 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Prostate cancer oligometastatic disease can be treated using stereotactic body radiotherapy (SBRT) in order to postpone start of systemic treatments such as androgen deprivation therapy (ADT). 68Ga-PSMA-PET/CT imaging allows for diagnosis of oligometastases at lower PSA values. We analysed a cohort of patients with prostate cancer lymph node oligometastases detected on PSMA-PET/CT.
Materials and methods
Ninety patients with metachronous oligometastatic prostate cancer received SBRT for 1–3 lymph node metastases diagnosed on 68Ga-PSMA-PET/CT. The primary end point was progression free survival (PFS), with disease progression defined as occurrence of either target lesion progression, new metastatic lesion or biochemical progression. Secondary outcomes were biochemical PFS (BPFS), ADT-free survival (ADT-FS), toxicity and quality of life (QoL). Baseline patient characteristics were tested for association with PFS and a preliminary risk score was created.
Results
Median follow-up was 21 months (interquartile range 10–31 months). Median PFS and BPFS were 16 and 21 months, respectively. Median ADT-FS was not reached (73% (95%-CI 62–86%) at 24 months). In multivariable analysis, younger age, higher PSA prior to SBRT and extrapelvic location were associated with shorter PFS. Grade 1 fatigue was the most predominant acute toxicity (34%). Highest grade toxicity was grade 2 for acute and late events. QoL analysis showed mild, transient increase in fatigue at 1–4 weeks after SBRT.
Conclusion
A median PFS of 16 months was attained after SBRT for patients with PSMA-PET positive oligometastatic lymph nodes from prostate cancer. Higher pre-SBRT PSA, younger age and extrapelvic location were found to be predictors of shorter PFS.
Background
Stereotactic body radiotherapy (SBRT) has gathered increasing interest as a local treatment option for patients with oligometastatic prostate cancer. It reduces the chance of disease progression after 6 months from 61% with observation alone, to 19% with SBRT, and can defer the start of androgen deprivation therapy (ADT) with a median period of 8 months [Citation1,Citation2]. Moreover, a survival benefit has been shown compared to palliative standard-of-care for patients with oligometastases from a range of primary tumour histologies [Citation3].
With the advent of 68-gallium prostate-specific membrane antigen (PSMA) positron emission tomography (PET) imaging, prostate cancer oligometastases can be diagnosed with greater sensitivity compared with choline PET imaging, especially in patients with PSA <2 ng/mL [Citation4–6]. In the ORIOLE trial, patients were treated on metastatic lesions diagnosed on conventional imaging, but also underwent PSMA-PET imaging prior to treatment. Patients who did not have any additional (untreated) lesions on PSMA-PET showed fewer new metastases at 6 months after SBRT (16 vs. 63%) [Citation2].
However, even with PSMA-PET imaging, failure remains frequent after SBRT for oligometastases: recently reported progression-free survival (PFS) rates at 12 and 24 months were 46–73% and 16–73%, respectively [Citation6–12]. To counteract undetected microscopic tumour spread, intermittent or continuous ADT can be added to metastasis-directed therapy (MDT), which can improve (biochemical) PFS [Citation13–15]. ADT, however, influences quality of life (QoL) and seems counterintuitive to the application of MDT in trying to postpone systemic therapy [Citation1,Citation16]. Prediction of oncological outcomes could help physicians and patients in their shared decision making regarding SBRT with/without ADT [Citation17]. Tumour biology shows great promise for oligometastatic patient selection but it is not ready to be used in a clinical setting [Citation2,Citation18,Citation19]. Our aim was to report outcomes after PSMA-PET directed SBRT for lymph node oligometastases and find predictors of PFS to improve patient selection using baseline characteristics.
Materials and methods
Patients and treatment
We included patients with up to five metachronous 68Ga-PSMA-PET/CT-detected prostate cancer lymph node oligometastases who were treated with SBRT and had >3 months of follow-up. All patients gave written informed consent for participation in a single centre, retrospective/prospective cohort study approved by the local medical ethics committee (www.trialregister.nl/trial/9252). Exclusion criteria were simultaneous local tumour recurrence (including seminal vesicle recurrence), non-nodal metastases, previous polymetastatic disease or ADT up to 24 months before the current diagnosis.
Patients were treated between October 2016 and October 2020, with a prescribed dose of 5 × 7 Gy or 3 × 10 Gy to 95% of the planning target volume (PTV). 68Ga-PSMA-PET/CT scans were acquired at multiple centres and were all assessed by nuclear medicine radiologists. Pre-treatment tumour delineation was based on PET/CT and MRI after image fusion with the planning CT scan. Gross Tumour Volume (GTV) consisted of the target lymph node(s) and GTVs were expanded with a 3-8 mm PTV margin, depending on nodal region, treatment machine, visibility of the target and distance between GTVs. Patients were treated on CBCT-linac (Agility, Elekta AB, Stockholm, Sweden) or on 1.5 T MR-linac (Unity, Elekta AB) [Citation20]. Since the clinical introduction of the MR-linac at our department in August 2018, patients were treated on the MR-linac except for patients with exclusion criteria for MR-linac delivery, such as an inability to lie still for 60 min. Vacuum cushion immobilisation was used for all patients until March 2019; we then investigated effect on target motion [Citation21]. After March 2020, patients with single pelvic targets did not receive immobilisation when treated on MR-linac, as the immobilisation was found to offer no advantage during MR-linac treatment for these patients [Citation21]. Follow-up after 3 months was at the discretion of the urologist for most patients; patients received a questionnaire every 6 months to register grade ≥3 late toxicities.
Patients undergoing SBRT from July 2018 onwards were included prospectively and were additionally monitored using QoL questionnaires (European Organisation for Research and Treatment of Cancer (EORTC) C30, EuroQol EQ-5D-5L and Multidimensional Fatigue Inventory (MFI)) before start of treatment, at 1 and 4 weeks, at 3 and 6 months after SBRT and then every 6 months thereafter [Citation22–24]. Completion of baseline QoL questionnaires was mandatory for further QoL questionnaire participation.
In case of repeat oligorecurrences after SBRT, patients were eligible to be treated with another cycle of SBRT, with a maximum of five metastatic lymph nodes per SBRT cycle. QoL questionnaires were restarted at each SBRT cycle.
Definition of baseline characteristics
Oligometastatic disease classification was according to the European Society for Radiotherapy and Oncology (ESTRO)-EORTC recommendation [Citation25]. Pelvic region was defined as caudal of the aortic bifurcation. Primary therapy was categorised into robotic-assisted laparoscopic prostatectomy (RALP), with or without salvage radiotherapy; and radiotherapy, either external beam radiotherapy (EBRT) or brachytherapy (BT). Previous lymph node dissection was investigated as a combination of lymph node dissections at the time of primary therapy and salvage lymph node dissections. Therapeutic free interval was time between the last treatment and current diagnosis. Time to first oligometastasis was measured from primary tumour diagnosis (date of biopsy, if available) to the first oligometastasis. PSA doubling time (PSADT) was calculated using the Memorial Sloan Kettering Cancer Centre tool (www.mskcc.org/nomograms/prostate/psa-doubling-time), with ≥3 PSA measurements over a period of ≥3 months and individual measurements ≥4 weeks apart [Citation26].
Oncological outcomes
The primary end point was progression free survival (PFS), defined as a composite endpoint as in the ORIOLE trial: either progression of a target lesion, a newly diagnosed metastasis or biochemical progression [Citation2]. Progression of a target lesion was defined as an increase in short axis diameter >20% and >5 mm [Citation1]. Secondary outcomes for this analysis were biochemical PFS (BPFS), ADT-FS, widespread PFS (WS-PFS), local control, acute and late toxicity and QoL. Biochemical progression was defined as a PSA rise >2 ng/ml above the lowest value after SBRT or the pre-SBRT value [Citation2]. ADT-FS was measured from end of SBRT to the start of ADT. Widespread progression was investigated as metastatic disease that is no longer amenable to further local treatment [Citation27]. For this study, widespread progression was defined as any progression that was not followed by another cycle of lymph node SBRT. Local control was defined as absence of progression of a target lesion. Physician-reported toxicity was according to the guidelines of the Common Terminology Criteria for Adverse Events v 5.0; acute toxicity was defined as toxicity within 3 months after SBRT.
Statistical analysis
The open source R software package (v 3.6.3) was used (R Foundation for Statistical Computing, Vienna, Austria; www.R-project.org) Data analysis and reporting was according to the TRIPOD statement [Citation28]. Multiple imputation was used for missing baseline characteristics. The mice package was used to create 100 imputed datasets; both baseline characteristics and oncological outcomes were used for imputation (Supplementary material: Table S1). Pooled results from imputed baseline characteristics were used for subsequent analyses. Sensitivity analysis included complete case analysis. No imputation was performed for QoL data.
Oncological outcomes were analysed using Kaplan–Meier survival analysis, with survival and survminer packages, with log-rank tests to compare differences between subgroups. Model development and validation was done with the rms package. Cox proportional hazard regression was used to identify baseline characteristics that were associated with PFS. Hazard ratios and 95% confidence intervals (CI) were calculated. Continuous variables were kept at their original scale. Significant variables in univariable analysis (p < 0.05) and age were compared with published literature to select variables for the multivariable model. Backward elimination of variables based on Akaike’s information criterion (AIC) was used to construct the final multivariable model, retaining only the parameters that yielded the lowest AIC value when combined, allowing one variable per 10 events [Citation29]. Correlation between variables was investigated. For continuous variables, proportional hazard assumption was assessed through Schoenfeld residuals and linearity through Martingale residuals. Proportional hazard assumption for categorical variables was checked using log-log curves.
We performed internal validation of the model using the rms package by creating 2000 bootstrap resamples of the imputed datasets and calculated the apparent and optimism-corrected C-statistic [Citation30]. Due to the small sample size in this study, we did not create a nomogram for PFS. We constructed risk groups for PFS, based on the linear predictor of the final multivariable model.
Results
We included 90 patients, out of which data was prospectively collected for 68% (Supplementary material: Figure S2). Patients had metachronous (89%) or repeat (11%) oligorecurrences, with a median time to first oligometastasis of 59 months (). Most patients had RALP as primary treatment (73%). Thirty-nine percent of patients had a pelvic lymph node dissection prior to this study, either at the time of primary treatment or as a salvage lymph node dissection. Patients with T3–T4 stage tumours had a previous lymph node dissection in 58% of the cases; for patients with T1–T2 stage tumours this was 29%. For each group, the median of removed lymph nodes was 12. None of the patients in our study previously received whole pelvic radiotherapy.
Table 1. Baseline patient characteristics at the time of the first SBRT treatment for lymph node oligometastases at our centre (N = 90).
Patients had up to three lymph node metastases for the first SBRT cycle in this study; metastatic disease was confined to the pelvis in 93% of patients. In total 116 SBRT cycles have been applied: 20 patients underwent a second SBRT cycle, five patients a third cycle and one patient a fourth cycle. A total of 177 lymph node metastases have been treated, of which 41 lymph nodes (23%) had a short axis diameter ≥10 mm on MRI, in 32 patients. Fifty patients have been treated using the MR-linac for at least one SBRT cycle.
Median follow-up time was 21 months (interquartile range 10–31 months). One patient died during follow-up due to newly diagnosed lung cancer. Disease progression was observed in 54 patients. Median (95% CI) survival times were 16 (12–19), 21 (17–34) and 19 (16–37) months for PFS, BPFS and WS-PFS, respectively (). Median ADT-FS was not reached, ADT-FS at 24 months was 73% (95% CI 62–86%). In univariable analysis, higher T-stage, RALP as primary treatment, lower PSA at current diagnosis and metastases limited to the pelvic nodal region were associated with longer PFS (). Patients with a PSA ≤2 ng/mL before SBRT had longer PFS, especially when compared with PSA >4 ng/mL (median PFS 18 vs. 6 months); no difference was observed in PFS of patients with respect to PSADT or Gleason score (, Supplementary material: Figure S3).
Figure 1. Kaplan–Meier survival estimates of four oncological outcomes after SBRT for prostate cancer lymph node oligometastases. PFS: progression free survival (composite endpoint including also biochemical progression, start of ADT and death due to disease progression); biochemical PFS: PSA rise ≥2 ng/mL compared to lowest PSA value after SBRT (or baseline PSA); widespread PFS: disease progression not amenable to renewed SBRT treatment; ADT-free survival: survival until start of ADT. 95% confidence intervals were plotted as ribbons around the survival estimates.
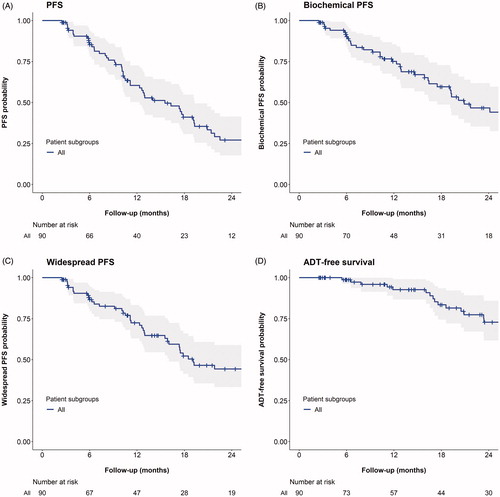
Table 2. Univariable and multivariable analysis of factors associated with progression free survival.
We selected age, PSA at current diagnosis, nodal region and type of primary treatment as parameters for the final multivariable model. Higher age, lower PSA and pelvic nodal region remained significantly associated with longer PFS. Calibration plots at 12, 18 and 24 months showed good concordance of the observed and predicted PFS (Supplementary material: Figure S4). Based on the final multivariable model, we constructed 2 risk groups (). The linear predictor L of the model can be calculated according to EquationEquation (1)(1)
(1) :
(1)
(1)
Where A is the patient age (years), P is the current PSA value (ng/mL), R is the nodal region (1 extrapelvic, 0 pelvic) and T is the treatment type (1 radiotherapy, 0 RALP).
Figure 2. Progression free survival for the risk group based on quantiles of the linear predictor of the final multivariable model. The patients were divided into two risk groups (creating a 2:1 distribution) based on the multivariable model derived in this study. The observed progression free survival was then plotted for each risk group, with 95% confidence intervals displayed as ribbons.
The linear predictor L can be calculated using the following formula:
Where A is the patient age (years), P is the current PSA value (ng/mL), R is the nodal region (1 extrapelvic, 0 pelvic) and T is the treatment type (1 radiotherapy, 0 RALP).
Low risk is depicted by L < 0.19, high risk is depicted by L> =0.19.
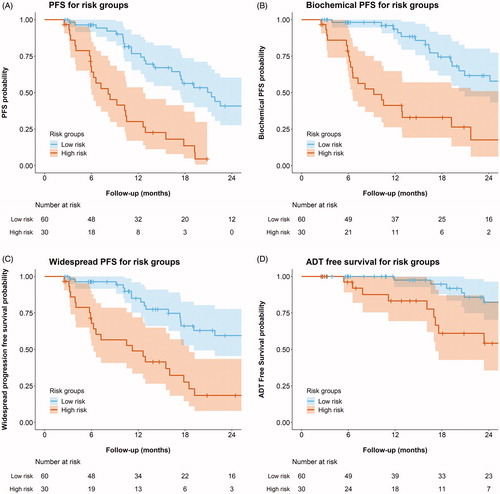
A 2:1 distribution of linear predictors was chosen after visual inspection, as it allowed the best stratification of observed PFS (compared with 1:2:1/1:1 distributions), with 60 patients classified as low risk and 30 as high risk. Low risk was depicted by score <0.19, high risk was depicted by score > =0.19. Median PFS (95% CI) was 21 (17–35) and 8 (6–13) months for low and high risk groups, respectively (). Similar differences between the risk groups were also observed for BPFS, WS-PFS and ADT-FS. The apparent C-statistic for model performance was 0.71, after internal validation the mean optimism-corrected C-statistic was 0.69.
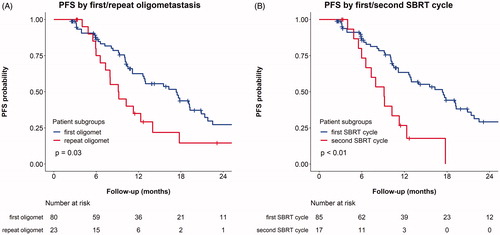
Progression of a target lesion was not observed. For five patients (6%), biochemical progression occurred without any further imaging available. After pelvic lymph node SBRT, 51% of patients with progression had metastases outside the pelvic lymph nodes. The number of patients with extrapelvic or non-nodal progression was similar for both risk groups and was also similar for patients with and without a previous lymph node dissection (data not shown). Twelve percent of patients had progression limited to lymph nodes in the same pelvic sub-region as before (classified as lower pelvic region left and right (comprised of internal and external iliac and obturator regions), presacral or mesorectal region and common iliac region left and right). A shorter PFS was observed after SBRT for repeat oligometastatic disease compared with newly diagnosed oligometastatic disease: median PFS (95% CI) was 9 (7–18) vs. 17 (13–21) months, respectively ().
Figure 3. Kaplan–Meier survival estimates of progression free survival (PFS), stratified by oligometastatic disease characteristics: A) first vs. repeat oligometastases* and B) first vs. second SBRT cycle for oligometastases. In subfigure A, ‘first oligomet’ refers to patients with a first diagnosis of oligometastases (metachronous oligorecurrences, N = 80) and ‘repeat oligomet’ refers to patients with repeat oligometastases (repeat oligorecurrences, N = 23). PFS was recalculated from the end of the second SBRT cycle for 13 patients who underwent repeated SBRT. In subfigure B, data from four patients who had undergone a single SBRT cycle for previous oligometastatic disease in another centre was combined with the data from the second SBRT cycle of the 13 patients who underwent two sequential SBRT cycles at our centre. One patient was excluded from the graph in subfigure B as he had already undergone two previous SBRT cycles at another centre. *Repeat oligometastases = previously treated oligometastases, regardless of treatment modality. Log-rank test p-values for comparisons between subgroups are included.
One patient had a PSA bounce after SBRT, with a PSA increase of 2.9 ng/mL. No suspect lesions were seen on PSMA-PET and PSA spontaneously declined afterwards.
Grade 1 and 2 acute toxicity were reported in 41 and 3% of the treatments, with grade 1 fatigue in 34% of treatments (Supplementary material: Table S2). Grade 2 acute toxicity comprised non-infective cystitis in two patients after previous salvage EBRT to the prostate bed, and grade 2 fatigue in one patient. Grade 1 and 2 late toxicity was reported for 15 and 7% of patients. Grade 2 late toxicity comprised non-infective cystitis for three patients, proctitis with bloody stools in two patients, an increase in urinary incontinence in two patients and lower back pain for one patient. All patients with late grade 2 toxicity had undergone previous salvage EBRT. No grade 3 or higher events were observed. No significant differences were observed in toxicity stratified by number of SBRT cycles (Supplementary material: Tables S3 and S4).
QoL data was available from 58 SBRT cycles of 49 patients. Patients reported an increase in fatigue at 1–4 weeks after SBRT which was resolved at 3–6 months (Supplementary material: Figure S4). Overall health status and physical functioning were unaffected. Investigation of fatigue subdomains showed mainly reduced activity after SBRT, which was resolved at 6 months (Supplementary material: Figure S5) [Citation23].
Discussion
With a PSMA-PET cohort of patients with prostate cancer lymph node oligometastases, we report median PFS and BPFS of 16 and 21 months after SBRT, with 73% of patients free of ADT at 24 months. Toxicity was limited to grade 1–2, with mild, transient fatigue reported in both toxicity and QoL. Our definition of PFS was comparable to the definition used in the ORIOLE trial, in which median PFS after SBRT was not reached with a median follow-up of 19 months [Citation2]. In the ORIOLE trial, patients had 1–3 metastases diagnosed with conventional imaging (CT/MRI/bone scintigraphy). Patients in our study had lymph node metastases with a median short axis diameter of 8 mm, so most lesions would probably not have been identified with conventional imaging yet. The shorter PFS in our PSMA-PET diagnosed population shows that the risk of microscopic tumour spread beyond detectable (oligo)metastases may not have been reduced due to the use of PSMA-PET imaging. A wide range of median (B)PFS has been reported after PSMA-PET directed SBRT monotherapy, from 10 to 22 months [Citation6,Citation7]. These outcomes are very comparable to (B)PFS after choline-PET directed SBRT [Citation1,Citation6,Citation31]. ADT-FS seems to be longer when using PSMA-PET to guide SBRT compared with choline-PET, but this may be an effect of stage migration with more sensitive PSMA-PET imaging at lower PSA levels [Citation6]. Thus, PSMA-PET imaging may have influenced the patient population treated for prostate cancer oligometastases but it has not increased the median period of PFS after SBRT.
To aid in patient selection, we have developed a preliminary risk classification that divides patients into low and high risk of progression after SBRT, with median PFS of 21 and 8 months, respectively. Higher age, lower PSA values and metastatic disease limited to the pelvic lymph nodes were associated with longer PFS after SBRT. Previous reports on predictors of PFS from literature were taken into account in building the model. We did not find an association between primary tumour Gleason score and PFS, which was consistent with previous studies [Citation10,Citation15,Citation31]. Higher primary tumour T-stage was significantly associated with longer PFS based on univariable analysis in our cohort, whereas an opposite relation was found previously [Citation10]. We excluded T-stage from our multivariable model for this reason. The association between number of target metastases (1-2 vs. 3) and PFS was borderline significant in univariable analysis (p = 0.05), it was eliminated from the final multivariable model as it did not contribute to the model based on AIC value. Lower pre-SBRT PSA, especially PSA <2 ng/mL, was associated with longer PFS in our study (Supplementary material: Figure S3), which is consistent with other reports [Citation7,Citation32]. Patients with extrapelvic versus only-pelvic lymph node metastases had median PFS of 7 vs. 17 months, which coincides with median PFS of 6 vs. 15–18 months reported previously [Citation31]. Finally, radiotherapy versus RALP as primary therapy for prostate cancer was included in our model as a predictor of shorter PFS; although it was not significantly associated with PFS in multivariable analysis, it did contribute to the model. Primary therapy as predictor of PFS is in line with results from a large retrospective analysis [Citation33]. This finding could be related to general patient characteristics, such as age and comorbidity, that may have influenced the choice of therapy at the time of primary tumour diagnosis: an overall survival benefit of patients treated with RALP versus radiotherapy was found in a meta-analysis of non-randomised studies, but was not observed in the randomised PROTECT trial [Citation34,Citation35]. For prediction purposes, this potential bias associated with primary therapy can be incorporated in a risk score, a causal relationship is not necessary for this purpose. However, these results will need to be validated in an external cohort to ascertain the applicability in other clinical situations than the one on which the model was based. It seems from literature this bias is persistent, therefore it has been included in the prediction models.
An important limitation of the current study is the small sample size, which made it impossible to construct a nomogram to predict PFS for individual patients. Furthermore, we used univariable analysis to guide model development, which underlines the need for external validation of this model. Follow-up after SBRT was non-standardized; in 4% of the cases with biochemical progression no PSMA-PET scan was available during follow-up. Interpretation of ADT-FS is limited by the lack of uniform clinical management with regards to the start of ADT, usually ADT was started with a PSA doubling time of approximately 3 months, a PSA of approximately 20 or symptomatic progression. This limits the ability to compare our results with the ADT-FS of 21 months that was reported in the STOMP trial, in which stricter guidelines for start of ADT were used [Citation1]. Finally, we did not investigate biological tumour characteristics in this study, which could further improve patient selection [Citation2].
In our study, half of the patients with progression after SBRT had extrapelvic or non-nodal metastases at the time of first progression. This is concordant with previous reports, with 54% − 69% extrapelvic or non-nodal progressions after choline-PET directed SBRT [Citation32,Citation33]. Thus, the pattern of progression does not seem to have changed as a result of PSMA-PET guidance and our risk classification could not predict the pattern of progression. Thus the addition of an elective treatment field, such as whole pelvic radiotherapy (WPRT), thus remains a clinical debate in which prevention of pelvic nodal relapses should be weighed against the increased risk of toxicity [Citation33]. The STORM/PEACE V trial (NCT0356924) may shed additional light on this: it randomises patients between metastasis-directed therapy with 6 months of ADT with or without WPRT [Citation36]. Furthermore, the POP-RT trial (NCT02302105) has recently shown a prolonged disease free survival after addition of WPRT to prostate EBRT in the primary treatment of patients with a high risk of pelvic lymph node involvement [Citation37]. A large reduction of pelvic relapses and also a reduction in extrapelvic relapses was observed in the WPRT group as compared to prostate-only EBRT. An increased use of WPRT for patients with high risk of pelvic lymph node involvement in the future could impact the population of patients with prostate cancer oligometastases compared to our series, given the number of patients with pelvic-only disease in our study (91%). However, 73% of our study population consisted of patients that underwent RALP as primary treatment and we have estimated that only about 41% of our patients would have had a large enough risk of lymph node involvement to have been eligible for inclusion in the POP-RT trial.
Addition of 6–12 months of ADT to the SBRT has been shown to defer the onset of new metastases after SBRT and has even shown a survival benefit in the setting of salvage lymph node dissections [Citation14,Citation38]. ADT could suppress microscopically spread tumour cells that are likely present in many oligometastatic patients, even with PSMA-PET imaging. The addition of 6 months of ADT to SBRT is currently investigated in the ADOPT trial (NCT04302454) and will be mandatory in both arms of the STORM/PEACE V trial (NCT0356924) [Citation36]. However, patients may still be reluctant to undergo temporary ADT due to the side effects [Citation16]. After the first 6 months of intermittent ADT, recovery of serum testosterone levels to non-castrate levels takes another 3-4 months and 6 months to recover to normal levels [Citation39,Citation40]. Especially for low risk patients, SBRT monotherapy might remain a valid treatment option for patients that are reluctant to receive (temporary) ADT.
In conclusion, we have shown that large differences exist in PFS after SBRT for prostate cancer lymph node oligometastases diagnosed with PSMA-PET. Patients with higher age, lower pre-SBRT PSA values and nodal metastases limited to the pelvis have longer PFS (median 21 months) after SBRT. Toxicity was limited to grade 1–2 and only mild, transient fatigue was reported by patients as influencing their quality of life.
Supplemental Material
Download Zip (4 MB)Acknowledgements
The authors are grateful to Dr. Simon Woodings for comments on the manuscript.
Disclosure statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The overarching University Medical Centre Utrecht MR-linac scientific project, including employment of multiple authors, has been partly funded by Elekta AB (Stockholm, Sweden). Elekta did not have any part in the design, execution or analysis of this study. Max Peters declares a grant received in August 2017 from the Dutch Cancer Society for the phase 2 PRECISE study regarding focal salvage brachytherapy for radiorecurrent prostate cancer, outside the submitted work. No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36(5):446–453.
- Phillips R, Yue Shi W, Deek M, et al. Outcomes of observation vs stereotactic ablative radiation for oligometastatic prostate cancer: the ORIOLE phase 2 randomized clinical trial. JAMA Oncol. 2020;6(5):650–659.
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase ii randomized trial. J Clin Oncol. 2020;38(25):2830–2838.
- Fodor A, Lancia A, Ceci F, et al. Oligorecurrent prostate cancer limited to lymph nodes: getting our ducks in a row: nodal oligorecurrent prostate cancer. World J Urol. 2019;37(12):2607–2613.
- Moghul M, Somani B, Lane T, et al. Detection rates of recurrent prostate cancer: 68 Gallium (Ga)-labelled prostate-specific membrane antigen versus choline PET/CT scans. A systematic review. Ther Adv Urol. 2019;11:175628721881579.
- Mazzola R, Francolini G, Triggiani L, et al. Metastasis-directed therapy (SBRT) guided by PET-CT 18 F-CHOLINE Versus PET-CT 68 Ga-PSMA in castration-sensitive oligorecurrent prostate cancer: a comparative analysis of effectiveness. Clin Genitourin Cancer. 2021;19(3):230–236.
- Kneebone A, Hruby G, Ainsworth H, et al. Stereotactic body radiotherapy for oligometastatic prostate cancer detected via prostate-specific membrane antigen positron emission tomography. Eur Urol Oncol. 2018;1(6):531–537.
- Artigas C, Flamen P, Charlier F, et al. 68Ga-PSMA PET/CT-based metastasis-directed radiotherapy for oligometastatic prostate cancer recurrence after radical prostatectomy. World J Urol. 2019;37(8):1535–1542.
- Ong WL, Koh TL, Joon DL, et al. Prostate-specific membrane antigen-positron emission tomography/computed tomography (PSMA-PET/CT)-guided stereotactic ablative body radiotherapy for oligometastatic prostate cancer: a single-institution experience and review of the published literature. BJU Int. 2019;124(Suppl 1):19–30.
- Hurmuz P, Onal C, Ozyigit G, et al. Treatment outcomes of metastasis-directed treatment using 68Ga-PSMA-PET/CT for oligometastatic or oligorecurrent prostate cancer: Turkish Society for Radiation Oncology group study (TROD 09-002). Strahlenther Onkol. 2020;196(11):1034–1043.
- Kalinauskaite G, Senger C, Kluge A, et al. 68Ga-PSMA-PET/CT-based radiosurgery and stereotactic body radiotherapy for oligometastatic prostate cancer. PLOS One. 2020;15(10):e0240892.
- Marzec J, Becker J, Paulsen F, et al. 68Ga-PSMA-PET/CT-directed IGRT/SBRT for oligometastases of recurrent prostate cancer after initial surgery. Acta Oncol. 2020;59(2):149–156.
- Deek MP, Yu C, Phillips R, et al. Radiation therapy in the definitive management of oligometastatic prostate cancer: the Johns Hopkins experience. Int J Radiat Oncol Biol Phys. 2019;105(5):948–956.
- Chaw CL, deSouza NM, Khoo V, et al. Clinical outcomes of stereotactic body radiotherapy with immediate versus delayed hormone therapy in men with oligometastatic recurrence of prostate cancer. Clin Oncol. 2020;32(8):509–517.
- Kroeze SGC, Henkenberens C, Schmidt-Hegemann NS, et al. Prostate-specific membrane antigen positron emission tomography-detected oligorecurrent prostate cancer treated with metastases-directed radiotherapy: role of addition and duration of androgen deprivation. Eur Urol Focus. 2021;7(2):309–316.
- Nguyen PL, Alibhai SMH, Basaria S, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol. 2015;67(5):825–836.
- Fossati N, Suardi N, Gandaglia G, et al. Identifying the optimal candidate for salvage lymph node dissection for nodal recurrence of prostate cancer: results from a large, multi-institutional analysis. Eur Urol. 2019;75(1):176–183.
- Pitroda SP, Khodarev NN, Huang L, et al. Integrated molecular subtyping defines a curable oligometastatic state in colorectal liver metastasis. Nat Commun. 2018;9(1):1793.
- Dhondt B, De Bleser E, Claeys T, et al. Discovery and validation of a serum microRNA signature to characterize oligo- and polymetastatic prostate cancer: not ready for prime time. World J Urol. 2019;37(12):2557–2564.
- Werensteijn-Honingh AM, Kroon PS, Winkel D, et al. Feasibility of stereotactic radiotherapy using a 1.5 T MR-linac: multi-fraction treatment of pelvic lymph node oligometastases. Radiother Oncol. 2019;134:50–54.
- Werensteijn-Honingh AM, Jürgenliemk-Schulz IM, Gadellaa-Van Hooijdonk CG, et al. Impact of a vacuum cushion on intrafraction motion during online adaptive MR-guided SBRT for pelvic and para-aortic lymph node oligometastases. Radiother Oncol. 2021;154:110–117.
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376.
- Smets EM, Garssen B, Bonke B, et al. The Multidimensional Fatigue Inventory (MFI): psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39(3):315–325.
- Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–1736.
- Guckenberger M, Lievens Y, Bouma AB, et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020;21(1):e18-28–e28.
- Arlen PM, Bianco F, Dahut WL, Prostate Specific Antigen Working Group, et al. Prostate Specific Antigen Working Group guidelines on prostate specific antigen doubling time. J Urol. 2008;179(6):2181–2186.
- Poon I, Erler D, Dagan R, et al. Evaluation of definitive stereotactic body radiotherapy and outcomes in adults with extracranial oligometastasis. JAMA Netw Open. 2020;3(11):e2026312.
- Collins GS, Reitsma JB, Altman DG, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015;350:g7594.
- Heinze G, Wallisch C, Dunkler D. Variable selection – a review and recommendations for the practicing statistician. Biom J. 2018;60(3):431–449.
- Harrell FE, Jr Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Statist Med. 1996;15(4):361–387.
- Jereczek-Fossa BA, Fanetti G, Fodor C, et al. Salvage stereotactic body radiotherapy for isolated lymph node recurrent prostate cancer: single institution series of 94 consecutive patients and 124 lymph nodes. Clin Genitourin Cancer. 2017;15(4):e623–e632.
- Nicosia L, Franzese C, Mazzola R, et al. Recurrence pattern of stereotactic body radiotherapy in oligometastatic prostate cancer: a multi-institutional analysis. Strahlenther Onkol. 2020;196(3):213–221.
- De Bleser E, Jereczek-Fossa BA, Pasquier D, et al. Metastasis-directed therapy in treating nodal oligorecurrent prostate cancer: a multi-institutional analysis comparing the outcome and toxicity of stereotactic body radiotherapy and elective nodal radiotherapy. Eur Urol. 2019;76(6):732–739.
- Wallis CJD, Saskin R, Choo R, et al. Surgery versus radiotherapy for clinically-localized prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70(1):21–30.
- Neal DE, Metcalfe C, Donovan JL, ProtecT Study Group, et al. Ten-year mortality, disease progression, and treatment-related side effects in men with localised prostate cancer from the Protect Randomised Controlled Trial according to treatment received. Eur Urol. 2020;77(3):320–330.
- De Bruycker A, Spiessens A, Dirix P, et al. PEACE V – salvage treatment of oligorecurrent nodal prostate cancer metastases (STORM): a study protocol for a randomized controlled phase II trial. BMC Cancer. 2020;20(1):406.
- Murthy V, Maitre P, Kannan S, et al. Prostate-only versus whole-pelvic radiation therapy in high-risk and very high-risk prostate cancer (POP-RT): outcomes from phase III randomized controlled trial. J Clin Oncol. 2021;39(11):1234–1242.
- Bravi CA, Fossati N, Gandaglia G, et al. Long-term outcomes of salvage lymph node dissection for nodal recurrence of prostate cancer after radical prostatectomy: not as good as previously thought. Eur Urol. 2020;78(5):661–669.
- Tunn UW, Canepa G, Kochanowsky A, et al. Testosterone recovery in the off-treatment time in prostate cancer patients undergoing intermittent androgen deprivation therapy. Prostate Cancer Prostatic Dis. 2012;15(3):296–302.
- Langenhuijsen JF, Badhauser D, Schaaf B, et al. Continuous vs. intermittent androgen deprivation therapy for metastatic prostate cancer. Urol Oncol. 2013;31(5):549–556.
