Abstract
Background
Glandular metastases (GM) have been associated with improved survival in metastatic clear cell renal cell carcinoma (m-ccRCC). We aimed to molecularly characterize m-ccRCC with GM.
Material and methods
We performed a retrospective cohort study on all m-ccRCC patients with available tissue at our institution, diagnosed with metastatic disease from 2000 to 2019. We determined previously described angiogenesis- and immune-related gene expression signatures (GES) and ccrcc molecular subtypes through whole transcriptome RNA sequencing of primary tumors and metastases. We tested differences in GES and molecular subtypes across groups and studied overall (OS) and progression-free survival (PFS) using Kaplan–Meier survival analysis and Cox regression models.
Results
Primary tumors of patients who developed GM (n = 55) had higher IMmotion Angio (p < 0.001) and JAVELIN Angio (p = 0.003) GES as well as a higher proportion of angiogenic ccrcc2 molecular subtypes (p = 0.008) than primary tumors of patients with non-GM (n = 128). Metastatic lesions in glandular organs (n = 32) also had higher IMmotion Angio (p = 0.008) and JAVELIN Angio (p = 0.02) GES and were more frequently of the ccrcc2 molecular subtype (p = 0.03), compared to metastatic lesions in non-glandular organs in patients who did not develop any GM (n = 231), but not compared to metastatic lesions in non-glandular organs in patients who also developed GM (n = 18). Patients with GM had better OS (HR 0.49, p < 0.001) and PFS on first-line vascular endothelial growth factor receptor tyrosine kinase inhibitors (VEGFR-TKIs) (HR 0.64, p = 0.045) than patients with non-GM. PFS on first- or any-line immuno-oncology (IO) was not different. IMmotion Angio, JAVELIN Angio GES, and ccrcc2 molecular subtype were associated with better OS and PFS on first-line VEGFR-TKIs, but not PFS on first or any-line IO.
Conclusions
Patients with m-ccRCC who develop GM are molecularly characterized by heightened angiogenesis, translating into better prognosis and better outcomes on VEGFR-TKIs, but not IO. Based on these findings, VEGFR-TKIs should be included in the first-line treatment of m-ccRCC patients with GM.
Introduction
The therapeutic landscape of metastatic clear cell renal cell carcinoma (m-ccRCC) is evolving at an unprecedented pace, and multiple first-line therapy options have recently become available. Currently, guidelines recommend the use of the immuno-oncology (IO) combination ipilimumab and nivolumab in international metastatic RCC database consortium (IMDC) intermediate and poor-risk patients or the use of a vascular endothelial growth factor receptor tyrosine kinase inhibitor (VEGFR-TKI) and IO combination therapy across all IMDC risk groups for treatment-naive m-ccRCC [Citation1]. As the treatment armamentarium is rapidly expanding, the need for personalized treatment strategies becomes more and more apparent. However, while the field of molecular characterization of RCC has gained a lot of interest in recent years, molecular biomarkers are yet to be implemented in routine clinical practice.
Earlier, the presence of glandular metastases (GM) has been associated with improved survival in m-ccRCC, but underlying molecular mechanisms remain to be elucidated [Citation2]. We hypothesized that the presence of GM could be a clinical representation of distinctive underlying disease biology, which could guide patient selection for first-line systemic treatment in m-ccRCC. Therefore, we aimed to study the molecular characteristics of primary tumors of patients who developed at least one GM compared to primary tumors of patients who developed non-GM. Additionally, we compared the characteristics of metastatic lesions in glandular organs compared to metastatic lesions in non-glandular organs.
Material and methods
Patients and samples
Following institutional review board approval (s63833), patients were identified from the clinical m-ccRCC database at our institution, diagnosed with the metastatic disease between 2000 and 2019. All patients with available tissue were included, and all patients were treatment-naive. Baseline clinical data including age, sex, IMDC risk category, timing, number and location of metastasis, and types of systemic treatments received, were collected as well as oncological outcomes, including progression-free survival (PFS) on first-line VEGFR-TKIs, PFS on first-line IO, PFS on any-line IO and overall survival (OS). Primary tumors of patients who developed at least one GM, with or without additional metastases in non-glandular organs, were compared to primary tumors of patients who did not develop any GM. We considered the pancreas, thyroid, contralateral adrenal gland, breast, and parotid as GM. Patients with ipsilateral adrenal gland metastasis were not considered as GM, since some patients might have undergone ipsilateral adrenalectomy at the time of surgery of the primary tumor, while others did not. Additionally, we compared the properties of metastatic lesions across three groups: (1) metastatic lesions in glandular organs, (2) metastatic lesions in non-glandular organs in patients who also developed GM and (3) metastatic lesions in non-glandular organs in patients who did not develop GM. The tissue of metastatic lesions was obtained from surgical metastasectomy. For survival analyses, we considered the patient cohort of whom primary tumor samples were available.
RNA sequencing
Archived formalin-fixed, paraffin-embedded (FFPE) primary tumor samples and samples of resected metastases were retrieved and upon review of H&E slides, tissue blocks with the highest tumoral content were selected. Unstained slides (5 μm thickness) were produced and macrodissected to only include tumoral tissue. RNA was extracted using the Maxwell RSC RNA FFPE kit (Promega) according to the manufacturer’s instructions. Subsequently, cDNA libraries were prepared using the Forward QuantSeq 3′ mRNA-Seq Library Prep Kit for Illumina (Lexogen) according to the manufacturer’s instructions using 5 μl of RNA and 16 PCR cycles. cDNA concentrations and fragment length were measured with the QubitTM dsDNA HS assay (Thermofisher) and Bioanalyzer HS DNA electrophoresis (Agilent). Illumina cBOT was used for clonal cluster generation and RNA sequencing was performed using the HISeq 4000 kit (Illumina) according to the manufacturer’s instructions.
Determination of GES and ccrcc1–4 molecular subtypes
Adaptors and optical duplicates were removed from raw sequencing read before aligning to the human reference genome hg19 with HiSat2 (v2.1.0) and quantified using featureCounts (v1.6.4) [Citation3,Citation4]. Downstream analyses were carried out with R (v4.0.3, The R foundation for statistical computing, Vienna, Austria). Counts were processed using DESeq2 (v1.26.0) and normalized using the VarianceStabilizingTransformation function [Citation5]. IMmotion Angio, IMmotion Teff, and IMmotion Myeloid GES, as well as JAVELIN Angio and JAVELIN Immuno GES, were calculated as described in the biomarker analyses of the IMmotion 150 and JAVELIN Renal 101 trials [Citation6,Citation7]. The previously described ccrcc1–4 molecular subtypes were determined using a centroid-based classifier, developed using the lol.classify.nearestCentroid function as implemented in the lolR (v2.1.0) package [Citation8,Citation9]. This classifier was trained on an in-house reference cohort of 139 primary tumor samples, sequenced in a first, single batch, in which the ccrcc1–4 molecular subtypes were determined based on unsupervised hierarchical WARD.D2 clustering of 855 genes (Supplemental Tables 1 and 2) using the stats (v3.6.2) package to obtain four clusters, representing the ccrcc1–4 molecular subtypes. These genes were selected based on differential gene expression between molecular subtypes in the previously described cohort of fresh frozen (FF) primary tumor samples and additional genes of interest, representing key biological features [Citation8–10].
Gene set enrichment analysis (GSEA)
To identify up- and down-regulated biological pathways underlying glandular tropism we performed GSEA, using the GSEA (v4.0.3) JAVA software, to compare primary tumor samples with GM to primary tumor samples with non-GM as well as metastatic lesions in glandular organs to metastatic lesions in non-glandular organs, leveraging the MSigDb (v7.2) Hallmark gene sets [Citation11,Citation12]. Gene sets were considered enriched if q < 0.25 and p < 0.05.
Statistical analyses
Categorical variables were compared using Fisher’s exact t-test and continuous variables were compared using the Mann–Whitney U test with Benjamini-Hochberg correction to account for multiple comparisons, using the stats (v3.6.2) package. [Citation13] Correlation between GES was assessed with Spearman’s rank correlation, using the stats (v3.6.2) package. OS, since diagnosis and PFS were estimated using Kaplan–Meier survival analysis with the logrank test and predictors thereof, were studied in Cox proportional hazards regression models using the survival (v3.2.7) and survminer (v0.4.9) packages. GES were treated as continuous variables in regression analyses [Citation14]. All tests were two-sided and a p-value of <0.05 was considered statistically significant.
Results
We included 55 primary tumor samples of m-ccRCC patients who developed at least one GM (28 contralateral adrenal gland, 20 pancreas, 3 pancreas + contralateral adrenal gland, 1 pancreas + contralateral adrenal gland + breast, 1 pancreas + thyroid, 1 contralateral adrenal gland + thyroid, 1 pancreas + thyroid + parotid gland) and 128 primary tumor samples of patients who developed metastases in non-glandular organs, as well as 32 metastatic lesions in glandular organs from 21 patients (8 contralateral adrenal glands, 17 pancreases, and 7 thyroids) and 249 metastatic lesions in non-glandular organs from 132 patients (95 lungs, 44 retroperitoneal lymph nodes, 30 bone, 18 contralateral kidneys, 13 skin, 9 brains, 7 liver, 7 pleura, 7 intestine, 4 muscle, 4 peritonea, 3 gingivae, 3 gall bladder, 2 ovaries, 2 ureters, 1 bladder). Of these 249 metastatic lesions in non-glandular organs, 18 were found in 10 patients who also had GM present (Supplemental Figure 1). Baseline patient characteristics of patients with primary tumor samples are shown in . There were no significant differences in age, sex, or IMDC risk category when comparing patients who developed GM vs. those who did not, nor was there a difference in the proportion of patients presenting with synchronous metastases. However, patients who developed metachronous GM had a significantly longer interval from RCC diagnosis to the development of metastases than those who developed metachronous non-GM. Of the 130 patients treated with first-line VEGFR-TKIs, 70 (53.9%) were treated with sunitinib, 52 (40%) with pazopanib, and 8 (6.2%) with sorafenib. Of the 18 patients treated with first-line IO, 17 (94.4%) were treated with ipilimumab/nivolumab and 1 (5.6%) with nivolumab, and of the 56 patients treated with any-line IO, 37 (66.1%) were treated with nivolumab, 2 (3.6%) with avelumab, and 17 (30.4%) with ipilimumab/nivolumab. Baseline patient characteristics of patients from which metastatic lesions were assessed are shown in Supplemental Table 3.
Table 1. Baseline patient characteristics of patients with primary tumor samples.
Differences in GES and ccrcc molecular subtypes for primary tumors are shown in . Primary tumors of patients who developed GM (, yellow boxes) had significantly higher IMmotion Angio (p < 0.001) and JAVELIN Angio (p = 0.003) GES than primary tumors with only non-GM (, teal boxes). IMmotion Teff (p = 0.26), IMmotion Myeloid (p = 0.88) and JAVELIN Immuno (p = 0.10) GES were not significantly different (). The proportion of patients with an angiogenic ccrcc2 molecular subtype was also higher in primary tumors of patients who developed GM (72.7% vs. 47.7%, p = 0.008) (). There were no differences in IMmotion Angio (p = 0.77), IMmotion Teff (p = 0.47), IMmotion Myeloid (p = 0.41), JAVELIN Angio (p = 0.93) or JAVELIN Immuno (p = 0.12) GES or the proportion of angiogenic ccrcc2 molecular subtypes (p = 0.77) when comparing primary tumors who developed metastases in the pancreas to primary tumors of patients who developed metastases in the contralateral adrenal gland.
Figure 1. Boxplots depicting the distribution of gene expression signatures and grouped barplots depicting the number of ccrcc molecular subtypes for primary tumors with vs. without glandular metastases (A–F) and for metastatic lesions in glandular organs vs. metastatic lesions in non-glandular organs when glandular metastases are present in the same patient vs. metastatic lesions in non-glandular organs when glandular metastases are absent in the same patient (G–L).
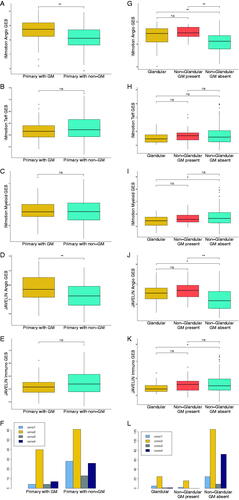
Differences in GES and ccrcc molecular subtypes for metastatic lesions are shown in . We compared three groups: (1) metastatic lesions in glandular organs (yellow boxes), (2) metastatic lesions in non-glandular organs in patients who also developed GM (red boxes), and (3) metastatic lesions in non-glandular organs in patients who did not develop GM (teal boxes). Metastatic lesions in non-glandular organs in patients who also developed GM (red boxes) displayed similar angiogenic GES and ccrcc2 molecular subtypes (78.1% vs 88.8%, p = 0.46) as compared to metastatic lesions in glandular organs (yellow boxes). Contrarily, metastatic lesions in non-glandular organs without any GM present in the same patient had lower angiogenic GES and fewer angiogenic ccrcc2 subtypes (78.1% vs. 54.1%, p = 0.03).
Both angiogenesis-related GES (IMmotion Angio and JAVELIN Angio) and both immune-related GES (IMmotion Teff and JAVELIN Immuno) were significantly positively correlated with each other (Supplemental Figure 2(A–B)).
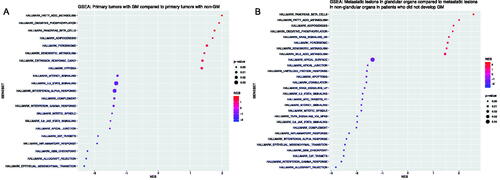
Significantly up- and down-regulated gene sets for primary tumors with GM compared to those with non-GM as well as metastatic lesions in glandular organs vs. those in non-glandular organs in patients who did not develop any GM according to GSEA are shown in .
Figure 2. Dotplot depicting gene set enrichment results for primary tumors with glandular metastases vs. primary tumors with non-glandular metastases (A) and metastatic lesions in glandular organs vs. metastatic lesions in non-glandular organs in patients who did not develop GM (B).
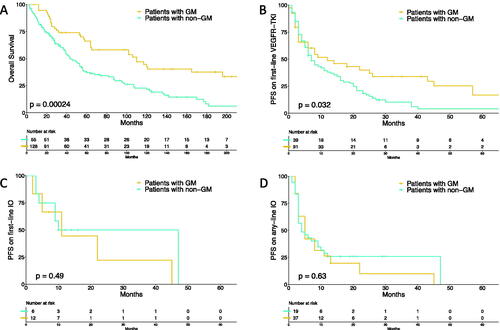
Patients with GM had significantly better OS (n = 183, HR 0.49, 95% CI 0.33–0.72, p < 0.001) and PFS on first-line VEGFR-TKIs (n = 130, HR 0.64, 95% CI 0.42–0.99, p = 0.045) than patients with non-GM, while PFS on first-line IO (n = 18, HR 1.49, 95% CI 0.44–5.06, p = 0.52) or any-line IO (n = 56, HR 1.15, 95% CI 0.63–2.11, p = 0.65) did not significantly differ. Kaplan–Meier curves for OS, PFS on first-line VEGFR-TKIs, PFS on first-line IO, and PFS on any-line IO, stratified by the presence of GM, are shown in . Of note, analyses on first-line IO therapy were solely exploratory, since the number of patients receiving first-line IO was small (n = 18). Objective response rates on first-line VEGFR-TKI and on any-line IO were 56.8% vs. 39.1% (p = 0.08) and 22.2% vs. 37.8% (p = 0.36), respectively, for patients with GM, compared to those with non-GM.
Figure 3. Kaplan–Meier curves depicting overall survival (A), progression-free survival on first-line vascular endothelial growth factor receptor tyrosine kinase inhibitors (B), progression-free survival on first-line immuno-oncology treatment (C) and progression-free survival on any-line immuno-oncology treatment (D), stratified for presence of glandular metastases.
In a Cox proportional hazards regression model, the IMmotion Angio and JAVELIN Angio GES itself, as well as the angiogenic ccrcc2 molecular subtype, were both associated with better OS and PFS on first-line VEGFR-TKIs, but not PFS on first-line IO or any-line IO (Supplemental Table 4). This was also the case, when included in a multivariable analysis on OS and PFS on first-line VEGFR-TKIs, including the IMDC risk stratification, with the exception of the JAVELIN Angio GES, which was not statistically significant for PFS on first-line VEGFR-TKIs ().
Table 2. Multivariable Cox proportional hazards regression models for OS and PFS on 1st line VEGFR-TKI for angiogenesis-related GES and ccrcc2 molecular subtype.
Discussion
In this study, we aimed to molecularly characterize m-ccRCC with glandular tropism, through whole transcriptome sequencing of both primary tumors and metastatic lesions. These underlying biologic features could eventually lead to distinct treatment approaches in a rapidly evolving treatment landscape.
We found that both primary tumors that develop GM and metastatic lesions in glandular organs are characterized by high expression of angiogenesis-related GES and a higher proportion of angiogenic molecular subtypes compared to primary tumors with non-GM and metastatic lesions in non-glandular organs in patients who did not develop GM. Conversely, immune-related GES were not significantly different and immune-related pathways were downregulated in these tumors. Interestingly, metastatic lesions in non-glandular organs exhibit similar characteristics as metastatic lesions in glandular organs when they are present in a patient who also developed GM.
Thus, the clinical phenotype m-ccRCC with glandular tropism is likely to be an indicator of heightened angiogenesis on a molecular level, which is translated in a favorable prognosis and better-expected outcomes on first-line VEGFR-TKIs.
This is in line with the earlier described outcomes of the angiogenic ccrcc2 molecular subtype, which was enriched in primaries with GM as well as metastatic lesions in glandular organs, and the angiogenic molecular subsets, recently described by Motzer et al. [Citation8,Citation15]. Both these angiogenic molecular subtypes are also enriched in the IMDC favorable risk groups, despite there being no significant difference in IMDC risk categories when comparing patients with GM to patients with non-GM in our cohort [Citation15,Citation16]. Additionally, Singla et al. have previously shown that RCCs with metastases specifically to the pancreas, which are typically indolent, are characterized by increased angiogenesis [Citation17,Citation18].
We used GSEA to explore whether biologically relevant gene sets were over- or under-represented in primary tumors that developed GM and in metastatic lesions in glandular organs. Primary tumors of patients who developed GM as well as metastatic lesions in glandular organs had significant upregulation of the fatty acid metabolism and the oxidative phosphorylation gene sets, which is also characteristic for the angiogenic molecular subsets of RCC, recently described by Motzer et al. [Citation15]. Interestingly, these gene sets were expressed at higher levels in non-responders to nivolumab in a recent study by Ross-Macdonald et al. on the CheckMate 009 trial cohort [Citation19].
Conversely, prognostically poor, immune-related gene sets including epithelial-to-mesenchymal transition, allograft rejection, inflammatory response, interleukin-6 signaling, and interferon-gamma response as well as gene sets pertaining to the cell cycle such as the mitotic spindle and G2M checkpoint gene sets, were significantly downregulated in patients who developed GM. This was also the case in the angiogenic RCC molecular subsets described by Motzer et al., again indicating the angiogenic and indolent nature of primary tumors with glandular tropism and metastatic lesions in glandular organs [Citation15]. Moreover, exactly these gene sets were significantly upregulated in responders to nivolumab in the study by Ross-Macdonald et al., indicating an inverse pattern of gene set over- or under-representation when comparing GSEA results from patients with GM in our study to GSEA results from nivolumab responders in the Ross–Macdonald study [Citation19]. This downregulation of immune-related pathways was not translated in clear differences in immune-related GES in our cohort or in different outcomes on IO in our exploratory analyses. Still, both the IMmotion Myeloid and JAVELIN Immuno GES were significantly lower in metastatic lesions in glandular organs compared to lesions in non-glandular organs. Moreover, both primary tumors who developed GM and metastatic lesions in glandular organs were rarely of the prognostically poor, immune-inflamed ccrcc4 molecular subtype. Accordingly, Singla et al. have shown earlier that RCCs with metastases specifically to the pancreas are not only characterized by increased angiogenesis but also by an uninflamed stroma, resulting in poorer outcomes on nivolumab, compared to patients without pancreatic metastases [Citation17]. However, in our cohort, we did not find significantly different outcomes on IO therapy in patients harboring GM, compared to those harboring non-GM. Thus, the possible impact of these underlying molecular characteristics of m-ccRCC with glandular tropism on treatment selection and outcome warrants further investigation.
Additionally, there is a considerable interplay between the immune system and angiogenesis in RCC. Structurally immature and nonfunctional tumor vasculature inhibits effective immune cell infiltration and renders the tumor micro-environment hypoxic [Citation20,Citation21]. Moreover, VEGF – a key driver of angiogenesis – plays a critical role in both local and systemic immunosuppression [Citation21,Citation22]. Therefore, the addition of antiangiogenic therapies targeting these processes can significantly increase sensitivity to IO therapy resulting in synergistic anti-tumor effects [Citation23]. This might have important treatment implications, since patients with GM, who are molecularly characterized by heightened tumoral angiogenesis, are most likely to be particularly good candidates for IO/VEGFR-TKI combination therapy, as they would benefit significantly from the alleviation of these immunosuppressive effects. Thus, patients with treatment-naïve m-ccRCC who display glandular tropism might be better candidates for first-line IO/VEGFR-TKI combination therapy rather than ipilimumab/nivolumab (IO/IO). Consequently, our findings could guide treatment selection for m-ccRCC patients with glandular tropism, while the widespread implementation of biomarkers for personalized m-ccRCC treatment is awaited.
The present study is limited by its retrospective design and the inherent associated biases. Additionally, the number of patients treated with IO in our cohort was too small to draw definitive conclusions on the effectiveness of these therapies in m-ccRCC patients with glandular tropism and are solely exploratory. Moreover, all patients in our analysis underwent prior nephrectomy since we used this tissue for RNA sequencing. Likewise, patients who are clinically selected to undergo surgical metastasectomy, generally have a more favorable disease course, which might lead to an overrepresentation of metastatic lesions with high angiogenic gene expression and angiogenic ccrcc2 molecular subtypes. Nevertheless, despite this possible sampling bias, we still found a higher proportion of angiogenic GES and angiogenic molecular subtypes in metastatic lesions in glandular organs and non-glandular organs in patients who also have GM, compared to those in non-glandular organs. Lastly, since we did not have data available on patients treated with IO/VEGFR-TKI combination therapy, we could not formally compare clinical outcomes of patients with and without GM treated with this combination therapy and only hypothesize that patients with GM would benefit from the inclusion of VEGFR-TKIs in first-line therapy.
Conclusion
Patients with m-ccRCC who display glandular tropism are characterized by heightened angiogenesis on a molecular level, translating into a better prognosis and better outcomes on VEGFR-TKIs, but not on IO treatment. Based on these findings, VEGFR-TKIs should be included in the first-line treatment of m-ccRCC patients with GM, since these patients would benefit most from anti-angiogenic therapies.
Supplemental Material
Download Zip (169.4 KB)Disclosure statement
Eduard Roussel received an unrestricted research grant from Ipsen and Pfizer. Lisa Kinget has received a grant from ‘Kom op tegen kanker’ (Belgium). Annelies Verbiest has received a grant from ‘Fonds voor Wetenschappelijk Onderzoek Vlaanderen’ (Belgium). Benoit Beuselinck received an unrestricted research grant from Bristol–Myers–Squibb and honorarium from Merck, Pfizer, Bristol–Myers–Squibb, Ipsen, and Astra-Zeneca and is a senior clinical investigator of ‘Fonds voor Wetenschappelijk Onderzoek Vlaanderen’ (Belgium). All other authors have nothing to disclose.
References
- Bedke J, Albiges L, Capitanio U, et al. Updated European Association of Urology guidelines on renal cell carcinoma: nivolumab plus cabozantinib joins immune checkpoint inhibition combination therapies for treatment-naïve metastatic clear-cell renal cell carcinoma. Eur Urol. 2021;79(3):339–342.
- Gravis G, Chanez B, Derosa L, et al. Effect of glandular metastases on overall survival of patients with metastatic clear cell renal cell carcinoma in the antiangiogenic therapy era. Urol Oncol Semin Orig Investig. 2016;34(4):167.e17–167.e23.
- Kim D, Paggi JM, Park C, et al. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol. 2019;37(8):907–915.
- Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30(7):923–930.
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550.
- McDermott DF, Huseni MA, Atkins MB, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med. 2018;24(6):749–757.
- Motzer RJ, Robbins PB, Powles T, et al. Avelumab plus axitinib versus sunitinib in advanced renal cell carcinoma: biomarker analysis of the phase 3 JAVELIN renal 101 trial. Nat Med. 2020;26(11):1733–1741.
- Beuselinck B, Job S, Becht E, et al. Molecular subtypes of clear cell renal cell carcinoma are associated with sunitinib response in the metastatic setting. Clin Cancer Res. 2015;21(6):1329–1339.
- Beuselinck B, Verbiest A, Couchy G, et al. Pro-angiogenic gene expression is associated with better outcome on sunitinib in metastatic clear-cell renal cell carcinoma. Acta Oncol. 2018;57(4):498–508.
- Roussel E, Verbiest A, Kinget L, et al. Molecular subtypes and gene expression signatures as prognostic features in fully resected clear cell renal cell carcinoma: a tailored approach to adjuvant trials. Clin Genitourin Cancer. 2021. DOI:https://doi.org/10.1016/j.clgc.2021.07.005
- Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102(43):15545–15550.
- Liberzon A, Birger C, Thorvaldsdóttir H, et al. The molecular signatures database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1(6):417–425.
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57(1):289–300.
- Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25(1):127–141.
- Motzer RJ, Banchereau R, Hamidi H, et al. Molecular subsets in renal cancer determine outcome to checkpoint and angiogenesis blockade. Cancer Cell. 2020;38(6):803–817.
- Verbiest A, Renders I, Caruso S, et al. Clear-cell renal cell carcinoma: molecular characterization of IMDC risk groups and sarcomatoid tumors. Clin Genitourin Cancer. 2019;17(5):e981–e994.
- Singla N, Xie Z, Zhang Z, et al. Pancreatic tropism of metastatic renal cell carcinoma. JCI Insight. 2020;5(7):e134564.
- Turajlic S, Xu H, Litchfield K, et al. Tracking cancer evolution reveals constrained routes to metastases: TRACERx renal. Cell. 2018;173(3):581–594.
- Ross-Macdonald P, Walsh AM, Chasalow SD, et al. Molecular correlates of response to nivolumab at baseline and on treatment in patients with RCC. J Immunother Cancer. 2021;9(3):e001506.
- Takakura N. Vascular reconstitution in the tumor for more effective tumor immunotherapy. Cancer Sci. 2021;112(4):1348–1356.
- Lee WS, Yang H, Chon HJ, et al. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med. 2020;52(9):1475–1485.
- Huang Y, Goel S, Duda DG, et al. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013;73(10):2943–2948.
- Rassy E, Flippot R, Albiges L. Tyrosine kinase inhibitors and immunotherapy combinations in renal cell carcinoma. Ther Adv Med Oncol. 2020;12:1758835920907504.
