Abstract
Background
Inpatient rehabilitation therapy (IRT) is commonly offered to cancer patients during or after cancer treatment in Germany. However, little is known about utilization and long-term effects of this offer in colorectal cancer (CRC) patients. We aimed to assess IRT utilization, determinants of utilization and the association between IRT and survival in CRC patients.
Materials and methods
CRC patients diagnosed in 2005–2014 recruited in the population-based DACHS study in South West Germany were included. Determinants of IRT utilization were assessed by multivariable logistic regression. Hazard ratios (HRs) of the association of IRT with overall and disease-specific survival were estimated by adjusted Cox proportional hazards models. Modified landmark approach was applied to avoid immortal time biased results.
Results
Among the included CRC patients (n = 3704), 43.6% underwent IRT. Patients who did not live in a relationship with a partner, worked as employee and who reported higher levels of physical activity were more likely to undergo IRT. Patients were less likely to undergo IRT if they had private health insurance, were diagnosed with cancer stage IV, received no or laparoscopic cancer surgery or were treated in a hospital with medium vs. high surgical volume. The median follow-up time was 4.4 years (post-landmark). Utilization of IRT was associated with better overall (HR 0.81, 95% confidence interval 0.72−0.92) and disease-specific survival (HR 0.72, 95% confidence interval 0.61−0.85).
Conclusion
Almost every other CRC patient underwent IRT. Next to clinical characteristics, identified social and lifestyle characteristics seemed to play an essential role in the decision-making. Use of IRT was associated with better overall and disease-specific survival.
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer worldwide [Citation1]. Although prognosis has improved in the last decades in particular in Western countries, more than one third of the patients decease within the first five years after diagnosis [Citation2,Citation3]. In addition, the disease and its treatment can cause various temporary or permanent health impairments in survivors. Common persistent side effects after a CRC diagnosis include fatigue, reduced physical fitness, peripheral neuropathy and fecal incontinence [Citation4–7]. Somatic impairments and psychological problems such as depressive symptoms or fear of recurrence/disease progression can affect participation in all aspects of life severely [Citation8–10].
Rehabilitation therapy during or after cancer treatment aims to reduce morbidity, promote participation and improve quality of life. The access to cancer rehabilitation and the delivery models vary strongly by health care systems [Citation11,Citation12]. In contrast to very low rates of inpatient rehabilitation therapy (IRT) in most countries including the United States [Citation13,Citation14], Germany has implemented IRT as an integral part in the continuum of cancer care [Citation15]. Outpatient rehabilitation programs, on the other hand, only account for about 2% of all cancer rehabilitation measures [Citation16]. The nationwide availability and the access to IRT can be tied to the institutionalization of rehabilitation as a social state obligation that started with the introduction of Bismarck’s social legislation at the end of the nineteenth century [Citation17]. To the present day, the German pension insurance plays a significant role by funding rehabilitative measures next to health insurances and by evaluating and shaping rehabilitative care.
IRT for cancer patients is provided by specialized inpatient rehabilitation clinics as post-acute treatment directly after primary cancer treatment or as follow-up care usually within the first year after completion of treatment. The individualized and multidisciplinary 3-week programs consist of interventions such as dietetic treatment, stoma care, physical therapy/exercise, occupational therapy, patient education, and psychosocial support. CRC patients with rehabilitation needs file an application at the responsible insurance (pension or health insurance) in order to be admitted to a rehabilitation clinic. This process is usually initiated by hospital social services or treating physicians during or after primary cancer treatment.
Even though IRT is offered as standard care in Germany, information about utilization rates in CRC patients is sparse, and reasons for (non-) utilization are not well understood. Structural barriers, clinical factors and personal reasons have been discussed in this context. However, reported findings are based on study participants with other cancers [Citation18,Citation19], few or younger (<65 years) CRC-patients [Citation20,Citation21], or from secondary data on population-level [Citation22]. Moreover, no study has yet investigated associations of IRT use with long-term survival [Citation23]. Better knowledge about IRT use would help stakeholders, healthcare providers and payers to evaluate allocation of treatment and to identify underutilization among certain patients’ groups.
We aimed to assess trends and determinants of IRT utilization and its association with long-term survival in a large cohort of CRC patients from a population-based study in Southern Germany.
Materials and methods
Study design and population
In this patient cohort study, data from 4009 CRC patients diagnosed in 2005–2014 who participated in the DACHS study were included. The DACHS study (Darmkrebs, Chancen der Verhütung durch Screening) is a population-based case-control study with additional long-term follow-up of cases. Patients with a first diagnosis of CRC (C18–C20, International Classification of Diseases, revision 10), at least 30 years of age, from the Rhine-Neckar region of Germany were eligible and recruited by treating physicians from 22 participating hospitals in the study region. Written informed consent was obtained from all participants. The ethics committees of the Medical Faculty of Heidelberg and the state medical boards of Baden-Wuerttemberg and Rhineland-Palatinate approved the study.
Data collection and follow-up
Sociodemographic data, lifestyle-related information and the medical history of the participants were obtained during a face-to-face interview by trained interviewer at baseline. Tumor and surgery-specific information were extracted from discharge letters and pathology reports. About three years after diagnosis, therapy-related information was collected from medical reports and physicians administered questionnaires. General practitioners and/or oncologists, who were involved in the treatment, filled out questionnaires based on patient medical records. Follow-up time started from CRC diagnosis. Population registries provided data on vital status and date of death if applicable. Death certificates from the local health authorities were obtained in order to verify the cause of death. Further details of the DACHS study have been published elsewhere [Citation24,Citation25].
Ascertainment of IRT, potential determinants and survival outcomes
The use of IRT (yes/no) within the first three years after diagnosis was ascertained retrospectively next to the other therapy-related information. The obtained piece of information included any IRT (post-acute/follow-up care) due CRC-specific or therapy-related health impairments.
We considered potential determinants of utilization based on previous literature and clinical experience. Sociodemographic information, lifestyle characteristics and comorbidities were based on data collected during the interview. The Charlson Comorbidity Index (CCI) was used to score reported comorbidities and group participants into four groups from no (0) to severe comorbidity (3+) [Citation26]. Prediagnosis physical activity was assessed retrospectively for every decade of age (at age 20, 30,…, 80). The most recent decade preceding the patient’s age was used to derive the latest physical activity level (e.g., physical activity at age 60 for patient group aged 60–69). Metabolic task hours per week (MET-h/wk) were calculated based on type of activity and averages of minutes spent per week. We classified the level of physical activity into low, medium and high by tertiles of the study population. Cancer stage at diagnosis was classified according to the International Union against Cancer Classification (UICC). Apart from the administration of chemotherapy, radiotherapy, and palliative treatment (yes/no), we also considered type of surgery (none, laparoscopic, open) and receipt of ostomy (yes/no) as potential determinants of IRT use.
Based on the cause of death, we classified the events into disease-specific and non-disease-specific.
Inclusion and exclusion criteria
Participants with available information on rehabilitation use (yes/no) were included in the analysis. We excluded patients who underwent outpatient rehabilitation only. Regarding the survival analysis, we furthermore excluded participants with missing follow-up information or/and no tumor resection.
Statistical analysis
We assessed the relative frequency of utilization within the cohort and calculated the utilization rates by calendar year and by cancer stage. Rehabilitants and non-rehabilitants were compared with respect to potential determinants by Fisher’s exact test or Chi-square test of independence. Independent predictors of utilization were determined using a multivariable logistic regression model. We selected potential predictors into the model, when the previous (bivariate) analysis resulted in a p-value less than 0.2.
Potential associations of IRT with overall and disease-specific survival were estimated by multivariable Cox proportional hazards models and quantified by adjusted hazard ratios (HRs). As survival analyses based on follow-up time from time of diagnosis might lead to biased results in favor of rehabilitative treatment, because rehabilitants need to live through IRT initiation to become rehabilitants, we controlled for a potential immortal-time bias by the Modified Landmark Approach [Citation27,Citation28]. Based on available data on start of IRT for one third of the rehabilitants, the landmark was set at eight months after CRC diagnosis (equivalent to the 75th percentile). Patients, who died before the landmark were excluded from the survival analysis, and the follow-up time was reduced by eight months.
Cox regression models were adjusted for the following potential confounders (categorization ): age (continuous), sex, partnership, years of school education, employment status, type of health insurance, body mass index, smoking, physical activity level (tertiles), cancer stage, tumor location, Charlson Comorbidity Score, chemotherapy, radiotherapy, surgical technique and surgical volume of treating hospital. Time-dependent interaction terms were added to the models to assess the proportional hazards assumption beforehand. Significant interactions were found for IRT and four covariates (age, cancer stage, chemotherapy, comorbidities). We therefore included time-dependent interaction terms for the four respective covariates in the models. Furthermore, we investigated the association of IRT and survival outcomes over time. Hence, we conducted time-interval-specific survival analyses in addition to the main analyses. Follow-up time was divided into four intervals (<3 years, 3–<6 years, 6–<9 years, ≥9 years). Cox proportional hazards models were calculated based on interval-specific follow-up time, respective persons at risk and events. Survival analysis for the first interval was corrected for potential immortal time bias by the Modified Landmark Approach as described above.
Table 1. Sociodemographic, clinical, and lifestyle characteristics of rehabilitants and non-rehabilitants.
A p-value of <0.05 in two-sided testing was considered statistically significant. All analyses were performed on SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Subanalysis: IRT and functional status
In a subset of participants, treating hospitals gave information about perioperative functional performance by using one or more of the following scales: American Society of Anesthesiologists (ASA) grade [Citation29], Eastern Cooperative Oncology Group (EGOG) score [Citation30] and Karnofsky Performance Score (KPS) [Citation31]. We combined the respective scales into one functional performance measure with the levels excellent (ASA = I, ECOG = 0 or KPS = 90–100), fair (ASA = II, EGOG = 1 or KPS = 70–80) and poor (ASA = III–IV, ECOG = 2–4 or KPS = 10–60) [Citation32]. We repeated the analyses with data from the subgroup, considering functional status as additional potential determinant of utilization and potential confounder in the survival analyses. To avoid biased results due to selectively assessed functional performance in case of either very good or very poor health status, we only included participants in the subanalyses, when functional performance was rated for at least 75% of the patients that were recruited in the same hospital.
Results
Study cohort
Data from 4009 CRC patients, who were diagnosed from 2005 to 2014 and participated in the DACHS study were available for the present analysis. We excluded 302 (7.5%) participants due to missing rehabilitation status. In addition, we excluded three (<1.0%) patients, who underwent outpatient rehabilitation therapy. The final cohort consisted of 3704 CRC patients. In survival analyses, we furthermore excluded 58 (1.6%) patients who did not receive surgery, two (<1.0%) patients with unknown follow-up status, and 108 (2.9%) patients who died within the pre-landmark period. Thus, data from 3536 patients remained for survival analyses (Supplementary Figure S1). presents the distribution of sociodemographic, clinical and lifestyle characteristics within the cohort and among rehabilitants and non-rehabilitants separately. The mean age of the study cohort was 68.3 (±11.2) years. Participants were mostly male (60.9%), lived in a partnership (74.2%), were retired (64.5%), had colon cancer (59.2%), were diagnosed with cancer stage II (30.8%) or III (32.6%), and did not receive chemotherapy (55.4%).
Utilization of IRT among the cohort
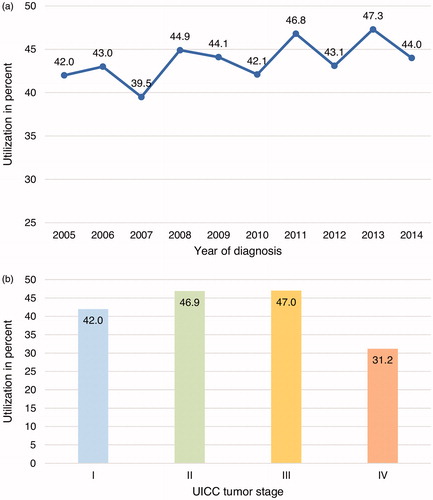
In total, 1615 (43.6%) participants underwent IRT within the first three years after diagnosis. Rehabilitants were admitted to 142 different rehabilitation facilities. We observed a small and non-significant variation of utilization over time with a minimum of 39.5% in 2007 and a maximum of 47.3% in 2013 (). Significant differences (p < 0.0001) in utilization were observed by cancer stage (). Highest uptake was reported among patients diagnosed with CRC stage II (46.9%) and III (47%) whereas in patients with stage I (42%) and IV (31.2%) utilization was less common.
Determinants of utilization
The comparison between rehabilitants and non-rehabilitants with respect to clinical, social and lifestyle characteristics identified several potential determinants of utilization (): age, sex, partnership, type of health insurance, tumor stage and location, palliative treatment, comorbidities, physical activity level, surgical volume (treating hospital). These candidate variables were selected for the multivariable logistic regression model, which identified eight independent predictors. Detailed results are presented in . Employed participants (OR 1.93, CI 1.51−2.46, vs. retired) and participants who did not live in a partnership (OR 1.33, CI 1.13−1.58) were more likely to undergo IRT. We furthermore observed a positive relationship between physical activity level and IRT use (OR 1.45, CI 1.21 − 1.74, highest vs. lowest). Strong inverse associations with IRT were observed for private health insurance (OR 0.61, CI 0.48 − 0.79), cancer stage IV (OR compared to stage I 0.63, CI 0.47– 0.84) and no tumor resection (OR compared to open surgery 0.37, CI 0.17 − 0.79). Weak inverse associations were estimated for laparoscopic versus open surgery (OR 0.81, CI 0.66 − 0.99) and treating hospitals with medium versus high surgical volume (OR 0.78, CI 0.66 − 0.92). The multivariable model included data from 97.4% of the participants. Cases with unknown insurance status (16.6%) were included in the model in contrast to cases with missing information on other predictor variables (3.6%). A sensitivity analyses based on complete cases only (80.2%) yielded comparable results (data not shown).
Table 2. Determinants of utilization of inpatient rehabilitation therapy: results of bivariate and multiple logistic regression analysis.
Survival outcomes
In total, 3536 participants with a median follow-up of 4.4 years (post-landmark) were included in the survival analysis. In the post-landmark period, 1169 (33.1%) deaths occurred, including 658 (56.3%) disease-specific deaths. shows survival curves comparing rehabilitants and non-rehabilitants. Both overall and disease-specific survival were significantly higher (p < 0.0001) for rehabilitants than for non-rehabilitants. IRT use was associated with a 19% lower overall (HR 0.81, CI 0.72 − 0.92) and a 28% lower disease-specific mortality (HR 0.72, CI 0.61 − 0.85) in the multivariable analyses ().
Figure 2. Crude survival curves for inpatient rehabilitation therapy (IRT) use and survival outcomes within the post-landmark period.
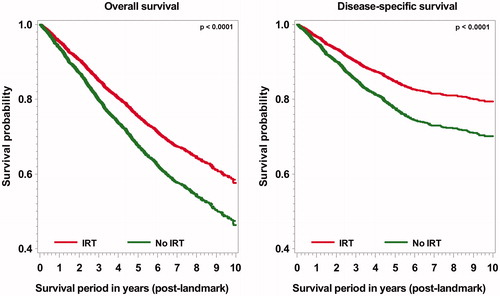
Table 3. Association of IRT and survival outcomes by observation periods.
Follow-up time-specific analyses
Time-interval-specific analyses indicated that the positive association of IRT with overall and disease-specific survival decreased over time and no significant associations were estimated for later observation periods (follow-up ≥ 6 years after diagnosis). presents survival outcomes for the respective observation periods.
Subanalysis: IRT and functional status
Perioperative functional status was available and classified for 1973 participants (53.3%) into the categories excellent (29.4%), fair (36.4%) and poor (34.2%). There was only a moderate correlation between functional status and age (r = 0.34, p < 0.001) indicating better status with lower age. We observed a positive association of better functional status and the use of IRT (poor vs. excellent) in the bivariate regression model. However, no significant association with IRT use was found in the multivariable analysis, which yielded comparable results to those derived from the whole study cohort (Supplementary Table S1). Although better functional status predicted better survival, controlling for it did not affect the association of IRT use with disease-specific and only affected marginally the association with overall survival (Supplementary Table S2).
Discussion
Our study indicates that more than 40% of patients diagnosed with CRC in this population-based study from Germany underwent IRT, mostly within the first eight months after the initial diagnosis. We did not observe significant variation over time in utilization but identified several clinical, sociodemographic and lifestyle-related factors that were associated with the use of IRT. Survival analyses suggest a positive association of IRT and overall/disease-specific survival within the first years after diagnosis.
Utilization and determinants
The proportion of rehabilitants among the study population was 43.6%. This finding is in line with results (45 − 51%) from observational studies that have included smaller samples of CRC patients [Citation20,Citation21] or that have approximated estimates by aggregated data [Citation22]. We observed a slightly lower utilization which might be explained by inclusion of older and (more) cancer stage IV patients, who receive IRT less often.
The proportion of rehabilitants only varied slightly over time and no time trend was observed. National data on utilization over time was only available for all types of cancer combined from the German Pension Insurance. Therefore, CRC-specific comparisons with population-wide data were not possible. Published age-standardized treatment rates per insured/retired person slightly increased from 2006 to 2010 and dropped again from 2010 to 2016 [Citation16]. Our (non-age-standardized) data did not reflect this pattern.
Apart from clinical factors, social and lifestyle-related factors were associated with the participation in an inpatient rehabilitation program. The important role of non-clinical characteristics came as no surprise, considering the conception of functions and disabilities as a ‘dynamic interaction between a person’s health condition, [...] environmental factors and personal factors‘ [Citation33]. We observed almost twice as high participation among patients that were employed at the time of diagnosis compared to retired participants. This finding reflects the principle of the German Social Code ‘Rehabilitation before Retirement’ and the effort by pension insurances plans to promote IRT in order to assess and at best preserve the ability to work. However, self-employed participants were less likely to undergo IRT. Although the negative association between self-employment and IRT did not remain significant in the multivariable model, possibly due to the low number of self-employed patients, this patient group might be at high risk of experiencing rehabilitative needs but decline treatment because of job responsibilities and financial constraints.
The odds of IRT were increased by 30% among study participants not living in a partnership. This result is in accordance with findings by Waldmann et al., who identified being single as the only independent predictor of IRT utilization in a small sample of younger CRC patients [Citation21]. Given reasons for non-utilization among 51 CRC patients in a study by Deck et al. included ‘to be not separated from the family’ and ‘leave his/her family alone’ [Citation20]. Many cancer patients seek social support by their partner and the partners might also act as temporary care givers at home.
We did not observe an (inverse) association between years of school education and IRT use as seen in women with breast cancer or in a Swiss cohort of cancer patients [Citation18,Citation34]. Nevertheless, our analysis identified private health insurance as a predictor for non-utilization. We have no information whether privately insured patients experienced less cancer-related disabilities or if they received rehabilitative care in settings other than IRT. However, in contrast to most statutory health-insured patients whose cancer rehabilitation costs are covered by the German pension insurance, rehabilitation costs of private-insured patients generally are partly or fully covered by their health insurance plans only, if they are included in patients’ individual contracts.
Our analysis suggests a positive association between higher levels of prediagnosis physical activity and IRT use. Since rehabilitative needs derive from individual expectations and demands, physically active persons might participate in a rehabilitation program in order to regain fitness. Moreover, they may be more open for rehabilitative treatment that usually includes extensive exercise therapy.
We identified several clinical factors that were associated with the use of IRT. Participants who received open surgery underwent IRT more often. Higher uptake might be explained by stronger physical impairment due to greater body structural damage and longer time of immobilization in comparison to less invasive laparoscopic techniques. Limited life expectancy probably accounts for the lower utilization of IRT observed in patients who did not receive tumor resection.
Despite the higher risk of persistent side effects with extensive treatment, we did not observe a relationship between chemotherapy or radiation and IRT use. When asked for reasons for non-utilization, cancer and breast cancer patients in previous studies have reported the wish for going back to normal life and distracting themselves from the disease by daily routine [Citation18,Citation20]. This wish might be pronounced in patients, who have already spent several months with cancer treatment.
Differences in IRT utilization were also present with respect to cancer stage. The lowest proportions of rehabilitants were seen in stage I and IV patients. Detection of CRC in early stages goes along with less functional impairments and consequently minor rehabilitative needs. Advanced cancer stage, on the other hand, has been described as a barrier to cancer rehabilitation due to short life expectancy despite functional loss and disability [Citation35,Citation36]. Progressing cancer may preclude patients getting admitted to IRT in case of insufficient physical capacity and poor chances of treatment success regarding the preservation of functions. Moreover, practitioners experience trouble referring multimorbid patient due to limited number of adequate therapy settings [Citation37].
Besides patient characteristics, the surgical volume of the clinics was associated with IRT use. Patients who had been operated in clinics with high surgical volume underwent IRT more often, suggesting that structural barriers for the access to IRT may exist. This is in line with findings by Deck et al. that about one fifth of the participating cancer patients were not informed about the possibility to undergo IRT [Citation20]. Large treatment centers may be more likely to have implemented standardized clinical pathways for acute and follow-up care, also involving social services, to detect disabilities and rehabilitative needs and promote IRT. Healthcare practitioners report further structural barriers involving the application process such as rigid deadlines in particular for post-acute IRT and the low availability to reach respective healthcare payers [Citation37].
IRT and survival
The use of IRT was associated with better overall and disease-specific survival. We used the Modified Landmark Approach to avoid immortal-time bias. Follow-up time-specific results suggest that the survival advantage in rehabilitants is strongest in the initial years after diagnosis and diminishes over time. Even though we adjusted for a number of potential confounders such as comorbidity, age and cancer stage, the association of IRT and survival might be partially explained by residual confounding. In particular, the possibility has to be kept in mind that patients with better prognosis may have been more likely to be referred to IRT. Therefore, our analysis does not allow to draw conclusions about a causal relationship between utilization of IRT and better survival. Although the observation that similar results were obtained in the subanalysis additionally adjusting for functional status does not support a major role of residual confounding by patients’ health status, residual confounding by additional factors including sociodemographic and lifestyle-related factors cannot be ruled out.
Strengths and limitations of the study
We were able to assess IRT utilization in a large cohort of CRC patients. Moreover, we identified several factors that predicted the use of IRT. However, we cannot clarify if the utilization was guided by and actually met individual rehabilitative needs. We have excluded patients from the analyses if they had participated in a comprehensive outpatient rehabilitation program but we did not obtain information about referrals to single rehabilitative services. For example, some patients might have received support by stoma nurses, physiotherapists, oncologists and psychologist in the ambulant sector. Furthermore, we could not distinguish between post-acute or follow-up IRT, and a small proportion of patients probably underwent IRT in a geriatric rehabilitation setting and not a cancer rehabilitation setting. Type of IRT would have been informative not only in in relation to prognosis but also with regard to utilization, since the timing and setting involve a different application process and sometimes different healthcare payers. However, in contrast to other medical indications, the German pension insurance funds cancer rehabilitation not only for employed but also for a proportion of retired patients. Treating physicians from cooperating clinics were able to recruit about half of the eligible patients for the DACHS study. Even though physicians cited the lack of time as the main reason for failed recruitment, we cannot rule out the possibility of a selection bias. Healthier patients might be more willing to participate in a study than older, multimorbid patients diagnosed with advanced cancer stage who receive IRT less often. Moreover, rehabilitation status could not be obtained for 7.5% of the participants, which lead to an exclusion from further analyses. Apart from this, the completeness of data was satisfying.
To our knowledge, this is the first study comparing short- and long-term survival between rehabilitants and non-rehabilitants with CRC. Based on the observational study design, we were able to investigate the prognostic role of IRT with respect to survival but cannot draw conclusions about IRT effects even though major efforts were undertaken to control for immortal-time bias and confounding by indication.
Conclusion
Nearly one-half of the CRC patients received IRT and its use was associated with better survival. Next to clinical factors, social and lifestyle-related factors seemed to play an essential role in the decision making of undergoing IRT. Our results suggest that hospital-related structural barriers exist and that the use of IRT might not be always guided by rehabilitative needs. Further research is needed to evaluate and optimize allocation of patients to either IRT or outpatient rehabilitation according to their rehabilitative needs. Moreover, future research is essential in order to evaluate and optimize effectiveness of various types of rehabilitation offers for short- and long-term prognosis, lifestyle changes and quality of life of CRC patients.
Supplemental Material
Download Zip (820.1 KB)Acknowledgments
We would like to thank Ute Handte-Daub and Ansgar Brandhorst for their technical assistance. We thank Isabelle Finke, Daniel Boakye and Tim Holland-Letz for their (statistical) advice. We are grateful to the study participants as well as the interviewers. We furthermore would like to thank the cooperating resident physicians and the below-listed clinics: Chirurgische Universitätsklinik Heidelberg, Klinik am Gesundbrunnen Heilbronn, St. Vincentiuskrankenhaus Speyer, St. Josefskrankenhaus Heidelberg, Chirurgische Universitätsklinik Mannheim, Diakonissenkrankenhaus Speyer, Krankenhaus Salem Heidelberg, Kreiskrankenhaus Schwetzingen, St. Marienkrankenhaus Ludwigshafen, Klinikum Ludwigshafen, Stadtklinik Frankenthal, Diakoniekrankenhaus Mannheim, Kreiskrankenhaus Sinsheim, Klinikum am Plattenwald Bad Friedrichshall, Kreiskrankenhaus Weinheim, Kreiskrankenhaus Eberbach, Kreiskrankenhaus Buchen, Kreiskrankenhaus Mosbach, Enddarmzentrum Mannheim, Kreiskrankenhaus Brackenheim.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Brenner H, Bouvier AM, Foschi R, EUROCARE Working Group, et al. Progress in colorectal cancer survival in Europe from the late 1980s to the early 21st century: the EUROCARE study. Int J Cancer. 2012;131(7):1649–1658.
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075.
- Arndt V, Merx H, Stegmaier C, et al. Quality of life in patients with colorectal cancer 1 year after diagnosis compared with the general population: a population-based study. J Clin Oncol. 2004;22(23):4829–4836.
- Thong MSY, Koch-Gallenkamp L, Jansen L, et al. Age-specific health-related quality of life in long-term and very long-term colorectal cancer survivors versus population controls – a population-based study. Acta Oncol. 2019;58(5):801–810.
- Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014;155(12):2461–2470.
- Jansen L, Herrmann A, Stegmaier C, et al. Health-related quality of life during the 10 years after diagnosis of colorectal cancer: a population-based study. J Clin Oncol. 2011; 29(24):3263–3269.
- Custers JAE, Gielissen MFM, Janssen SHV, et al. Fear of cancer recurrence in colorectal cancer survivors. Support Care Cancer. 2016;24(2):555–562.
- Jansen L, Koch L, Brenner H, et al. Quality of life among long-term (≥5 years) colorectal cancer survivors-systematic review. Eur J Cancer. 2010;46(16):2879–2888.
- Rothbarth J, Bemelman WA, Meijerink WJ, et al. What is the impact of fecal incontinence on quality of life? Dis Colon Rectum. 2001;44(1):67–71.
- Hellbom M, Bergelt C, Bergenmar M, et al. Cancer rehabilitation: a Nordic and European perspective. Acta Oncol. 2011;50(2):179–186.
- Stubblefield MD, Hubbard G, Cheville A, et al. Current perspectives and emerging issues on cancer rehabilitation. Cancer. 2013;119 Suppl 11:2170–2178.
- Cheville AL, Mustian K, Winters-Stone K, et al. Cancer rehabilitation: an overview of current need, delivery models, and levels of care. Phys Med Rehabil Clin N Am. 2017;28(1):1–17.
- Cheville AL, Beck LA, Petersen TL, et al. The detection and treatment of cancer-related functional problems in an outpatient setting. Support Care Cancer. 2009;17(1):61–67.
- Rick O, Dauelsberg T, Kalusche-Bontemps EM. Oncological rehabilitation. Oncol Res Treat. 2017;40(12):772–777.
- German-Pension-Insurance. Reha-Bericht 2018 [Rehabilitation-Report 2018]. Berlin (Germany); 2018. p. 73–88 (German).
- Wehner C. Die Rehabilitation der gesetzlichen Rentenversicherung in Geschichte und Gegenwart: Eine Einführung [The rehabilitation by the statutory pension insurance in history and present: an introduction]. In: Wehner C, editor. Aufbrüche in der Rehabilitation. Geschichte und Gegenwart der Rehabilitationin der gesetzlichen Rentenversicherung [Departures in rehabilitation. the past and present of rehabilitation in the statutory pension insurance]. Bochum (Germany): Documentation- and Research Agency of the Social Security Institutions; 2019. p. 7–24 (German).
- Geyer S, Schlanstedt-Jahn U. Gibt es soziale Ungleichheiten in der Inanspruchnahme der onkologischen Rehabilitation bei Mammakarzinompatientinnen? [Are there social inequalities in the utilisation of oncological rehabilitation by breast cancer patients?]. Gesundheitswesen. 2012;74(2):71–78 (German).
- Lehmann C, Beierlein V, Hagen-Aukamp C, et al. Psychosoziale Einflussfaktoren für die Inanspruchnahme medizinischer Rehabilitationsmaßnahmen bei Patienten mit einer Prostatakrebserkrankung [Psychosocial predictors of utilization of medical rehabilitation services among prostate cancer patients]. Rehabilitation. 2012;51(3):160–170.
- Deck R, Babaev V, Katalinic A. Gründe für die Nichtinanspruchnahme einer onkologischen Rehabilitation. Ergebnisse einer schriftlichen Befragung von Patienten aus onkologischen Versorgungszentren [Reasons for the non-utilisation of an oncological rehabilitation. results of a written survey with patients of oncological healthcare centers]. Rehabilitation. 2019;58(4):243–252.
- Waldmann A, Lautz E, Hampe J, et al. Popgen-Darmkrebs: Reha-Inanspruchnahme von jüngeren Patienten mit kolorektalem Tumor [Utilization of inpatient rehabilitation of younger patients with colorectal neoplasms – results of the project “Popgen-Colorectal Cancer”]. Rehabilitation. 2007;46(6):349–355.
- Nowossadeck E, Barnes B. Inanspruchnahme der onkologischen Rehabilitation im Verhältnis zur Krebsinzidenz. [Utilization of oncological rehabilitation in relation to cancer incidence]. Gesundheitswesen. 2016;78(08/09):A163.
- Scherer S, Jansen L, Boakye D, et al. Changes in health-related outcomes among colorectal cancer patients undergoing inpatient rehabilitation therapy: a systematic review of observational and interventional studies. Acta Oncol. 2021; 60(1):124–134.
- Brenner H, Chang-Claude J, Jansen L, et al. Reduced risk of colorectal cancer up to 10 years after screening, surveillance, or diagnostic colonoscopy. Gastroenterology. 2014;146(3):709–717.
- Hoffmeister M, Jansen L, Rudolph A, et al. Statin use and survival after colorectal cancer: the importance of comprehensive confounder adjustment. J Natl Cancer Inst. 2015;107(6):djv045.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Diss. 1987;40(5):373–383.
- Weberpals J, Jansen L, Silversmit G, et al. Comparative performance of a modified landmark approach when no time of treatment data are available within oncological databases: exemplary cohort study among resected pancreatic cancer patients. Clin Epidemiol. 2018;10:1109–1125.
- Suissa SJA. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008;167(4):492–499.
- Owens WD, Felts JA, Spitznagel EL. Jr. ASA physical status classifications: a study of consistency of ratings. Anesthesiology. 1978;49(4):239–243.
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655.
- Karnofsky DA, Abelmann WH, Craver LF, et al. The use of the nitrogen mustards in the palliative treatment of carcinoma. With particular reference to bronchogenic carcinoma. Cancer. 1948;1(4):634–656.
- Boakye D, Walter V, Martens UM, et al. Treatment selection bias for chemotherapy persists in colorectal cancer patient cohort studies even in comprehensive propensity score analyses. Clin Epidemiol. 2019;11:821–832.
- World-Health-Organization. International classification of functioning, disability and health. Geneva (Switzerland); ICF; 2001. p. 8.
- Ture M, Barth J, Angst F, et al. Use of inpatient rehabilitation for cancer patients in Switzerland: who undergoes cancer rehabilitation? Swiss Medical Weekly. 2015;145:w14214.
- Cheville AL, Troxel AB, Basford JR, et al. Prevalence and treatment patterns of physical impairments in patients with metastatic breast cancer. J Clin Oncol. 2008;26(16):2621–2629.
- Spill GR, Hlubocky FJ, Daugherty CK. Oncologists' and physiatrists' attitudes regarding rehabilitation for patients with advanced cancer. Pm R. 2012;4(2):96–108.
- Weis J, Dresch C, Bartsch HH, et al. Barrieren der Antragstellung in der Onkologischen Rehabilitation: Eine bundesweite Expertenstudie [Barriers in the application process for oncological rehabilitation: a nationwide expert study]. Rehabilitation. 2021;60(2):95–101.