Abstract
Background and purpose
To analyse the cumulative incidence of any failure (AF), prostate cancer-specific failure (PCSF), any death (AD), prostate cancer-specific death (PCSD), and local control in 2387 men with prostate cancer (PC), consecutively treated with combined high-dose-rate brachytherapy (HDRBT) and external beam radiotherapy (EBRT) from 1998 to 2010.
Material and methods
A retrospective, single-institution study of men with localised PC. The mean age was 66 years and 54.7% had high-risk PC according to the Cambridge prognostic group (CPG) classification. The treatment was delivered as EBRT (2 Gy × 25) and HDRBT (10 Gy × 2) with combined androgen blockade (CAB). The median follow-up was 10.2 years.
Results
The cumulative incidence of PCSD at 10 years was 5% [CI 95% 0.04–0.06]. The 10 years PCSD per risk group were: low (L) 0.4%, intermediate favourable (IF) 1%, intermediate unfavourable (IU) 4.3%, high-risk favourable (HF) 5.8%, and high-risk unfavourable (HU) 13.9%. The PCSF rate at 10 years was 16.5% [CI 95% 0.15–0.18]. The PCSF per risk group at 10 years were: L 2.5%, IF 5.5%, IU 15.9%, HF 15.6%, and HU 38.99%. PCSF occurred in 399 men, of whom 15% were found to have local failure. The estimated frequency of local failure in the entire cohort was 1.2%.
Conclusions
HDRBT combined with EBRT is an effective treatment with long-term overall survival and excellent local control for patients with PC. The low rate of local recurrence among men with relapse suggests that these patients were micro metastasised at time of treatment, which calls for improved methods to detect disseminated disease.
Introduction
Modern radiotherapy (RT) for prostate cancer (PC) is based on the concept of dose-escalated RT and has developed during recent decades [Citation1–5]. As a consequence of a low a/β ratio, PC tumours have high sensitivity to dose fractionation [Citation6], which supports the use of hypo-fractionated RT to achieve dose escalation. To meet the challenge to deliver hypo-fractionated RT within the limits of normal tissue tolerance for organs at risk, high-dose-rate brachytherapy (HDRBT) is an attractive treatment approach [Citation7–9].
Since 1998, a combination of two sessions of HDRBT and conformal external beam radiotherapy (EBRT) has been an established treatment option for PC at Karolinska University Hospital. The principles of treatment, 5-year outcome and toxicity data have been published previously for a subset of the patients [Citation10].
In the present retrospective, single-institution study, the aim was to analyse the 10-year outcome in men with PC, consecutively treated from 1998 to 2010 with combined EBRT and HDRBT, in terms of local control, PC specific failure (PCSF), PC specific death (PCSD), and death from any cause. In addition, the effects on treatment efficacy with respect to risk-group classification and time period of treatment were investigated.
Material and methods
Patient cohort
A total of 2893 consecutive patients with localised PC, who were treated from May 1998 to December 2010 with EBRT and HDRBT at Karolinska University Hospital were included in this retrospective, single-institution study cohort. After application of exclusion criteria, 2387 patients remained for analyses (). The study was approved by the Ethic committee of the Stockholm region (2006/620-31/1).
Figure 1. Consort diagram of the cohort of 2893 consecutive men treated with combined radiotherapy in Stockholm 1998–2010 and reasons for exclusion from the study cohort.
All patients had histopathologically verified PC, assessed by the Gleason grading system [Citation11]. The T-stage was defined according to the TNM classification, 5th–7th edition [Citation12]. Further staging was performed with surgical lymph node dissection (LND) and bone scan, initially in all men with prostate-specific antigen (PSA) >10 µg/L. However, from July 2000, only patients with high-risk characteristics (Gleason ≥ 4 + 3, PSA ≥20 µg/L) and from July 2007 those with T3 tumour were selected for these staging procedures. Only men with T-stage ≤ T3a and negative LND and bone scan were treated with the combined RT approach.
Radiotherapy (RT)
The EBRT planning target volume (PTV) covered the prostate gland and the seminal vesicles with a 2.0 cm margin, except posterior, where it was 1.5 cm. From 2005, the margins were reduced to 1.5 and 1.0 cm, respectively. The total EBRT dose was 50 Gy delivered in 25 fractions and the 95% iso-dose had to comprise the PTV. The constraint to organs at risk was limited to a restriction to have less than 50 Gy to the posterior rectal wall. The HDRBT target dose was 20 Gy, administered in two 10 Gy fractions, 2 weeks apart, covering the prostate gland and the base of the vesicles with a 3 mm margin (PTV HDRBT). The V100% was minimum 90% of the prescribed nominal dose to the prostate and vesicle base. V200% was limited to <10% of PTV HDRBT. Urethra was defined as a 5 mm circle. Dmax urethra was set to < 110% and V100% to < 70% of the prescribed dose. Constraints to the rectal mucosa (upper surface of the ultrasound probe) were set to Dmax <60% of the prescribed dose. No dose constraints were applied to the bladder. The applied RT technique has been described in detail previously [Citation10].
Hormonal treatment
Most patients (95%), received neoadjuvant and concomitant combined androgen blockade (CAB) for 6 months. The indication for CAB treatment changed over time. From November 2008, 3 months of adjuvant CAB was added in high-risk patients.
Follow-up
The follow-up procedures varied over the years and were more intense in the early period 1998–2005. The minimum follow-up for all patients was performed at 3 months after treatment, comprising physical examination and blood samples including PSA. Thereafter, all were followed up every 6 months for 2 years and then annually until 10 years after treatment. Biochemical failure was defined as PSA nadir + 2 ng/mL according to the Phoenix definition [Citation13]. Patients with biochemical failure were referred to fine-needle aspiration biopsies of the residual prostate mass to diagnose any local recurrence.
Risk group classification
Patients were divided into five risk groups according to the Cambridge prognostic group (CPG) risk classification [Citation14]. These criteria originate from the D’Amico criteria but have been further developed to better reflect the differences in prognosis in the intermediate-risk group. Furthermore, the CPG also includes stage T3 and T4 tumours and was developed to specifically address death from PC. The risk groups were defined as follows: low risk (L) PSA <10 and Gleason score ≤6 and cT1- cT2, intermediate-risk favourable (IF) Gleason score 3 + 4 or PSA 10–20 and cT1- cT2, intermediate-risk unfavourable (IU) PSA 10–20 and Gleason score 3 + 4 and cT1- cT2 or Gleason score 4 + 3 and cT1- cT2, high-risk favourable (HF) PSA >20 or Gleason score 8 or cT3 and high-risk unfavourable (HU) minimum 2 factors of; PSA > 20, Gleason score 8, cT3 or Gleason score ≥9 or cT4.
Statistical considerations
Data in this study were continuously collected from patients’ medical records from 2005 until October 2019. All data were stored using Medlog system software (Information Analysis System, NV 89402, USA). Cause of death was categorised into two groups; (1) Death from other cause (DOC), including men with no evidence of PC at the time of death and men who died of other causes after biochemical failure, with or without hormonal treatment, and with no sign of progression during such treatment. (2) Men with PSA progression during hormonal treatment and progressive metastatic PC were classified as PCSD. Any cause of death (AD) consists of both DOC and PCSD.
Failure was defined as either death due to other causes as first event (DOCF) or PC specific failure (PCSF) as first event. PCSF was defined as biochemical failure according to the Phoenix definition, or the occurrence of PC metastases. Any failure (AF) was defined as the first event of PCSF or DOCF. The pre-defined primary outcome was PCSD, with other outcomes considered secondary. In the analysis of time to death, survival times were calculated from the date of the first HDRBT treatment to the date of death, or for patients who were still alive, to the date of the last clinical visit. In the analysis of time to failure, survival times were calculated from the date of the first HDRBT treatment to the date of first failure, and for event-free patients to the date of the last clinical visit. Descriptive statistics such as means, standard deviations, counts, and percentages were used to characterise the study cohort. Distributional differences in the risk group were tested using the chi-square test of independence for categorical variables. Graphs of cumulative incidence were employed to illustrate risk over time. Competing risks were considered when calculating cumulative incidence functions. Univariate and multivariate modelling of the risk group was performed using competing risk regression. The multivariate models were stratified for the covariates: age (<65, >65 years), time of treatment (1998–2005, 2006–2010), comorbidity (0, 1–2, 3–9), and hormonal treatment (yes, no). Results from these models are presented as sub-hazard ratios (sHRs) or hazard ratios (HRs) for models with no competing risk (AD, AF), together with 95% confidence intervals. Reported p values from these models refer to the Wald test for the comparison of within-group categories and to the likelihood ratio test for the overall group effect. The significance level was set to .05. All statistical analyses were conducted using the Stata version 16 statistical software (StataCorp, College Station, TX, USA).
Results
In total, 2387 men were included in this study, with a median follow-up of 10.2 years (25 percentile 8.7 years and 75 percentile 12.5 years) until October 2019, when the database was closed. Patient characteristics according to risk group are shown in . A comparison of men treated before or after 31 December 2005 showed a mean age of 66 years in both groups and only small variations in prognostic markers ‘PSA at diagnosis’ and T-stage (data not shown). There was, however, a difference between the two periods concerning Gleason grade. In the early cohort the Gleason grade was; 6 (42%), 3 + 4 (28%), 4 + 3 (13%), ≥8 (16%), compared to 6 (25%), 3 + 4 (39%), 4 + 3 (22%), and ≥8 (14%) in the later cohort. Despite this, there were no major differences in risk group distribution between the cohorts ().
Table 1. Patient characteristics by Cambridge prognostic group risk classification.
Prostate cancer-specific death (PCSD) and any death (AD)
In total, 1662 (69%) men were still alive at the time of data collection, of whom 1491 (90%) had no sign of recurrence. During follow-up 725 (30%) men died, 145 (6%) due to PC. The majority, 580 (24%) died from causes other than PC. This group included 90 men (4%) who died from other causes after a PC relapse. Survival status according to risk group is shown in . The median time to death was 13.7 years and the cumulative incidence of AD was 23%, corresponding to an overall survival at 10 years of 77%. At 5, 10, and 15 years, respectively, the cumulative incidence of PCSD was 1.5%, CI 95% [0.003, 0.01], 5%, CI 95% [0.005, 0.04], and 10.2%, CI 95% [0.01, 0.08]. Comparing AD and PCSD between men treated from 1998 to 2005 and from 2006 to 2010 showed no difference between the two time periods (sHR 1.1, CI 95% [0.94, 1.3] p = .23).
Table 2. Survival and failure status by CPG risk classification.
AD and PCSD according to risk group are presented in . Numbers at risk from 0 to 15 years per risk group are presented in Supplementary Table 1a. There were statistically significant differences between risk groups in AD and PCSD, but no difference in the risk of DOC. In the L and IF risk groups, the risk of dying from PC was 0.4%, CI 95% [0.0003, 0.02] and 1%, CI 95% [0.004, 0.02], respectively, at 10-year follow-up. The corresponding numbers for the IU, HF, and HU groups were 4.3%, CI 95% [0.02, 0.07], 5.8%, CI 95% [0.03, 0.06], and 13.9%, CI 95% [0.11, 0.17], respectively.
Figure 2. Cumulative incidence estimates of death and failure by risk group. (a) Cause of death, total cohort; (b) prostate cancer-specific death by risk group (PCSD); (c) any death by risk group (AD), (d) cause of first failure, total cohort; (e) prostate cancer-specific failure (PCSF) as first failure by risk group (PCSF); (f) any failure by risk group (AF). Death of other cause (DOC), death of other cause as first failure (DOCF), risk group; L: low; IF: intermediate favourable; IU: intermediate unfavourable; HF: high risk favourable; HU: high risk unfavourable. Numbers at risk per risk-group regarding AD and AF are presented in Supplementary Table 1a and b, respectively.
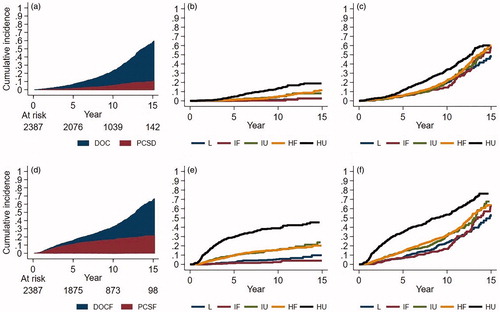
Competing risk regression analysis by risk group, considering PCSD and DOC, using IF as reference, showed a statistically significant increase in the risk of PCSD and AD associated with higher risk group, p < .0001 and p < .001, respectively. This finding was confirmed in the adjusted model, stratified for age, treatment period, comorbidity, and hormonal treatment (p < .0001 and p < .0001, respectively) (). Moreover, there were no differences between the risk groups in DOC; p = .8 and .9 in the uni and multivariate analysis, respectively. The association between higher risk group and increased risk of PCSD and AD, was analysed according to study time, split in three time periods, and was found to be predictive up to 10 years (p < .0001 and p < .0001 respectively), Supplementary Table 2.
Table 3. Competing risk multivariate regression analysis (sHR, CI 95%, p) for failure and death, by Cambridge prognostic group risk classification.
Prostate cancer-specific failure (PCSF), any failure (AF)
At the time of death or analysis of data in this study 1988 (83%) had no evidence of disease. Failure status by CPG risk group is shown in . The cumulative incidence of AF at 5 and 10 years was 15.7% [CI 95% 0.01–0.14] and 32.3%, [CI 95% 0.01–0.30], corresponding to a progression-free survival at 5 and 10 years of 84.3% and 68%, respectively. The corresponding figures for PCSF at the same time points were 10.8%, CI 95% [0.10–0.12] and 16.5%, CI 95% [0.15–0.18]. PCSF according to risk group at 5 and 10 years was; L 1.3 and 2.5%, IF 2.3 and 5.5%, IU 9.7 and 15.9%, HF 9.4 and 15.6% and HU 29.3 and 38.9%, respectively, . Numbers at risk per risk group for 0–15 years are presented in Supplementary Table 1b. In the group of men with PCSF as first failure (n = 399) fine-needle biopsies were performed in 49%. Of these men, 29 (15%) out of 196 were found to have residual cancer in the prostate gland. The occurrence of local failure in the group of men with PCSF corresponds to an estimated local failure rate of 1.2% in the entire cohort. The rate of local failure according to risk group is shown in . Competing risk regression analysis by risk group, taking account of PCSF and DOCF as first failure, using IF as reference, confirmed an increased risk of PCSF and AF associated with higher risk group, but not for DOCF as first failure (p < .0001, p < .0001, and p = .2, respectively). The adjusted model, stratified for age, treatment period, comorbidity, and hormonal treatment confirmed these results (). Further analysis of PCSF and DOCF according to time after radiotherapy, confirmed the association between higher risk group and increased risk of PCSF and AF up to 10 years (p < .0001 and p < .0001, respectively), Supplementary Table 2.
Discussion
To the best of our knowledge, the present real-world data study is the largest single centre study, reporting the outcome of 2387 consecutive patients with PC, treated with combined EBRT and HDRBT with CAB treatment for 6–9 months. This treatment approach resulted in an overall low risk of local failure (1.2%), PCSF (10.5%), and PCSD (5%) at a median follow-up of 10 years, although 55% of patients were diagnosed with typical high-risk PC.
In a review from 2015, De Bari et al. evaluated the results of 12 studies, including 2 randomised clinical trials, investigating combined HDRBT and EBRT treatment [Citation15]. They reported biochemical-free survival (BFS) rates at 5 years of 90–100%, 69–97%, and 63–97% in low, intermediate and high-risk patients, respectively. Since then several authors have reported 10-year results confirming stable levels of BFS rates at 10 years for low and intermediate PC, 93–100% and 79–91%, respectively. In the high-risk group, a slight decrease in BFS was generally reported, compared to the 5-years figures, 62–84% [Citation16–18]. Concerning the PC-specific survival at 10 years, several studies, report on survival rates of 94–98% in intermediate and high-risk cohorts [Citation8,Citation9,Citation16–21]. Although the treatment results are excellent, there are differences in the reported incidence of biochemical failure between studies, especially in the high-risk group. It is likely that these differences can be attributed to differences in the definition of risk groups, treatment technique, or to the use of androgen ablation therapy. Still our results, from a large population of consecutively treated patients, support the use of EBRT and HDRBT as a safe and efficient treatment option for men with PC.
The CPG risk classification was used in our analysis, which previously demonstrated differences in prognosis between five risk groups in men with PC [Citation22]. This classification was also found to have strong prognostic value in terms of the risk of PC failure and death up to 10 years in the present cohort. The prognostic value remained when adjusted for age, time of treatment, comorbidity, and hormonal treatment. Unexpectedly, the patients in our cohort were regrouped from five into three new distinct risk categories. The L and IF groups formed a low-risk group. The IU and HF groups constituted a new intermediate-risk group. The outcome in the IU group was similar to the outcome in the HF group in terms of PCSF and PCSD. The risk of PCSF and PCSD was considerably lower in these two groups when compared to the HU group, which formed the new high-risk category.
In the HU group, the risk of recurrence was three times higher at 5 years compared to the IU and HF groups, after which the increased risk levelled out. Two other studies showed a similar pattern of recurrence in the high-risk group, with most failures before 5 years, followed by a plateau [Citation16,Citation23]. In our study, the presence of local failure was investigated by fine-needle aspiration biopsies in 49% of cases with PCSF. Local failure was confirmed by positive biopsies in 15% of these patients, corresponding to an estimated 1.2% risk of local failure in the entire cohort. Given that local failure was estimated to approximately 1% in all treated patients, while PCSF was 16.5% at 10 years for the whole cohort, it reasonable to anticipate that the major reason for relapse is due to micro metastatic disease at time of commence of RT. More sensitive methods for detecting disseminated disease, such as PSMA-PET/CT or ctDNA may improve the precision in selecting patients for this treatment modality and should be evaluated in prospective studies.
The main limitations of this study are the retrospective approach, as well as the changes in diagnostic procedure and classifications of patients that occurred over time. In particular, changes in Gleason grading in 2005 led to one-third of tumours being upgraded, compared to the 1992 version of Gleason grading [Citation11]. A strength of the study is that complete data were available for >99% of patients. Another strength is the large consecutive patient population, treated at a single centre and followed-up uniformly for 10 years from the start of treatment, providing reliable 10-years estimates.
Conclusion
HDRBT combined with EBRT is an effective treatment with long-term overall survival for patients with localised and locally advanced PC. The estimated local failure rate was 1.2%, indicating excellent local control. The low rate of local recurrence among patients with PCSF suggests that these patients were most likely micro metastasised at the time of commencing the RT, which calls for improved methods to detect disseminated disease prior to treatment. The rate of PCSF and PCSD was significantly correlated with the CPG risk-group classification, but the patients in our cohort were regrouped into three new distinct risk categories. This finding could be of clinical importance for avoiding both under and overtreatment of men in the intermediate and high-risk groups.
Supplemental Material
Download MS Word (23.8 KB)Supplemental Material
Download MS Word (22.3 KB)Acknowledgments
The authors thank Carina Holmberg research nurse for her work completing our database. After completion of this manuscript Professor Bo Lennernäs sadly passed away. He was instrumental in the introduction of HDR brachytherapy as a curative treatment in Sweden.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Zelefsky MJ, Yamada Y, Fuks Z, et al. Long-term results of conformal radiotherapy for prostate cancer: Impact of dose escalation on biochemical tumor control and distant metastases-free survival outcome. Int J Radiat Oncol Biol Phys. 2008;1571:1028.
- Dearnaley DP, Jovic G, Syndikus I, et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2014;15(4):464–473.
- Heemsbergen WD, Al-Mamgani A, Slot A, et al. Long-term results of the Dutch randomized prostate cancer trial: impact of dose-escalation on local, biochemical, clinical failure, and survival. Radiother Oncol. 2014;110(1):104–109.
- Kuban DA, Tucker SL, Dong L, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70(1):67–74.
- Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from Proton Radiation Oncology Group/American College of Radiology 95-09. J Clin Oncol. 2010;28(7):1106–1111.
- Fowler JF. The radiobiology of prostate cancer including new aspects of fractionated radiotherapy. Acta Oncol. 2005;44(3):265–276.
- Hoskin PJ, Rojas AM, Bownes PJ, et al. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother Oncol. 2012;103(2):217–222.
- Hsu I, Rodgers JP, Shinohara K, et al. Long-term results of NRG oncology/RTOG 0321: a phase II trial of combined high dose rate brachytherapy and external beam radiation therapy for adenocarcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2020;110:700–707.
- Martinez AA, Gonzalez J, Ye H, et al. Dose escalation improves cancer-related events at 10 years for intermediate- and high-risk prostate cancer patients treated with hypofractionated high-dose-rate boost and external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79(2):363–370.
- Kälkner KM, Wahlgren T, Ryberg M, et al. Clinical outcome in patients with prostate cancer treated with external beam radiotherapy and high dose-rate iridium 192 brachytherapy boost: a 6-year follow-up. Acta Oncol. 2007;46(7):909–917.
- Delahunt B, Egevad L, Samaratunga H, et al. Gleason and Fuhrman no longer make the grade. Histopathology. 2016;68(4):475–481. Review.
- Sobin LH, Wittekind CH, editors. International union against cancer. TNM classification of malignant tumours. 5th ed. New York (NY): John Wiley & Sons; 1997.
- Roach M, 3rd, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965–974.
- Gnanapragasam VJ, Bratt O, Muir K, et al. The Cambridge Prognostic Groups for improved prediction of disease mortality at diagnosis in primary non-metastatic prostate cancer: validation study. BMC Med. 2018;16(1):31.
- De Bari B, Daidone A, Alongi F. Is high dose rate brachytherapy reliable and effective treatment for prostate cancer patients? A review of the literature. Crit Rev Oncol Hematol. 2015;94(3):360–370.
- Prada PJ, González H, Fernández J, et al. Biochemical outcome after high-dose-rate intensity modulated brachytherapy with external beam radiotherapy: 12 years of experience. BJU Int. 2012;109(12):1787–1793.
- Galalae RM, Zakikhany NH, Geiger F, et al. The 15-year outcomes of high-dose-rate brachytherapy for radical dose escalation in patients with prostate cancer – a benchmark for high-tech external beam radiotherapy alone? J Brachy. 2014;13(2):117–122.
- Åström L, Grusell E, Sandin F, et al. Two decades of high dose rate brachytherapy with external beam radiotherapy for prostate cancer. Radiother Oncol. 2018;127(1):81–87.
- Ishiyama H, Kamitani N, Kawamura H, et al. Nationwide multi-institutional retrospective analysis of high-dose-rate brachytherapy combined with external beam radiotherapy for localized prostate cancer: an Asian Prostate HDR-BT Consortium. J Brachy. 2017;16(3):503–510.
- Helou J, D’Alimonte L, Loblaw A, et al. High dose-rate brachytherapy boost for intermediate risk prostate cancer: long-term outcomes of two different treatment schedules and early biochemical predictors of success. Radiother Oncol. 2015;115(1):84–89.
- Wedde TB, Småstuen MC, Brabrand S, et al. Ten-year survival after high-dose-rate brachytherapy combined with external beam radiation therapy in high-risk prostate cancer: a comparison with the Norwegian SPCG-7 cohort. Radiother Oncol. 2019;132:211–217.
- Zelic R, Garmo H, Zugna D, et al. Predicting prostate cancer death with different pretreatment risk stratification tools: a head-to-head comparison in a nationwide cohort study. Eur Urol. 2020;77(2):180–188.
- Galalae RM, Martinez A, Mate T, et al. Long-term outcome by risk factors using conformal high-dose-rate brachytherapy (HDR-BT) boost with or without neoadjuvant androgen suppression for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2004;1558(4):1048–1055.
