Background
Advanced rectal cancers are treated with neo-adjuvant (chemo)radiotherapy followed by surgery, but 5–10% experience locoregional recurrence after initial treatment. Radical (R0) salvage surgery is the main treatment and the most important prognostic factor for overall survival [Citation1,Citation2]. Neo-adjuvant re-irradiation has been introduced to increase R0 resection rates [Citation3,Citation4].
Several, mainly retrospective, studies have described re-irradiation of localized rectal cancer recurrences [Citation5–8]. Two systematic reviews have evaluated toxicity and outcomes of re-irradiation in rectal cancer [Citation9,Citation10]. Re-irradiation doses were generally around 40 Gy. The studies report similar levels of grade 3–4 toxicity, (Guren: 9–20% grade 3–4 acute toxicity; Lee: grade ≥ 3 acute and late toxicities in 11.7% and 25.5% for resected and non-resected patients, respectively).
Proton therapy allows for highly conformal treatment. The absence of the low-dose-bath reduces excess dose to surrounding organs at risk (OAR).
Only a few clinical studies have published data on re-irradiation for rectal recurrence with proton therapy [Citation11–13], with doses ranging from 16 Gy relative biological effectiveness (RBE) to 64.8 Gy RBE. All reported acceptable toxicities; Acute toxicity grade 1–3 (14–60%), late toxicity grade 1–4 (14–43%).
The risk of toxicity is the dose-limiting factor in re-irradiation. In this study we compared intensity modulated proton therapy (IMPT) with volumetric modulated arc photon therapy (VMAT) for re-irradiation of pelvic recurrences from rectal cancer with the aim of decreasing dose to surrounding OARs.
Material and methods
Patients
All patients enrolled in the Re-RAD-I study (External beam radiotherapy for pelvic recurrences in rectal cancer patients previously treated with radiotherapy) (VEK no: 1-10-72-417-14) were eligible for comparative IMPT planning. One patient was excluded due to insufficient retrieval of photon plan. Patients were treated at Aarhus University Hospital, Denmark and Oslo University Hospital, Norway. The primary neo-adjuvant radiotherapy included 3 D conformal technique (n = 11), 5-field IMRT (n = 2), and 2-arc VMAT (n = 2). Primary dose was 25 Gy in 5 fractions (n = 4), 48 Gy in 24 fractions (n = 1), 50 Gy in 25 fractions (n = 5), 52 Gy in 26 fractions (n = 4), or 60 Gy in 30 fractions (n = 1).
Re-irradiation was planned with 40.8 Gy in 1.2 Gy per fraction, twice-a-day and concomitant capecitabine 825 mg/m2 twice daily. Planning CT scans were done in supine position (2.5–3 mm slices) with intravenous contrast. MR scans in treatment position included T2 weighted axial and sagittal sequences. Clinical target volume (CTV) was delineated according to the protocol, a symmetrical margin of 10 mm (trimmed to anatomical barriers) was added for the internal target volume (ITV) plus an additional 7 mm margin for the planning target volume (PTV). OARs were delineated according to RTOG guidelines [Citation14] or anatomy. The re-irradiation photon plans included VMAT techniques; 1 arc (n = 2), 2 half-arcs (n = 1), and 2 arcs (n = 12), Eclipse (Varian Medical Systems, Inc, Palo Alto, CA, USA). The plans were normalized to target (PTV) and PTV dose was between 95–107% of the prescribed dose.
The Re-RAD-I study was approved by the local ethical committee and The Danish Data Protection Agency. In Norway, by the Regional Ethical Committee South-East (2015/1527) and the Institutional Protocol Review Board. All patients gave written informed consent.
Proton therapy dose planning, IMPT
Three posterior fields (gantry angles 150°, 180° and 210°) were used for IMPT plans. A 5 cm range shifter was used depending on the water equivalent thickness (WET) to the CTV (<4 cm) for individual fields.
To steer the optimization, relative tumor volumes (RTVs) based on the CTVs were used. RTVs take into account a 5 mm setup error, a calibration curve error of 3.5% and 1 mm proximal and distal margin, respectively.
IMPT with multi field optimization (MFO) robust optimization and evaluation was done for 14 scenarios; 2 scenarios with 0 mm and 12 scenarios with 5 mm setup error and, all with 3.5% calibration curve error. An RBE factor of 1.1 was used. CTV dose coverage criterion for the 14 robust optimized scenarios fulfilled a worst-case scenario of V95%≥98%.
IMPT plans were optimized in Eclipse v13.7 (Varian Medical Systems, Inc, Palo Alto, CA, USA) and normalized to target (CTV) mean of 40.8 Gy (RBE). Dose to OAR was kept as low as reasonably possible.
Data analysis
The mean and near maximum (D0.03ccm) organ doses were compared for VMAT vs. IMPT plans including: bowel loops, bladder, sacral bone, penile bulb and vagina. Ureter and femoral heads were analyzed as ipsilateral and contralateral structures. CTV volumes differed considerably, therefore we compared benefit of IMPT for CTVs < or ≥ the median CTV volume of 115 cm3.
Wilcoxon's signed rank test or Wilcoxon's rank sum were used for comparison (MATLAB ver. R2020b). A Bonferroni corrected p-value of ≤0.0024 (0.05/21) was considered statistically significant.
Results
Comparative dose planning was done for fifteen patients (8 males, 7 females).
Median CTV volume for re-irradiation was 115 cm3, but with considerable variation (range: 19.5–1080.6 cm3).
For the VMAT plans the median target (PTV) coverage was D98% = 94.4% (interquartile range (IQR): 90.1–96.7%); for the IMPT plans the median worst-case scenario target (CTV) coverage was D98% = 96.0% (IQR: 95.3–96.8%).
Mean doses (Dmean) were lower with IMPT compared to VMAT for all delineated OARs. For bowel loops, bladder and sacral bone, median Dmean was significantly reduced from 3.2 Gy to 0.4 Gy, from 8.2 Gy to 1.7 Gy, and from 8.7 Gy to 4.2 Gy, respectively. For ureters Dmean was lowered from 10.6 Gy to 9.3 Gy ipsilaterally and significantly from 5.7 Gy to 0.03 Gy, contralaterally. Near max doses (D0.03ccm) differed according to OAR position relative to target. For OARs close to the target, no difference in maximum dose was seen. All OAR Dmean and D0.03ccm doses are presented in . shows mean dose-volume histograms (DVHs) for bowel loops, bladder and sacral bone including standard deviations for VMAT versus IMPT plans.
Figure 1. (a) Mean DVHs and standard deviations (SD) of bowel loops, bladder, and sacral bone. VMAT photon (red), IMPT proton (blue). (b) Box plots for the Δ mean-dose (Δ mean-dose = VMAT – IMPT dose) for bowel loops, bladder and sacral bone for recurrence CTV volume < (red) or ≥ (blue) than the median volume of 115 cm3. Box plots define the interquartile range (IQR) with the 25th and 75th percentile as top and bottom. The whiskers length is defined as 1.5 times the interquartile range and outliers beyond are marked.
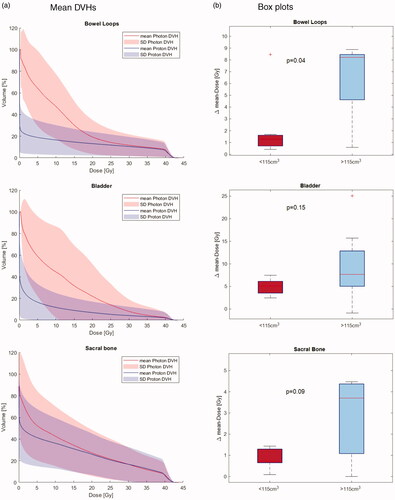
Table 1. Organ at risk doses for the recurrence treatment plans, VMAT vs. IMPT.
Comparing CTVs < or ≥115 cm3, a non-significant benefit of IMPT (Δmean-dose = VMAT – IMPT dose) was seen for CTVs >115 cm3 for bowel loops (8.2 Gy versus 1.3 Gy, p = 0.04), bladder (7.7 Gy versus 4.6 Gy, p = 0.15), and sacral bone (3.7 Gy versus 0.7 Gy, p = 0.09), as illustrated in .
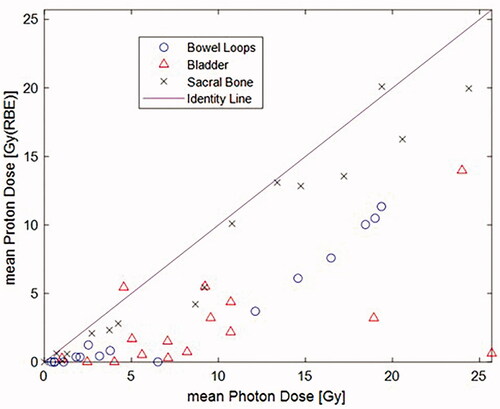
displays a scatter plot for mean photon (Gy) versus mean proton (Gy/RBE) dose for bowel loops, bladder and sacral bone. All but two are below the identity line, and in favor of protons.
Discussion
We report the results of comparative VMAT and IMPT dose planning for re-irradiation of rectal cancer pelvic recurrences. This is the first comparative dose planning study for this patient group, and allows us to assess the potential benefit of IMPT relative to VMAT. We found that mean doses to delineated OARs were lower with IMPT and allowed for sparing of OARs. D0.03ccm was also lower for OAR not in the vicinity of the target, otherwise comparable between the two groups. Larger recurrences showed a trend for greater dosimetric benefit.
Proton therapy can be relevant for re-irradiation when the surrounding healthy tissue has received maximum or near maximum tolerated doses. There are limited data on normal tissue tolerances for pelvic re-irradiation [Citation15,Citation16], but it is probable that any dose sparing achievable with IMPT could contribute to a decreased toxicity due to high cumulative doses. Protons do, however, not reduce maximum dose to OAR in close proximity of the target, as our finding supports. It is the low dose bath to the OARs surrounding the target volume that can be reduced with IMPT.
Only few studies on proton re-irradiation of rectal cancer recurrences have been published. The studies are heterogeneous with a mixture of rectal and other pelvic cancers, different re-irradiation doses (16–64.8 Gy (RBE)), schedules, and both neo-adjuvant or definitive intent. Generally, acute and late toxicities seemed manageable [Citation13,Citation14]. Re-irradiation doses for both the proton and photon re-irradiation studies are often kept around 40 Gy, to minimize combined doses to OARs. However, higher doses are associated with better pathological response in rectal cancer [Citation17]. As studies indicate that toxicity after re-irradiation is acceptable, dose-escalation with proton therapy might be relevant, in order to increase the R0 resection rates or as an option for definitive treatment for the un-resectable recurrences.
Posterior fields are often used in pelvic proton therapy, to reduce impact from changing bowel gas/fillings, weight and other variable conditions [Citation13,Citation18]. This can result in slightly higher dose to the parts of the sacral bone close to the CTV. However, we found comparable D0.03ccm and lower Dmean with IMPT.
In conclusion, IMPT for pelvic re-irradiation resulted in lower mean doses to OARs, which could translate into clinical benefit with decreased side effects, or enable dose escalation regimens in future trials.
Acknowledgements
The authors would like to thank Espen Rusten for assistance with extracting dose data from the treatment planning system for the Norwegian patients.
Disclosure statement
The authors report no conflicts of interest
References
- Guren MG, Kørner H, Pfeffer F, et al. Nationwide improvement of rectal cancer treatment outcomes in Norway, 1993–2010. Acta Oncol. 2015;54(10):1714–1722.
- Nielsen M, Rasmussen P, Pedersen B, et al. Early and late outcomes of surgery for locally recurrent rectal cancer: a prospective 10-year study in the total mesorectal excision era. Ann Surg Oncol. 2015;22(8):2677–2684.
- Palmer G, Martling A, Cedermark B, et al. A population-based study on the management and outcome in patients with locally recurrent rectal cancer. Ann Surg Oncol. 2007;14(2):447–454.
- Valentini V, Morganti AG, Gambacorta MA, et al. Preoperative hyperfractionated chemoradiation for locally recurrent rectal cancer in patients previously irradiated to the pelvis: a multicentric phase II study. Int J Radiat Oncol Biol Phys. 2006;64(4):1129–1139.
- Mohiuddin M, Lingareddy V, Rakinic J, et al. Reirradiation for rectal cancer and surgical resection after ultra high doses. Int J Radiat Oncol Biol Phys. 1993;27(5):1159–1163.
- Mohiuddin M, Marks GM, Lingareddy V, et al. Curative surgical resection following reirradiation for recurrent rectal cancer. Int J Radiat Oncol Biol Phys. 1997;39(3):643–649.
- Lingareddy V, Ahmad NR, Mohiuddin M. Palliative reirradiation for recurrent rectal cancer. Int J Radiat Oncol Biol Phys. 1997;38(4):785–790.
- Mohiuddin M, Marks G, Marks J. Long-term results of reirradiation for patients with recurrent rectal carcinoma. Cancer. 2002;95(5):1144–1150. 2002.
- Lee J, Kim CY, Koom WS, et al. Practical effectiveness of re-irradiation with or without surgery for locoregional recurrence of rectal cancer: a meta-analysis and systematic review. Radiother Oncol. 2019;140:10–19.
- Guren MG, Undseth C, Rekstad BL, et al. Reirradiation of locally recurrent rectal cancer: a systematic review. Radiother Oncol. 2014;113(2):151–157.
- Moningi S, Ludmir E, Polamraju P, et al. Definitive hyperfractionated, accelerated proton reirradiation for patients with pelvic malignancies. Clin Transl Radiat Oncol. 2019;19:59–65.
- Koroulakis A, Molitoris J, Kaiser A, el al. Reirradiation for rectal cancer using pencil beam scanning proton therapy: a single institutional experience. Adv Radiat Oncol . 2021; 6(1):100595.
- Berman AT, Both S, Sharkoski T, et al. Proton reirradiation of recurrent rectal cancer: dosimetric comparison, toxicities, and preliminary outcomes. Int J Part Ther. 2014;1(1):2–13.
- Gay HA, Barthold HJ, O'Meara E, et al. Pelvic normal tissue contouring guidelines for radiation therapy: a radiation therapy oncology group consensus panel atlas. Int J Radiat Oncol Biol Phys. 2012;83(3):e353–e362.
- Das P, Delclos ME, Skibber JM, et al. Hyperfractionated accelerated radiotherapy for rectal cancer in patients with prior pelvic irradiation. Int J Radiat Oncol Biol Phys. 2010;77(1):60–65.
- Abusaris H, Hoogeman M, Nuyttens J. Re-irradiation: outcome, cumulative dose and toxicity in patients retreated with stereotactic radiotherapy in the abdominal or pelvic region. Technol Cancer Res Treat. 2012;11(6):591–597.
- Appelt A, Pløen J, Vogelius I, et al. Radiation dose-response model for locally advanced rectal cancer after preoperative chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2013;85(1):74–80.
- Gort EM, Beukema JC, Matysiak W, et al. Inter-fraction motion robustness and organ sparing potential of proton therapy for cervical cancer. Radiother Oncol. 2021;154:194–200.