?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
Thyroid hypofunction is a late effect observed in several groups of cancer survivors, but has to date not been evaluated in-depth in testicular cancer survivors (TCSs). We investigated the prevalence of thyroid hypofunction in long-term TCSs and compared the findings with those of a comparison group from the general population.
Patients and Methods
Norwegian TCSs diagnosed with unilateral testicular cancer in the period 1980–1994 (N = 1,436) were grouped according to their cancer treatment (Surgery only; Radiotherapy only; Cisplatin-based chemotherapy, eventually combined with radiotherapy). They were invited to participate in three surveys covering up to three decades post-diagnosis. Serum thyrotropin (s-TSH) from samples collected from the last survey were analyzed. S-TSH results were also available from a health survey of the general population performed in a county in mid-Norway (the HUNT3 Survey [comparison group]). Data on the prescription of thyroid hormone replacement therapy (levothyroxine) from the Norwegian Prescription Database were obtained for the TCSs and the comparison group’s participants. Thyroid hypofunction was defined as ‘untreated’ (overt or subclinical) hypothyroidism (with s-TSH ≥3.5 mIU/L and no regular prescription of levothyroxine) or ‘treated’ hypothyroidism with regular prescription of levothyroxine.
Results
Three decades after diagnosis the prevalence of thyroid hypofunction (i.e., both treated and untreated) was 11% in the TCSs and the prevalence ratio was 1.9 indicating an almost doubled prevalence in the TCSs compared to the comparison group (prevalence ratio 1.91, 95% CI [1.54; 2.38]). However, there were no significant differences in the risk of thyroid hypofunction related to the TCSs’ treatment modality.
Conclusion
TCSs may have an increased prevalence of thyroid hypofunction compared to the general population. Hypothyroidism has negative consequences related both to primary hypogonadism and to cardiovascular disease. As both conditions are overrepresented in TCSs, regular monitoring of thyroid hormones may be advisable.
Introduction
Testicular Cancer Survivors (TCSs) have an increased risk of several treatment-related side effects, and these conditions are often similar to those developing during the physiological aging process [Citation1]. These Adverse Health Outcomes (AHOs) may include disturbances of hormonal axes, in particular the pituitary-gonadal axis. However, the prevalence of thyroid dysfunction in this group of cancer survivors is largely unknown, except for a few studies with conflicting results [Citation2–5]. Thyroid hypofunction is an AHO encountered after radiotherapy (RT) in Hodgkin’s lymphoma and breast cancer survivors where radiation to the thyroid gland is supposed to be the culprit [Citation6–9]. Chemotherapy applied in combination with RT to the head and neck region may have a synergistic effect, but chemotherapy per se is seldom suspected to cause thyroid hypofunction [Citation7].
The cytotoxic agent cisplatin has been linked to increased risk of e.g., cardiovascular disease, peripheral neuropathy, nephropathy, and gonadal dysfunction [Citation10–12], but not shown to cause thyroid dysfunction [Citation13]. However, as hypothyroidism increases the risk of cardiovascular disease [Citation14], it is especially interesting to assess thyroid function in TCSs. In the general population thyroid hyperfunction has low prevalence, but the prevalence of thyroid hypofunction is higher [Citation15,Citation16]. Thyroid hypofunction increases with aging, as does the prevalence of AHOs in TCSs [Citation1,Citation17,Citation18]. Therefore findings on thyroid hypofunction in TCSs should be compared to those from aging men in the general population to assess the incidence and excess risk of this condition in cancer survivors. Data from a survey in Norwegian TCSs (treated 1980–1994) [Citation17,Citation19] and from a survey in the general population (the HUNT3 Survey [Citation20]) enable such exploratory and comparative studies.
With this background, we aimed to
Evaluate the prevalence of thyroid hypofunction (i.e., both treated and untreated) in aging TCSs compared to males from the general population.
Assess whether different treatment modalities are associated with subsequent thyroid hypofunction in TCSs.
Patients and methods
Testicular cancer survivors
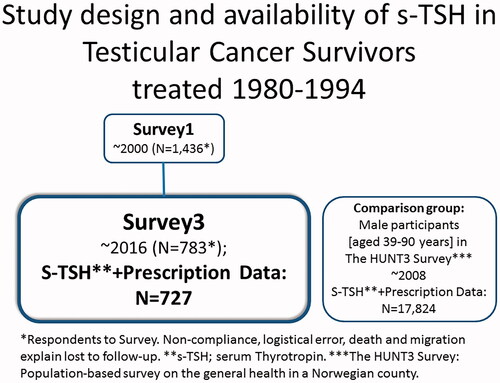
Survivors treated for unilateral TC in Norway from 1980 to 1994 were included in the national Norwegian Testicular Cancer Survivor study with three follow-up surveys, the first took place in ∼2000 (Survey 1; S1) and the latest in ∼2016 (Survey 3; S3) [Citation19]. At S3, they had their thyroid function measured in serum samples at Oslo University Hospital. The Cancer Registry of Norway provided the date of death before S3.
Treatment
From 1980 to 1994 post-orchiectomy treatment of TC has in Norway consisted of three main treatment strategies; surveillance or additional surgery, cisplatin-based chemotherapy (CBCT), radiotherapy (RT), or a combination of these treatments modalities [Citation19]. The TCSs were divided into three treatment groups according to their cumulative treatment, resulting from primary and eventual salvage therapy.
Group 1: Surgery only (orchiectomy with or without retro-peritoneal lymph node dissection)
Group 2: RT
Group 3: CBCT ± infra-diaphragmatical RT
Comparison group: the HUNT3 survey
A comparison group was formed from men aged 39 to 90 years (corresponding to the age range in the TCSs) with available serum Thyrotropin (s-TSH) values participating in a large study of the general population performed in a county in mid-Norway eight years before S3 [Citation16] (The HUNT3 Survey: 2006–2008). The HUNT3 Survey participants had consistent findings regarding hypothyroidism as was expected based on data from primary care in the county [Citation21]. The HUNT Study area has comparable distributions of demographic variables to the rest of the country, except for lack of large cities, lower immigration, and somewhat lower average income and education level [Citation22].
Outcomes
Norwegian prescription database
Since 2004, all medication prescribed to non-institutionalized Norwegian inhabitants is registered in the Norwegian Prescription Database (NorPD) (www.reseptregisteret.no) based on an individual identification number. Among TCSs we identified the men who had received prescribed thyroid medication based on data from NorPD (drug class and date of dispensing), available for TCSs at S3 (∼2016), and the comparison group from the HUNT3 Survey (∼2008). We subsequently excluded the few men (<1%) using antithyroid medication. We thus identified entries for medication due to hypothyroidism for the TCSs who were S1 responders and who were still alive at the time of S3, independent of their participation in S3. We did a similar approach for the comparison group at the time of the HUNT3 Survey. Usually, thyroid hormone replacement therapy (levothyroxine) is used life-long. Therefore we defined that prescription of levothyroxine for ≥12 months indicated treatment of hypothyroidism.
s-TSH
In S3, s-TSH was measured on the Cobas 6000 (Roche Diagnostics, Mannheim, Germany), results were reduced by 15% to match results from the HUNT3 survey based on an in-house assay comparison showing 10–20% difference (data not shown) and reported by Thienport et al. [Citation23]. As unadjusted s-TSH values are relevant for routine practice, these are also presented herein. However, in all analyses comparing TCSs to the comparison group, we have used the adjusted TSH levels to avoid overestimation of thyroid hypofunction. In the HUNT3 Survey, s-TSH samples were measured by the use of Architect ci8200 (Abbott, Longford, Ireland) [Citation16].
Thyroid hypofunction
Based on data from NorPD and the serum analysis of TSH, we defined total thyroid hypofunction as
prescription of levothyroxine for more than 12 months; treated hypothyroidism or
elevated s-TSH, defined as s-TSH ≥3.5 mIU/L [Citation15] without regular levothyroxine prescription; untreated hypothyroidism.
Statistical analyses
The outcomes were the prevalence and the prevalence ratio (PR) of thyroid hypofunction (both treated and untreated hypothyroidism) in TCSs responding to S3 and in the comparison group. The crude outcomes were defined as follows:
The PR was calculated for TCSs and the comparison group:
Subsequently, the PRs for TCSs in different treatment groups (comparing selected treatment groups) were calculated:
We also, based on prescription data only, calculated the PR of treated hypothyroidism in all TCSs included in the surveys and alive at the time of S3, compared to the comparison group in the HUNT3 Survey.
Data were described with medians and ranges for continuous variables and counts with percentages for categorical variables. The analyses of the primary outcome were based on S3, the survey with the longest observation time of the TCSs, and the estimates compared to the HUNT3 Survey.
To accommodate for differences in age distribution in the TCSs and the comparison group, we fitted generalized linear models with a logit link to model age-adjusted PRs. The median age of the TCSs at S3 was used as a cutoff to form two age groups in the statistical model. ‘Group’ (TCSs vs. comparison group) and ‘age’ (in two categories) were entered as fixed effects. The results are presented as point estimates with 95% confidence intervals (CI).
To evaluate the effect of cumulative exposure to CBCT on the primary outcome, we fitted linear regression models using the logarithmic transform of the dependent variable to fulfill the assumptions of normally distributed residuals. All tests were two-sided and the significance level was set to 5%. The study was considered exploratory, so we did not adjust for multiple testing.
Data were analyzed using SPSS version 26 and Stata version 14.2.
Ethical considerations
The present study was approved by the Committee for Medical Research Ethics of the Southern Health Region of Norway (2015/1264) and the Norwegian Data Inspectorate (16/01431-2/CDG).
Results
Patient characteristics
Of the 1,436 TCSs responding to S1, s-TSH was available for N = 727 at S3 (). The proportions of TCSs with the diagnosis of seminoma and non-seminoma were similar (). The median age at diagnosis was 31 years (range 14–64). At S3 the median age was 60 years (range 39–90) and at S3, the time period since diagnosis was median 27 years (range 21–36). Twenty percent of the TCSs were in the Surgery Group, 40% in the RT Group, and finally 40% in the CBCT ± RT Group. The medical characteristics were similar in TCSs at S1 and the s-TSH-evaluable TCSs at S3, respectively () [Citation19]. Loss-to follow-up was due to non-compliance, logistical error, death or migration. The men in the comparison group (N = 17,824) were slightly younger than the TCSs with a median age of 58 (range 39–90) years (p = 0.001).
Table 1. Pretreatment, treatment and follow-up characteristics.
Thyroid hypofunction
At S3, the prevalence of untreated hypothyroidism was 5.9% (12% using the unadjusted s-TSH level), the majority was biochemical (subclinical) hypothyroidism (i.e., s-TSH ≥3.5–10 mIU/L and serum-free thyroxine in the lower reference interval) (). The total prevalence of thyroid hypofunction (i.e., both treated and untreated) among the 727 TCSs, was 11% () (17% when using an unadjusted s-TSH level). In the comparison group, thyroid hypofunction was found in 5.5% (N = 976) with 2.2% (N = 379) of participants having regular levothyroxine prescription recorded in NorPD and 3.3% (N = 597) having elevated s-TSH without recorded treatment.
Table 2. Prevalence of Thyroid hypofunction; untreated (grouped by s-TSH-level) and treated in Testicular Cancer Survivors (TCSs).
Compared to the comparison group adjusted for age, the TCSs included in S3 had an almost doubled prevalence of thyroid hypofunction (using the adjusted s-TSH levels for analyses) (PR 1.91, 95%CI [1.54; 2.38]) (). The prevalence of untreated hypothyroidism was 1.7 times higher in TCSs compared to the comparison group (PR 1.72, 95%CI [1.28; 2.33]) (). There was also a higher prevalence of treated hypothyroidism in the TCSs compared to the comparison group (PR 2.22, 95%CI [1.58;3.11]). The crude prevalences of thyroid hypofunction (both treated and untreated) were almost doubled both in the TCSs (14%) and the comparison group (7.5%) aged ≥60 years compared to those aged <60 years (TCSs; 7.7% and comparison group; 3.7%). This resulted in a similar PR comparing TCS and the comparison group for those < and those ≥60 years. (PR 1.8 in the age group ≥60 years, vs. PR 2.1 in the age group <60 years) ().
Table 3. Prevalence and age-adjusted prevalence ratios (PR) of thyroid hypofunction (untreated and treated) in testicular cancer survivors (TCSs)∼30 years after diagnosis (survey 3) compared to an age-adjusted comparison group (CG)a.
The prescription practice analysis which included all available TCSs from S1 still alive at S3 (N = 1,224) compared to the comparison group (age-adjusted), confirmed the findings of a higher prevalence of treated hypothyroidism in the TCSs (data not shown).
The effect of treatment modality on thyroid hypofunction
When analyzing the age-adjusted risk for thyroid hypofunction in the different treatment groups, the results did not show any clinically relevant differences (). There was no association between total CBCT exposure and s-TSH at S3 (p = 0.525).
Table 4. Treatment-related prevalence ratios for thyroid hypofunction in testicular cancer survivors ∼30 years after diagnosis (survey 3).
Discussion
In the present study, we found thyroid hypofunction in 11% (17% based on unadjusted TSH level) of TCSs ∼30 years after diagnosis. Furthermore, we show for the first time that TCSs had an almost doubled prevalence of thyroid hypofunction (both treated and untreated) when compared with a comparison group from the general population. Our analyses showed a twice as high prevalence of thyroid hypofunction in TCSs ≥ 60 years when compared to those who were younger. However, when collated to the comparison group, the prevalence ratios of thyroid hypofunction were almost identical across the two age categories. This comparison is interesting, especially as s-TSH values increase with age [Citation24], whereas reference ranges are not age-adjusted [Citation25,Citation26]. Nevertheless, several limitations should be considered as discussed below.
Adverse health outcomes in TCSs
Thyroid hypofunction is not an established AHO in TCSs and was not found in a large study of endocrine late effects managed in a hospital setting by Jensen et al. [Citation5]. However, they did not evaluate prescription practice or s-TSH values in their analyses. Moreover, as primary hypothyroidism is generally managed in primary care, their study was not designed to assess thyroid hypofunction per se. The probable pathophysiological mechanisms underlying the thyroid hypofunction observed in the present study are not fully understood. Disturbance of the thyroid hormonal axis is a common AHO after RT to the head and neck region affecting both the central pituitary axis (secondary dysfunction) and the thyroid gland (primary dysfunction) [Citation6,Citation9]. However, in TCSs radiotoxic effect causing secondary or primary thyroid dysfunction, is less likely as both the pituitary and thyroid glands are distant to the RT target region. A cytotoxic effect from chemotherapy in the hormone-producing cells of the thyroid gland could be a cause of thyroid hypofunction [Citation27] or even thyroid cancer [Citation28], albeit not well-documented after CBCT in the literature. In our TCSs, the different treatment groups had non-significant differences in risk of thyroid hypofunction, and there were no associations between cumulative cisplatin exposure and subsequent hypothyroidism (data not shown). We thus cannot directly associate the treatment modality to the risk of subsequent hypothyroidism.
Thyroid hypofunction and testicular dysgenesis syndrome
As neither RT nor CBCT is an established cause of thyroid hypofunction in TCSs, we speculate whether the TCSs as a group could be vulnerable to thyroid hypofunction irrespective of the testis cancer treatment. Hormonal disturbances related to the underlying pathology of testicular dysgenesis syndrome could theoretically also affect the risk of thyroid hypofunction. Environmental endocrine-disrupting chemical exposure in utero is suspected of affecting thyroid hormones [Citation29–32] in addition to being an etiological factor of the testicular dysgenesis syndrome. This inherent risk is possibly enhanced by orchiectomy in TCSs. As such, these conditions could be a common cause for the thyroid hypofunction observed in the TCSs in addition to the effects from cancer treatment. Environmental disrupting chemicals are also debated as possible causes of both thyroid and testicular cancer [Citation33]. The increased risk of thyroid cancer reported in TCSs [Citation34,Citation35] could support this possibility.
Primary hypothyroidism and primary hypogonadism
Thyroid hormones are essential for normal organ function and it is well-known that primary hypothyroidism lowers the free testosterone and sex hormone-binding hormonal globulin in men from the general population [Citation36]. Furthermore, the gonadal hormones normalize during treatment with levothyroxine [Citation36]. After orchiectomy, TCSs are prone to primary hypogonadism, and hypothyroidism could augment existing gonadal dysfunction. Clinicians should therefore be especially aware of the risk of hypothyroidism in TCSs with primary hypogonadism.
Thyroid hypofunction and cardiovascular disease
The association of hypothyroidism with cardiovascular disease has been recognized for centuries [Citation37,Citation38], and subclinical hypothyroidism has been linked to cardiovascular morbidity and mortality [Citation39,Citation40]. Even high normal s-TSH levels (i.e., within the reference range) were associated with increased cardiac mortality in women, but were not associated with increased risk of hospitalization due to cardiovascular disease in the HUNT population [Citation41]. Furthermore, the association of high normal s-TSH levels and cardiac mortality was not found in a later meta-analysis [Citation42]. As TCSs have an increased risk of cardiovascular disease [Citation10,Citation43,Citation44], untreated hypothyroidism could be an additional load to their risk profile. In the general population, treatment of hypothyroidism should be considered in symptomatic individuals when s-TSH is elevated, though still <10 mIU/L [Citation14]. This strategy should be implemented in the follow-up of long-term TCSs. With s-TSH >10 mIU/L, levothyroxine is generally recommended [Citation14,Citation45].
Strength and limitations
A strength of our study is the long follow-up time, stretching over almost three decades after diagnosis with an acceptable compliance rate. Each individual who lives in Norway is given a unique identification number. Using this ID number we were able to link data on TCSs and the comparison group with data from NorPD, thus our cohorts’ data on the collection of prescriptions are complete.
There are several limitations, one of the most important being a gradual selection bias toward more healthy participants during follow-up [Citation46].
The method-associated difference between the two s-TSH assays used in this study is considered a limitation. We addressed this limitation by adjusting s-TSH levels for S3 as described in the Methods section. The down-scaling of TSH level limits the risk of overestimation of untreated hypothyroidism in the TCSs when compared to the comparison group. However, we have probably to some degree over-adjusted the s-TSH results in the TCSs, especially as the reference ranges in the two assays were equal before adjustment.
The comparison of S3 to the HUNT3 Survey has limitations regarding the timing of the survey and blood sampling. The decreasing prevalence of elevated s-TSH reported from the HUNT2 (1995–1997) to the HUNT3 Surveys [Citation16], was probably caused by increased awareness of elevated s-TSH in the primary health care as the total prevalence of thyroid hypofunction was unaltered. The increase in the prescription of levothyroxine has continued after the HUNT3 Survey in 2008 and to the time of S3 in 2016 (http://www.reseptregisteret.no/Prevalens.aspx). However, the influence of this bias may be partly balanced by a higher prescription rate in the county of the HUNT3 Survey compared to the mean level in the rest of the country where the TCSs were recruited from. Moreover, the increase in the prescription of levothyroxine has been moderate and is not expected to affect the outcome of the present study which is total thyroid hypofunction (i.e., both treated and untreated hypothyroidism).
Clinical implications
TCSs have an increased risk of several AHOs, cardiovascular morbidity being one of the most serious. As even slight or moderate thyroid hypofunction may affect the risk of cardiovascular disease, increased prevalence of thyroid hypofunction in TCSs found in our study, represents a concern and should be addressed. The Norwegian National guidelines on follow-up in TCSs recommend regular assessment of the cardiovascular risk profile including relevant biochemistry, but not thyroid hormones. Based on our results and the general risk profile in TCSs, increased awareness of thyroid hypofunction among clinicians is important, particularly in patients with cardiovascular disease and those with hypogonadal and/or hypothyroid symptoms. Since the majority of TCSs with untreated hypothyroid function had biochemical (subclinical) hypothyroidism, future studies should include symptomatical evaluation of thyroid function.
Conclusions
Thyroid hypofunction could be suspected in at least one of ten TCSs ∼30 years after diagnosis in our study. Furthermore, these TCSs had a higher prevalence of thyroid hypofunction compared to the comparison group from the general population. However, as this is an observational study only, our findings need confirmation. TCSs are prone to primary hypogonadism which may make them extra vulnerable to clinical consequences of primary hypothyroidism. As untreated hypothyroidism may interact with the risk of cardiovascular disease and gonadal dysfunction, regular monitoring of s-TSH in aging TCSs could be advisable.
The comparison group: The Trøndelag Health Study (HUNT) is a collaboration between HUNT Research Center (Faculty of Medicine and Health Sciences, Norwegian University of Science and Technology NTNU), Trøndelag County Council, Central Norway Regional Health Authority, and the Norwegian Institute of Public Health.
Acknowledgments
The authors wish to thank all the men in the cohort, without the good adherence to these studies, the validity of our results would be limited. In addition, the patient’s general practitioners have contributed with clinical information. Grethe Skjolde, Siri Lothe Hess, and Vigdis Opperud have administered patient recruitment and bioengineers at OUH have analyzed blood samples in S3.
Disclosure statement
The authors report no conflicts of interest.
References
- Travis LB, Beard C, Allan JM, et al. Testicular cancer survivorship: research strategies and recommendations. J Natl Cancer Inst. 2010;102(15):1114–1130.
- Salminen EK, Leskinen S. Thyroid function following chemotherapy in testicular cancer. Acta Oncol. 1997;36(1):85–86.
- Stuart NS, Woodroffe CM, Grundy R, et al. Long-term toxicity of chemotherapy for testicular cancer-the cost of cure. Br J Cancer. 1990;61(3):479–484.
- Leitner SP, Bosl GJ, Bajorunas D. Gonadal dysfunction in patients treated for metastatic germ-cell tumors. J Clin Oncol. 1986;4(10):1500–1505.
- Jensen MV, Rugbjerg K, de Fine Licht S, et al. Endocrine late effects in survivors of cancer in adolescence and young adulthood: a Danish population-based cohort study. JAMA Netw Open. 2018;1(2):e180349.
- Reinertsen KV, Cvancarova M, Wist E, et al. Thyroid function in women after multimodal treatment for breast cancer stage II/III: comparison with controls from a population sample. Int J Radiat Oncol Biol Phys. 2009;75(3):764–770.
- Vogelius IR, Bentzen SM, Maraldo MV, et al. Risk factors for radiation-induced hypothyroidism: a literature-based meta-analysis. Cancer. 2011;117(23):5250–5260.
- Hancock SL, Cox RS, McDougall IR. Thyroid diseases after treatment of Hodgkin's disease. N Engl J Med. 1991;325(9):599–605.
- Seland M, Bjoro T, Furre T, et al. Hormonal dysfunction is frequent in cancer survivors treated with radiotherapy to the head and neck region. J Cancer Surviv. 2015;9(4):630–640.
- Haugnes HS, Wethal T, Aass N, et al. Cardiovascular risk factors and morbidity in long-term survivors of testicular cancer: a 20-year follow-up study. J Clin Oncol. 2010;28(30):4649–4657.
- Haugnes HS, Aass N, Fossa SD, et al. Components of the metabolic syndrome in long-term survivors of testicular cancer. Ann Oncol. 2007;18(2):241–248.
- Hjelle LV, Bremnes RM, Gundersen PO, et al. Associations between long-term serum platinum and neurotoxicity and ototoxicity, endocrine gonadal function, and cardiovascular disease in testicular cancer survivors. Urol Oncol. 2016;34:487.e13–487.e20.
- Nuver J, Smit AJ, Wolffenbuttel BH, et al. The metabolic syndrome and disturbances in hormone levels in long-term survivors of disseminated testicular cancer. J Clin Oncol. 2005;23(16):3718–3725.
- Floriani C, Gencer B, Collet TH, et al. Subclinical thyroid dysfunction and cardiovascular diseases: 2016 update. Eur Heart J. 2017;39:203–507.
- Bjoro T, Holmen J, Kruger O, et al. Prevalence of thyroid disease, thyroid dysfunction and thyroid peroxidase antibodies in a large, unselected population. The Health Study of Nord-Trondelag (HUNT). Eur J Endocrinol. 2000;143(5):639–647.
- Asvold BO, Vatten LJ, Bjoro T. Changes in the prevalence of hypothyroidism: the HUNT study in Norway. Eur J Endocrinol. 2013;169(5):613–620.
- Sprauten M, Haugnes HS, Brydoy M, et al. Chronic fatigue in 812 testicular cancer survivors during long-term follow-up: increasing prevalence and risk factors. Ann Oncol. 2015;26(10):2133–2140.
- Fung C, Fossa SD, Williams A, et al. Long-term morbidity of testicular cancer treatment. Urol Clin North Am. 2015;42(3):393–408.
- Nome RV, Cvancarova SM, Bjoro T, et al. Longitudinal kidney function outcome in aging testicular cancer survivors. Acta Oncol. 2020;59(4):467–474.
- Krokstad S, Langhammer A, Hveem K, et al. Cohort profile: the HUNT study, Norway. Int J Epidemiol. 2013;42(4):968–977.
- Langhammer A, Krokstad S, Romundstad P, et al. The HUNT study: participation is associated with survival and depends on socioeconomic status, diseases and symptoms. BMC Med Res Methodol. 2012;12:143.
- Holmen J, Midthjell K, Krüger Ø, et al. The Nord-Trøndelag Health Study 1995-97 (HUNT 2): objectives, contents, methods and participation. Norsk Epidemiol. 2003;13:19–32.
- Thienpont LM, Van Uytfanghe K, De Grande LAC, et al. Harmonization of serum thyroid-stimulating hormone measurements paves the way for the adoption of a more uniform reference interval. Clin Chem. 2017;63(7):1248–1260.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489–499.
- Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on thyroid hormone replacement. Thyroid. 2014;24(12):1670–1751.
- Pearce SH, Brabant G, Duntas LH, et al. ETA guideline: management of subclinical hypothyroidism. Eur Thyroid J. 2013;2(4):215–228.
- Ishiguro H, Yasuda Y, Tomita Y, et al. Long-term follow-up of thyroid function in patients who received bone marrow transplantation during childhood and adolescence. J Clin Endocrinol Metab. 2004;89(12):5981–5986.
- Fung C, Fossa SD, Milano MT, et al. Solid tumors after chemotherapy or surgery for testicular nonseminoma: a population-based study. J Clin Oncol. 2013;31(30):3807–3814.
- Bajkin I, Bjelica A, Icin T, et al. Effects of phthalic acid esters on fetal health. Med Pregl. 2014;67(5–6):172–175.
- Meeker JD, Calafat AM, Hauser R. Di(2-ethylhexyl) phthalate metabolites may alter thyroid hormone levels in men. Environ Health Perspect. 2007;115(7):1029–1034.
- Kim M, Jeong JS, Kim H, et al. Low dose exposure to di-2-ethylhexylphthalate in juvenile rats alters the expression of genes related with thyroid hormone regulation. Biomol Ther. 2018;26(5):512–519.
- Romano ME, Eliot MN, Zoeller RT, et al. Maternal urinary phthalate metabolites during pregnancy and thyroid hormone concentrations in maternal and cord sera: the HOME Study. Int J Hyg Environ Health. 2018;221(4):623–631.
- Lauretta R, Sansone A, Sansone M, et al. Endocrine disrupting chemicals: effects on endocrine glands. Front Endocrinol. 2019;10:178–178.
- Travis LB, Fossa SD, Schonfeld SJ, et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst. 2005;97(18):1354–1365.
- Hellesnes R, Kvammen O, Myklebust TA, et al. Continuing increased risk of second cancer in long-term testicular cancer survivors after treatment in the cisplatin era. Int J Cancer. 2020;147(1):21–32.
- Meikle AW: The interrelationships between thyroid dysfunction and hypogonadism in men and boys. Thyroid. 2004;14(Suppl 1):S17–S25.
- Cappola AR, Ladenson PW. Hypothyroidism and atherosclerosis. J Clin Endocrinol Metab. 2003;88(6):2438–2444.
- Udovcic M, Pena RH, Patham B, et al. Hypothyroidism and the heart. Methodist Debakey Cardiovasc J. 2017;13(2):55–59.
- Rodondi N, den Elzen WPJ, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304(12):1365–1374.
- Asvold BO, Bjoro T, Vatten LJ. Associations of TSH levels within the reference range with future blood pressure and lipid concentrations: 11-year follow-up of the HUNT study. Eur J Endocrinol. 2013;169(1):73–82.
- Asvold BO, Bjoro T, Platou C, et al. Thyroid function and the risk of coronary heart disease: 12-year follow-up of the HUNT study in Norway. Clin Endocrinol. 2012;77(6):911–917.
- Asvold BO, Vatten LJ, Bjoro T, et al. Thyroid function within the normal range and risk of coronary heart disease: an individual participant data analysis of 14 cohorts. JAMA Intern Med. 2015;175(6):1037–1047.
- Fossa SD, Gilbert E, Dores GM, et al. Noncancer causes of death in survivors of testicular cancer. J Natl Cancer Inst. 2007;99(7):533–544.
- Lauritsen J, Hansen MK, Bandak M, et al. Cardiovascular risk factors and disease after male germ cell cancer. J Clin Oncol. 2020;38(6):584–592.
- Biondi B, Cooper DS. Thyroid hormone therapy for hypothyroidism. Endocrine. 2019;66(1):18–26.
- Fossa SD, Dahl AA, Myklebust TA, et al. Risk of positive selection bias in longitudinal surveys among cancer survivors: lessons learnt from the national Norwegian Testicular Cancer Survivor Study. Cancer Epidemiol. 2020;67:101744.