Abstract
Background
Multiple meta-analyses have demonstrated that routine surveillance following colorectal cancer surgery improves survival outcomes. There is limited data on how recurrence patterns and post-recurrence outcomes vary by individual tumor stage.
Methods
Using a multi-site community cohort study, we examined the potential impact of primary tumor stage on the sites of recurrence, management of recurrent disease with curative intent, and post-resection survival. We also explored changes over time.
Results
Of 4257 new colon cancers diagnosed 2001 through 2016, 789 (21.1%) had stage I, 1584 (42.4%) had stage II, and 1360 (36.4%) had stage III colon cancer. For consecutive 5-year periods (2001–2005, 2006–2010, 2011–2016), recurrence rates have declined (23.4 vs. 17.1 vs. 13.6%, p < 0.001), however, the resection rates of metastatic disease (29.3 vs. 38.6 vs. 35.0%, p = 0.21) and post-resection 5-year survival (52.0 vs. 51.8 vs. 64.2%, p = 0.12) have remained steady. Primary tumor stage impacted recurrence rate (3.8 vs. 12 vs. 28%, p < 0.0001 for stage 1, 2, and 3), patterns of recurrence, resection of metastatic disease, (50 vs. 42 vs. 30%, p < 0.0001) and post-resection 5-year survival (92 vs. 64 vs. 44%, p < 0.001).
Conclusion
In this community cohort we defined significant differences in recurrence patterns and post-resection survival by tumor stage, with a diminishing rate of recurrence over time. While recurrence rates were lower with stage I and II disease, the high rate of metastatic disease resection and excellent post-resection outcomes help to justify routine surveillance in these patients.
Introduction
Colorectal cancer is the third most common cancer diagnosed, and the fourth leading cause of cancer-related death worldwide [Citation1]. Surveillance following resected non-metastatic disease includes clinical review and carcinoembryonic antigen (CEA) testing every 3–6 months for 5 years, and annual computed tomography (CT) scans for at least 3 years [Citation2]. This strategy is supported by three large meta-analyses of randomized studies that each concluded that intensive surveillance is associated with improved overall survival [Citation3–5]. There is, however, limited data on the impact of surveillance in the modern era, where CT scans of chest, abdomen, and pelvis are now routine at baseline and where systemic therapy and surgical options continue to evolve. There is a paucity of data examining stage-based variation in recurrence patterns, resection of recurrent disease, and post-resection survival outcomes.
Examining data from a large community cohort of patients with non-metastatic colon cancer, collected since 2001, we report a comprehensive analysis of trends over time and the evident impact of evolving clinical practice. We also report a comprehensive stage-based analysis of outcomes, including sites of recurrence, the proportion of patients with the resectable disease, and post-resection survival.
Methods
Consecutive adult patients with newly diagnosed and histologically confirmed colorectal cancer were identified in a search of the ACCORD (Australian Comprehensive Cancer Outcomes and Research Database) registry. This is a prospective database collecting comprehensive clinical, pathology, treatment, and outcome data across all stages of the disease on consecutive patients with colorectal cancer at six hospitals in Melbourne, Australia. Data captured includes the method of recurrence detection (clinical vs. CEA vs. imaging) and the sites of recurrent disease. Patients with primary rectal cancer or with metastatic disease at presentation were excluded from the current analysis. Patients were followed until 1 December 2018. Surveillance was as per local (NHMRC) guidelines [Citation6], which includes clinical review and carcinoembryonic antigen (CEA) testing every 3–6 months for 5 years, annual computed tomography (CT) scans for at least 3 years, and surveillance colonoscopy. The study was approved by the Institutional Human Ethics Committee at BioGrid, Melbourne.
Statistical analysis
Descriptive statistics were used to describe clinical characteristics and details of recurrence. Categorical variables are presented as observed counts and weighted percentages according to the overall size of each group, and continuous variables as median with corresponding range. Survival was estimated by the Kaplan–Meier method. Overall survival (OS) was defined as the time from diagnosis to death or censored at the date of the last follow-up. Progression-free survival (PFS) was defined as the time from diagnosis to the date of known recurrence (determined by imaging and/or histology). Residual overall survival (ROS) was defined as the time from recurrence to death or censored at the date of the last follow-up. Multivariate analyses were conducted with known variables including age at diagnosis, stage at diagnosis, recurrence, and receipt of salvage surgery.
Subgroup analyses were performed between patients based on the stage of disease at diagnosis, as well as treatment at recurrence, including salvage surgery (surgical removal of all remaining or recurrent tumors), using relevant intergroup statistics (e.g., chi-squared, Mann–Whitney, cox proportional hazards). Trends over time were examined for the 5-year periods of 2001–2005, 2006–2010, and 2011–2016. Statistical significance was defined as p-value <0.05. All statistical analyses were performed using R programming v3.5.1.
Results
From 1/1/2001 to 30/12/2016, 6487 patients with colorectal cancer had been entered on the registry, including 4527 (69.8%) with colon cancer. De novo metastatic disease was present in 794 of these cases (17.5%). For the population of stage 1 − 3 colon cancer (n = 3733), the median follow-up time was 7.8 years (range: 1.9–18.1 years). Included were 789 (21.1%) with stage 1, 1584 (42.4%) with stage 2, and 1360 (36.4%) with stage 3 disease. shows the clinical characteristics. Over half were male with a median age of 70 years (range: 16–101).
Table 1. Clinical characteristics according to the stage.
Over time
The proportion of patients who recurred decreased over time for the 5-year periods examined (as shown in ). The largest absolute difference was seen for stage 3, with 5-year recurrence rates dropping from 37.5% for 2001–2005, down to 29.3% for 2006–2010 (p = 0.036) and 23.9% for 2011–2016 (p < 0.001). For stages 1 and 2, there was no significant difference in recurrence rates between 2001–2005 and 2006–2010. More modest decreases in absolute values were seen when comparing across all time periods, with these being statistically significant (stage 1: 5.1–2.8%, p < 0.0001; stage 2: 16.5–10.6%, p = 0.04).
Figure 1. Recurrence overtime for all (A), stage 1 (B), stage 2 (C), or stage 3 patients (D); and stage-based variation of recurrence (E).
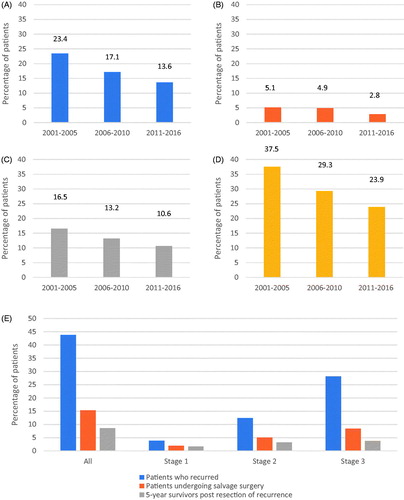
The proportion of patients with recurrence undergoing resection of metastatic disease did not significantly change over time (29.3% for 2001–2005 vs. 38.6% for 2006–2010 vs. 35.0% for 2011–2016). However, the proportion of all patients diagnosed with colon cancer undergoing curative intent salvage surgery significantly declined in the later time period [6.9% for 2001–2005 vs. 6.6% for 2006–2010 (p = 0.93) vs. 4.8% for 2011–2016 (p = 0.04)].
The sites of recurrent disease remained stable over time. In patients with isolated (no other sites of metastatic disease) lung or liver recurrence, the rates of resection over time were not significantly different (lung only: 37.5 vs. 41.5 vs. 53.8%, p = 0.46; liver-only: 46.7 vs. 50.0 vs. 56.8%, p = 0.48), but appeared to be trending higher.
By disease stage
The stage-based variation in tumor recurrence is shown in . The proportion of patients who developed recurrent disease significantly increased with advancing stage (3.8 vs. 12.4 vs. 28.1%, p < 0.0001). There was no significant association between stage and median time to recurrence (19.0 vs. 15.1 vs. 15.9 months, p = 0.16). The proportion of patients with recurrent disease that underwent curative intent salvage surgery decreased with advancing stage (50.0 vs. 42.3 vs. 30.1%, p < 0.0001).
outlines the stage-based variation in location of recurrence. Patients with stage 1 disease were most likely to develop recurrent disease in the liver (88.0 vs. 68.0 or 53.6%, p < 0.0001), and least likely to develop recurrent disease in the lung (12.0 vs. 30.7 or 38.5%, p < 0.0001). Whereas patients with stage 3 disease more likely developed recurrent disease involving the lymph nodes (8.0 vs. 8.7 vs. 19.7% for stage 1, 2, and 3, p = 0.004) and peritoneum (3.9 vs. 8.1 vs. 20.4% for stage 1, 2, and 3, p = 0.007).
Table 2. Stage-based variation in the location of recurrence.
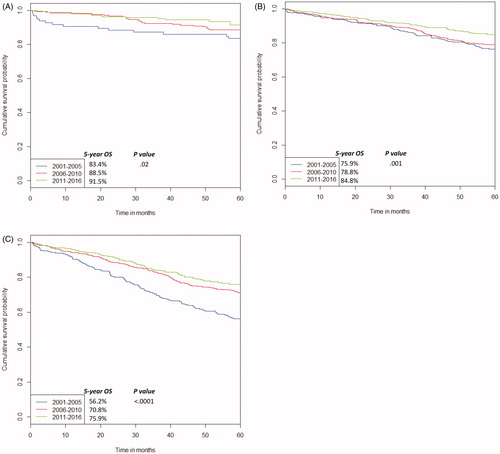
shows the variation in overall survival (OS) for all patients. Death without documented disease recurrence occurred in 9.9% of all patients. Consistent with this being largely due to death from co-morbidities, this rate was similar across disease stage, including 7.5% of stage 1 cases vs. 11.5% of stage 2 and 9.5% of stage 3 cases (p = NS). There was a significant improvement in OS for each stage over the time periods (stage 1: 83.4 vs. 88.5 vs. 91.5%, HR 0.68, 95%CI 0.48–0.94, p = 0.02, stage 2: 75.9 vs. 78.8 vs. 84.8%, HR 0.77, 95%CI 0.65–0.90, p = 0.001, stage 3: 56.2 vs. 70.8 vs. 75.9%, HR 0.70, 95%CI 0.61–0.80, p < 0.0001). There was also a significant difference in stage-based survival, irrespective of time period (HR 1.63, 95%CI 1.46–1.81, p < 0.0001). In a multivariate analysis, the strongest predictors for worsened overall survival were age ≥65 years (HR 2.21, 95%CI 0.38–12.87, p < 0.0001), developing recurrent disease (HR 7.25, 95%CI 6.17–8.53, p < 0.0001) and not undergoing salvage surgery (HR 2.87, 95%CI 2.24–3.67, p < 0.0001).
Figure 2. Variation in overall survival (OS) over time for stage 1 (A), stage 2 (B), and stage 3 (C).
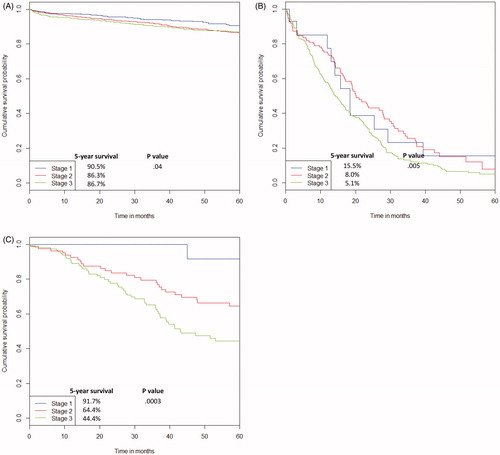
shows the Kaplan Meier survival curve for OS for patients who did not recur and post-recurrence survival in patients who recurred with or without curative intent salvage surgery. In patients who did not recur during follow-up, the 5-year survival was 90.5% for stage 1, 86.3% for stage 2, and 86.7% for stage 3. In patients who recurred and underwent salvage surgery, the residual 5-year survival was 91.7% for stage 1, 64.4% for stage 2, and 44.4% for stage 3 (HR 2.065, 95%CI 1.40–3.06, p = 0.0003). The survival outcomes of patients who recurred and did not undergo salvage surgery were also examined. Here again, there was a significant difference in residual 5-year survival post-recurrence with advancing stage of disease (15.5 vs. 8.0 vs. 5.1%, HR 1.37, 95%CI 1.10–1.69, p = 0.005). Within each tumor stage, survival was significantly improved by undergoing salvage surgery (stage 1: HR 0.08, 95%CI 0.02–0.35; stage 2: HR 0.18, 95%CI 0.12–0.29; stage 3: HR 0.26, 95%CI 0.19–0.35). In a multivariate analysis, undergoing salvage surgery was the greatest predictor for improved residual survival (HR 0.34, 95%CI 0.28–0.41, p < 0.0001).
Figure 3. 5-Year overall survival for patients who did not recur (A); 5-year residual survival for those who recurred and did not undergo salvage surgery (B); and 5-year residual survival for those that recurred and underwent salvage surgery (C).
An overview of the stage-based impact of surveillance is illustrated in . For stage 1 vs. 2 vs. 3, disease recurrence was observed in 3.8 vs. 12.4 vs. 28.1% of all patients, the recurrent disease was resected in 1.9 vs. 5.0 vs. 8.4% of all patients, and ultimately 1.7 vs. 3.2 vs. 3.7% of all patients were 5-year survivors beyond metastatic disease resection. Overall, there is a markedly higher proportion of patients with stage 3 disease who recur and a markedly lower proportion who are long-term survivors, with the converse for stage 1.
By clinical characteristics
There was no age-based variation in recurrence rates (15.5% for ≥65 years vs. 17.9% for <65 years, p = 0.07) or median time to recurrence (15.9 months for ≥65 years vs. 15.8 months for <65 years, p = 0.32). However, younger patients (aged <65 years) were more likely to undergo salvage surgery (8.4% for <65 years vs. 4.3% for ≥65 years). The absolute difference was most pronounced for stage 3 disease (12.6 vs. 5.9%, p < 0.0001). There was no age-based variation in survival outcomes following resection with 5-year survival across all stages of 55.6% for younger patients and 56.7% for older patients (p = 0.85).
Discussion
In this analysis of a large community cohort, we have demonstrated declining recurrence rates over time, as recently reported by others, with the novel finding that stage at initial diagnosis impacts the pattern of recurrent disease. While the recurrence rates are lower with the earliest stages of the disease, compared to stage III cases, a higher proportion appears to be salvageable with resection of metastatic disease.
The 5-year recurrence rates reported here, 3.8 vs. 12.4 vs. 28.1% for stage 1, 2, and 3 diseases are consistent with recent population data from Sweden [Citation7]. A combination of factors likely contributes to the declining recurrence rates over time. While a CT of the chest, abdomen, and pelvis at diagnosis has become a standard of care, this is a relatively recent practice. For example, as recently as 2007 a survey of Australian clinicians reported that only a minority performed CT chest as part of preoperative investigations [Citation8]. Earlier guidelines [Citation9] recommended only a liver ultrasound and chest x-ray as baseline investigations, potentially missing small volume intra-abdominal or lung metastases. As such, some patients included in historic series with a poor prognosis due to initially undetected low volume metastatic disease are now removed from non-metastatic cohorts. Improvements in adjuvant chemotherapy, notably the introduction of oxaliplatin as a standard adjuvant therapy from around 2005 [Citation10], and surgical techniques [Citation11] would have also contributed to the significant decline in recurrence rates for stage 3 disease (from 37.5% in 2001–2005 to 29.3% for patients diagnosed in 2006–2010).
Resection rates for metastatic recurrences were stable, at 6.9 and 6.6% for each 5 year period between 2001 and 2010, despite the observed declining proportion of patients with recurrent disease. Notably, this is in a community cohort where consecutive patients were included and adherence to surveillance protocols is anticipated to be lower than that of clinical trials. This potentially results in late, symptomatic presentations of recurrent disease, which are no longer respectable. Despite this, comparisons of what was achieved in a real-world setting and what was reported in the pivotal studies are of interest. Earlier studies reported resection rates of 10% [Citation4] in the intensive surveillance arm, varying over time and with protocols used, including some that did not include regular CT. In the most recently reported study by Primrose et al. [Citation12], recruiting 2003–2009, 5.9% of colon cancer patients overall had a surgically treatable recurrence. Differences in practice by country notwithstanding, our data does demonstrate that the proportion of patients able to undergo resection of metastatic disease can be maintained at the level achieved in a randomized clinical trial that demonstrated a significant survival impact of surveillance.
There are several reasons why the number of patients undergoing surgical resection of metastatic disease is maintained despite fewer patients having a recurrence. The dominant factor is the evolving definition of what constitutes resectable disease, with many modern candidates for liver resection not meeting prior more conservative criteria. Chemotherapy to ‘convert’ initially unresectable patients to operative candidates [Citation13] is now also routinely considered. Older/frailer patients can more safely undergo major resections. Consistent with this, a trend toward an increasing proportion of the patients with recurrent disease undergoing liver or lung resection is seen over time in our series. Notably, this is not compromising the post-resection 5-year survival outcomes, which if anything is trending higher. This provides comfort that a more ‘aggressive’ approach is not leading to more operations that fail to improve patient outcome as was suggested by previous studies in a different era where earlier detection of recurrences was not associated with improved survival [Citation14].
Our data provide novel insights into the impact of the stage on each of the surveillance endpoints. An unexplained observation from the FACS trial [Citation15] was that patients with recurrence of a lower stage tumor were significantly more likely to be treatable with curative intent. Our data suggest one explanation for this is the varying patterns of recurrence observed for each stage of the disease. Notably, sites of metastatic disease which are not readily amenable to surgical resection, including distant lymph nodes (19.7 vs. 8.0%) and peritoneal disease (20.4 vs. 3.9%) were more frequent in stage 3 vs. stage 1 cases. Ultimately more patients with recurrence from stage I cancer had oligometastatic disease amenable to surgery with curative intent, including the highest rate of liver resections.
We also observed significant stage-based differences in outcome post-resection, a trend noted in the FACS study [Citation15] and other series of patients undergoing resection of colorectal liver metastases [Citation16,Citation17]. Consistent with this is our finding of stage-based differences in 5-year survival outcomes for the patients who did not undergo resection of recurrent disease. A similar trend has been observed in adjuvant therapy studies, with O’Connell et al. [Citation18] reporting that patients enrolled on adjuvant trials with initial stage 2 disease had longer post-recurrence survival than initial stage 3 patients. These apparent stage-based differences in biology and how they impact metastatic patterns remain poorly understood. Tumors with a RAS mutation are more likely to develop lung metastases [Citation19], but the frequency of RAS mutations is not known to vary by tumor stage.
Clearly, the benefit of surveillance in younger fitter patients will be greater, as more patients will be motivated to undergo curative-intent surgery and the potential life-years gained from preventing death from colorectal cancer are far greater. In a limited age-based comparison we observed higher resection rates in younger patients, defined as <65 years old. We would assume that older patients were less likely to undergo routine surveillance or resection of oligometastatic disease due to age, frailty, co-morbidity, and/or refusal. Notably, the oldest patient in our series to undergo liver resection was 84 years, after which he lived another 5.5 years. We would suggest that the decision not to follow-up patients with CEA and/or imaging be made on a case-by-case basis, rather than using any arbitrary age-based criteria.
Our study has several limitations. This is an unplanned retrospective review of a large prospective registry and compliance with follow-up guidelines is uncertain. These might contribute to the observed low rate of recurrence for stage 1 cancer of 3.8%, however, the recurrent disease should ultimately manifest clinically at a later timepoint. In other words, surveillance should only bring forward in time the demonstration of recurrence, not impact the eventual rate of recurrence, excepting the small number of cases that die of an intercurrent illness in the interval between surveillance detectable and clinically detectable disease. Whilst these absolute numbers are small, particularly in stage 1 patients, the impact on survival is great, such that surveillance should be considered to some degree. Several studies as well as a Cochrane Review have demonstrated a lack of benefit from high intensity compared with low-intensity surveillance [Citation20–22]. The issue of surveillance intensity was not addressed by this study, which instead concludes the clinical utility of screening in principle. The outcome data for patients diagnosed 2011–2016 and post-resection of recurrence is still maturing. All centers involved are large metropolitan centers with high caseloads and similar outcomes may not be observed elsewhere due to variations in care. There are a few novel findings that cannot be explained by our current understanding of tumor biology. Reproducing these results in an independent cohort of patients would be desirable. Molecular associations cannot be explored as data was not routinely captured.
The strength of our data is that it reflects real-world outcomes, demonstrating a similar impact when measured by the proportion of patients undergoing surgery for recurrent disease as a more recent randomized study of surveillance intensity. As such it presents a strong case for maintaining routine surveillance in the modern era as per current clinical guidelines. Emerging biomarkers, such as Oncotype DX [Citation23] and circulating tumor DNA [Citation24], promise to predict the risk of disease recurrence more accurately and may be incorporated into tumor staging and prognostication, to fine-tune surveillance. In the meantime, a degree of surveillance for all non-metastatic colon cancers should remain the standard of care.
Conclusions
Our data demonstrate a clear impact of the initial stage on patterns of recurrence, resection of recurrent disease, and post-resection survival. While we observed a decline in recurrence rates over time a more aggressive approach to treating recurrent disease is the likely explanation for a similar proportion of patients undergoing curative-intent resection. Ultimately, these data suggest that surveillance following colon cancer surgery remains beneficial for patients, with an appropriate case-by-case consideration based on increased age or very early-stage disease. As multidisciplinary care of recurrence episodes continues to improve, the benefit of surveillance could be enhanced.
Disclosure statement
The authors declare no conflicts of interest.
References
- Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–691.
- Meyerhardt JA, Mangu PB, Flynn PJ, et al. Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol. 2013;31(35):4465–4470.
- Renehan AG, Egger M, Saunders MP, et al. Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trials. BMJ. 2002;324(7341):813–813.
- Tjandra JJ, Chan MK. Follow-up after curative resection of colorectal cancer: a meta-analysis. Dis Colon Rectum. 2007;50(11):1783–1799.
- Pita-Fernandez S, Alhayek-Ai M, Gonzalez-Martin C, et al. Intensive follow-up strategies improve outcomes in nonmetastatic colorectal cancer patients after curative surgery: a systematic review and meta-analysis. Ann Oncol. 2015;26(4):644–656.
- Party CCACCGW. Clinical practice guidelines for the prevention, early detection and management of colorectal cancer. Sydney: Cancer Council Australia; 2017.
- Osterman E, Glimelius B. Recurrence risk after up-to-date colon cancer staging, surgery, and pathology: analysis of the entire Swedish population. Dis Colon Rectum. 2018;61(9):1016–1025.
- Kosmider S, Stella DL, Field K, et al. Preoperative investigations for metastatic staging of colon and rectal cancer across multiple centres–what is current practice? Colorect Dis. 2009;11(6):592–600.
- Benson AB, Desch CD, Flynn PJ, et al. 2000 Update of American Society of Clinical Oncology colorectal cancer surveillance guidelines. J Clin Oncol. 2000;18(20):3586–3588.
- PBS. PBAC outcomes – positive recommendations. Canberra: Australian Government; 2005. Available from: https://www.pbs.gov.au/info/industry/listing/elements/pbac-meetings/pbac-outcomes/2005-03/positive-recommendations
- Hohenberger W, Weber K, Matzel K, et al. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation–technical notes and outcome. Colorectal Dis. 2009;11(4):354–364.
- Primrose JN, Perera R, Gray A, et al. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA. 2014;311(3):263–270.
- Power DG, Kemeny NE. Chemotherapy for the conversion of unresectable colorectal cancer liver metastases to resection. Crit Rev Oncol Hematol. 2011;79(3):251–264.
- Rosati G, Ambrosini G, Barni S, et al. A randomized trial of intensive versus minimal surveillance of patients with resected Dukes B2-C colorectal carcinoma. Ann Oncol. 2016;27(2):274–280.
- Pugh SA, Shinkins B, Fuller A, et al. Site and stage of colorectal cancer influence the likelihood and distribution of disease recurrence and postrecurrence survival: data from the FACS randomized controlled trial. Ann Surg. 2016;263(6):1143–1147.
- Neeff HP, Drognitz O, Holzner P, et al. Outcome after repeat resection of liver metastases from colorectal cancer. Int J Colorectal Dis. 2013;28(8):1135–1141.
- Wong H-L, Boolell V, Kosmider S, et al. Impact of primary tumor stage on survival following resection of metachronous liver and/or lung metastases in colorectal cancer. J Clin Oncol. 2015;33(15_suppl):3557–3557.
- O'Connell MJ, Campbell ME, Goldberg RM, et al. Survival following recurrence in stage II and III colon cancer: findings from the ACCENT data set. J Clin Oncol. 2008;26(14):2336–2341.
- Tie J, Lipton L, Desai J, et al. KRAS mutation is associated with lung metastasis in patients with curatively resected colorectal cancer. Clin Cancer Res. 2011;17(5):1122–1130.
- Snyder RA, Hu C, Cuddy A, et al. Association between intensity of posttreatment surveillance testing and detection of recurrence in patients with colorectal cancer. JAMA. 2018;319(20):2104–2115.
- Wille-Jørgensen P, Syk I, Smedh K, et al. Effect of more vs less frequent follow-up testing on overall and colorectal cancer-specific mortality in patients with stage II or III colorectal cancer: the COLOFOL randomized clinical trial. JAMA. 2018;319(20):2095–2103.
- Jeffery M, Hickey BE, Hider PN. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2019;9:CD002200.
- You YN, Rustin RB, Sullivan JD. Oncotype DX(®) colon cancer assay for prediction of recurrence risk in patients with stage II and III colon cancer: a review of the evidence. Surg Oncol. 2015;24(2):61–66.
- Tie J, Wang Y, Tomasetti C, et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med. 2016;8(346):346ra92.
