practitioners in Denmark also have a screening obligation to forward patients to a specialist in case a mouth ulcer has been reported as being older than two weeks [Citation33]. Moreover, professional training is thought to be important in detecting early-stage cancer. The study from Thailand by Kerdpon et al. found that about half of the patients were mismanaged on their first consultation and therefore suggests that professional training to recognize potentially malignant lesions should be prioritized [Citation20].
Regarding total diagnostic delay, correlation with OS was examined as well. A study from China by Tong et al. found that there was correlation between 2-year OS and diagnostic delay of more than two months (p = 0.03), while a study from Australia and a study from Spain did not report a significant correlation [Citation16,Citation26,Citation27]. Moreover, a nonsignificant trend between delay in diagnosis and OS was reported in a study of head and neck cancers in general, by McGurk et al. [Citation34]. It could seem paradoxical that delay in diagnosis and OS is reported as noncorrelated, but the ‘silent’ tumor theory and tumor growth velocity (tumor aggressiveness) can likely both be explanations for this. This was presented in a study from Spain reporting on oral and oropharyngeal cancers [Citation35]. The study found a U-shaped curve in the correlation between total diagnostic delay and mortality: patients with short or long diagnostic delay had a higher mortality than those with medium delays. As the biology differs from tumor to tumor, patients with fast-growing tumors and rapid progression would demand shorter delay than those with slower growing ones [Citation35]. It is noteworthy that some tumors might be aggressive, while others are “slow”, independent of size. This, however, contradicts the TNM-classification by UICC, which is currently adopted as a unified standard to classify cancer by anatomic disease extent, thus providing an indication of prognosis [Citation36]. The three studies on total diagnostic delay focused on the time-period prior to the day of diagnosis and did not address the adjuvant therapy as a part of the analysis [Citation16,Citation26,Citation27]. However, it could be interesting to investigate further, whether the duration of primary therapy and delays in adjuvant treatment could be of importance. The question of adjuvant therapy has already been addressed in a study by Chen et al., who found it to be independently associated with improved OS for head and neck cancers [Citation37].
In general, recent studies have shown that the incidence of oral cavity cancer has increased during the past decades in Western Europe [Citation2,Citation3]. This results in a greater pressure on the healthcare system with a putative impact on the time from diagnosis to treatment. Notably, the treatment option could impact the length of TTI. The two studies from the USA (Fujiwara et al. n = 4,868 and Rygalski et al. n = 18,672) reported data on the time between diagnosis and surgery (with or without postoperative radiotherapy (RT) and adjuvant chemotherapy (Rygalski et al. only)), whereas the study from Italy and the study from Taiwan reported data on the time from diagnosis to RT, surgery or chemotherapy (radical concomitant radio-chemotherapy or neoadjuvant chemotherapy followed by RT, in the study from Italy) [Citation13,Citation22,Citation23]. The patients in the Danish study by Jensen et al. were either treated with surgery (with or without postoperative radiotherapy (RT)) or radiotherapy as primary treatment. Notably, the treatment option could impact the length of TTI. The Danish study by Jensen et al. and the Italian study by Polesel et al. both found that patients with cancer of the oral cavity had a shorter TTI when the definitive treatment was surgery alone [Citation17,Citation23]. More than 40% of the patients treated with RT in the Italian study had a TTI longer than 90 days [Citation23]. The study found that treatment planning for RT required time for accurate staging and definition of therapeutic plan. Moreover, RT devices were only available in research/academic institutes, thus resulting in waiting lists being quite long (approximately 3 weeks) [Citation23].
Multiple studies found that TTI correlated with OS [Citation18,Citation22,Citation23]. Rygalski et al. reported that patients with a TTI of more than 67 days had a worse overall survival than patients with a TTI <67 days. Therefore, the study suggests that all reasonable efforts should be made to expedite primary surgery. Interestingly, the Italian study by Polesel et al. concluded that there was no significant difference in OS for patients treated between 30 and 44 days, compared with those treated within 30 days [Citation23]. Similar findings were also reported in a study by Murphy et al., who investigated over 50,000 head and neck cancer patients in the USA [Citation5]. The study overall found that patients with a TTI of greater than 46 to 52 days had an increased risk of mortality, though, it is notable that the study reported that a TTI of 31 to 60 days did not result in a higher risk of death compared with a TTI of 0 to 30 days. Moreover, the Danish study by Jensen et al. reported that the effect of TTI was found non-statistically significant in multivariate analyses [Citation17]. Healthcare systems might thus be able to prolong TTI within a reasonable timeframe, without affecting outcome. Further, a Danish study on HPV positive and negative oropharyngeal squamous cell carcinoma patients reported that only a TTI greater than 60 days significantly predicted a higher risk of death (HPV negative patients; p = 0.03) [Citation7]. This suggests that prolonged TTI, in most cases, is found correlated with OS, though the important determinant is the number of days. Currently, different practices are adopted for reducing delay. In 2007, the Danish government decided that all cancer patients should be diagnosed and treated without delay by introducing the fast track accelerated clinical pathway. The diagnostic package for head and neck cancer was developed in collaboration with the Danish Head and Neck Cancer Group (DAHANCA), and the standards of the fast track pathway includes: 21 calendar days for diagnosis; seven days for planning of surgery; 11 days for planning radiotherapy; and therefore a total of 28 or 32 calendar days from suspicion of cancer to initiation of surgery or radiotherapy, thus ensuring the patient a shorter TTI [Citation38,Citation39]. The fast-track program aims at ensuring that all patients are treated as efficiently as possible independent of the stage at diagnosis.
In conclusion, this review found that patient delay only significantly correlated with advanced stage cancer in Asian studies, whereas the remaining studies found no correlation. Professional delay and total diagnostic delay were mostly found noncorrelated with advanced stage cancer. TTI was generally reported to be correlated with OS, but some studies did not report on such correlation. One study found that there was no clear advantage in OS for patients treated within 30 days compared to patients treated between 30 and 44 days. Efforts preventing TTI from becoming too prolonged is hereby preferred. The optimal length of TTI, for both the patient and the professionals, is still to be discussed.
Supplemental Material
Download MS Word (13.3 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Lambert R, Sauvaget C, De Camargo Cancela M, et al. Epidemiology of cancer from the oral cavity and oropharynx. Eur J Gastroenterol Hepatol. 2011;23(8):633–641.
- Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45(4–5):309–316.
- Karnov KKS, Grønhøj C, Jensen DH, et al. Increasing incidence and survival in oral cancer: a nationwide Danish study from 1980 to 2014. Acta Oncol. 2017;56(9):1204–1209.
- Simard EP, Torre LA, Jemal A. International trends in head and neck cancer incidence rates: differences by country, sex and anatomic site. Oral Oncol. 2014;50(5):387–403.
- Murphy CT, Galloway TJ, Handorf EA, et al. Survival impact of increasing time to treatment initiation for patients with head and neck cancer in the United States. J Clin Oncol. 2016;34(2):169–178.
- Guggenheimer J, Verbin RS, Johnson JT, et al. Factors delaying the diagnosis of oral and oropharyngeal carcinomas. Cancer. 1989;64(4):932–935.
- Grønhøj C, Jensen D, Dehlendorff C, et al. Impact of time to treatment initiation in patients with human papillomavirus-positive and -negative oropharyngeal squamous cell carcinoma. Clin Oncol (R Coll Radiol). 2018;30(6):375–381.
- Schutte HW, Heutink F, Wellenstein DJ, et al. Impact of time to diagnosis and treatment in head and neck cancer: a systematic review. Otolaryngol Head Neck Surg. 2020;162(40):446–457.
- Van Harten MC, Hoebers FJP, Kross KW, et al. Determinants of treatment waiting times for head and neck cancer in the Netherlands and their relation to survival. Oral Oncol. 2015;51(3):272–278.
- DAHANCA, Dansk Selskab for Hoved- og Hals Onkologi; DSHHO. Behandling af planocellulaert karcinom i mundhulen Nationale retningslinjer [Treatment Plan of Oral Cavity Squamous Cell Carcinoma, National Guidelines]. 2016 [cited 2021 May 19]. Available from: https://www.dahanca.dk/CA_Adm_Web_Page?WebPageMenu=1&CA_Web_TabNummer=0
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269.
- Abdo EN, A de A G, Barbosa AA, et al. Time elapsed between the first symptoms, diagnosis and treatment of oral cancer patients in Belo Horizonte, Brazil. Med Oral Patol Oral Cir Bucal. 2007;12:469–473.
- Fujiwara RW, Cadoni G, et al. Treatment delays in oral cavity squamous cell carcinoma and association with survival. Head Neck. 2014;36:1391.
- Seoane-Romero JM, Vázquez-Mahía I, Seoane J, et al. Factors related to late stage diagnosis of oral squamous cell carcinoma. Med Oral. 2012;17(1):e35–e40.
- Scott SE, Grunfeld EA, McGurk M. The idiosyncratic relationship between diagnostic delay and stage of oral squamous cell carcinoma. Oral Oncol. 2005;41(4):396–403.
- Thomas A, Manchella S, Koo K, et al. The impact of delayed diagnosis on the outcomes of oral cancer patients: a retrospective cohort study. Int J Oral Maxillofac Surg. 2020;50(5):585–590.
- Jensen JS, Jakobsen KK, Mirian C, et al. Impact of time to treatment initiation for patients with oral cavity squamous cell carcinoma: a population-based, retrospective study. Acta Oncol . 2020;60(4):491–496.
- Rygalski CJ, Zhao S, Eskander A, et al. Time to surgery and survival in head and neck cancer. Ann Surg Oncol. 2021;28(2):877–885.
- Rutkowska M, Hnitecka S, Nahajowski M, et al. Oral cancer: the first symptoms and reasons for delaying correct diagnosis and appropriate treatment. Adv Clin Exp Med. 2020;29(6):735–743.
- Kerdpon D. Factors related to advanced stage oral squamous cell carcinoma in southern Thailand. Oral Oncol. 2001;37(3):216–221.
- Kumar S, Heller RF, Pandey U, et al. Delay in presentation of oral cancer: a multifactor analytical study. Natl Med J India. 2001;14:13–17.
- Liao CT, Chen HN, Wen YW, et al. Association between the diagnosis-to-treatment interval and overall survival in Taiwanese patients with oral cavity squamous cell carcinoma. Eur J Cancer. 2017;72:226–234.
- Polesel J, Furlan C, Birri S, et al. The impact of time to treatment initiation on survival from head and neck cancer in north-eastern Italy. Oral Oncol. 2017;67:175–182.
- Sargeran K, Murtomaa H, Safavi SMR, et al. Delayed diagnosis of oral cancer in Iran: challenge for prevention. Oral Health Prev Dent. 2009. 7:69–76.
- Teppo H, Alho OP. Comorbidity and diagnostic delay in cancer of the larynx, tongue and pharynx. Oral Oncol. 2009;45(8):692–695.
- Tong XJ, Shan ZF, Tang ZG, et al. The impact of clinical prognostic factors on the survival of patients with oral squamous cell carcinoma. J Oral Maxillofac Surg. 2014;72(12):2497.e1–e10.
- Seoane P, Pita-Fernández S, Gómez, I, et al. Proliferative activity and diagnostic delay in oral cancer. Head Neck. 2010;32(10):1377–1384.
- Jafari A, Najafi S, Moradi F, et al. Delay in the diagnosis and treatment of oral cancer. J Dent (Shiraz). 2013;14:146–150.
- Hollows P, Mcandrew PG, Perini MG. Delays in the referral and treatment of oral squamous cell carcinoma. Br Dent J. 2000;188:3–6.
- Scott SE, Grunfeld EA, Main J, et al. Patient delay in oral cancer: a qualitative study of patients' experiences. Psychooncology. 2006;15(6):474–485.
- Tandlaegeforeningen. Saerligt for tandlaeger [Guidelines for Dentists]; 2020 [cited 2021 Apr 11]. Available from: https://www.tandlaegeforeningen.dk/patienter/tandsygdomme-gener-og-behandlinger/l-o/mundhulekraeftcancer/for-tandlaeger/
- Tandlaegeforeningen. Saerlovgivning, som afløser Tandlaegeoverenskomsten [Special Legislation on Dental Practisioners’ Tariffs]. [cited 2021 May 19]. Available from: https://www.rstfnet.dk/Dokumenter/saerlovgivning_03092018.pdf
- SST. Notat vedr. henvisning og visitation til sygehusbehandling, med saerligt fokus på kraeft [Note on Referral and Visitation for Hospital Treatment, with Special Focus on Cancer]; 2019 [cited 2021 May 19]. Available from: https://www.sst.dk/-/media/Udgivelser/2020/Henvisning-hospital/Notat-vedr-henvisning-og-visitation-til-sygehusbehandling-med-saerligt-fokus-paa-kraeft.ashx?la=da&hash=EDE68A43192157DB53DE2BEE2A338EDCF490A537
- McGurk M, Chan C, Jones J, et al. Delay in diagnosis and its effect on outcome in head and neck cancer. Br J Oral Maxillofac Surg. 2005;43(4):281–284.
- Lopez-Cedrún JL, Varela-Centelles P, Otero-Rico A, et al. Overall time interval (“Total diagnostic delay”) and mortality in symptomatic oral cancer: a U-shaped association. Oral Oncol. 2020;104:104626.
- UICC. TNM Atlas. [cited 2021 May 18]. Available from: https://www.uicc.org/resources/tnm
- Chen MM, Roman SA, Yarbrough WG, et al. Trends and variations in the use of adjuvant therapy for patients with head and neck cancer. Cancer. 2014;120(21):3353–3360.
- Sundhedsstyrelsen. Pakkeforløb for hoved- og halskraeft; 2020 [cited 2021 May 19]. Available from: https://www.sst.dk/-/media/Udgivelser/2020/Hoved-halskræft/220620-Pakkeforloeb-for-hoved--og-halskraeft.ashx?la=da&hash=0941AA8923C1F798F4AC98B23432B0E330CBC51B
- Lyhne NM, Christensen A, Alanin MC, et al. Waiting times for diagnosis and treatment of head and neck cancer in Denmark in 2010 compared to 1992 and 2002. Eur J Cancer. 2013;49(7):1627–1633.