Abstract
Purpose
A prior phase I study showed that the neo-adjuvant combination of pazopanib and radiotherapy was well tolerated, and induced promising pathological responses in soft-tissue sarcoma patients. Results of the subsequent prospective, multicenter phase II, PASART-2 trial are presented here, further investigating the efficacy and safety of this combination.
Patients and methods
Patients with high-risk, localized soft-tissue sarcoma received neo-adjuvant radiotherapy, 50 Gy in 25 fractions (PASART-2A) or with a subsequent dose de-escalation to 36 Gy in 18 fractions (PASART-2B). This was combined with 800 mg once daily pazopanib, which started one week before radiotherapy and finished simultaneously. After an interval of 4–8 weeks, surgical resection was performed. The primary endpoint was the rate of pathological complete responses (pCR), defined as ≤5% viable cells.
Results
25 patients were registered in the study, 21 in PASART-2A and 4 in PASART-2B. After central pathology review, the combination treatment led to a pCR in 5 patients (20%). 17 patients (68%) experienced grade 3+ toxicities during neo-adjuvant treatment, of which the most common were alanine aminotransferase (ALT) elevation, aspartate aminotransferase (AST) elevation, and hypertension, all asymptomatic. Grade 3+ acute post-operative toxicities occurred in 5 patients (20%), of which the most common was wound infection. All patients completed the full radiotherapy regimen and underwent surgery. Pazopanib was discontinued before completion in 9 patients (36%), due to elevated ALT and/or AST, and shortly interrupted in 2 patients (8%), due to hypertension.
Conclusion
Apart from asymptomatic hepatotoxicity, the study regimen was well tolerated. Although the pre-specified efficacy endpoint (30% pCR) was not met, a more than doubling of historical pCR rates after neo-adjuvant radiotherapy alone was observed, which warrants further investigation.
Background
Soft tissue sarcomas are rare malignant tumors of mesenchymal origin, that can arise anywhere in the body. The latest World health organization (WHO) classification describes more than 40 subtypes, which are distinguished by pathologists primarily based on their histological appearance [Citation1], supplemented with immunohistochemistry and molecular analysis where appropriate.
The standard treatment for localized high-risk soft tissue sarcoma is surgery combined with radiotherapy [Citation2]. Pre-operative radiation has a more favorable late toxicity profile compared to postoperative radiotherapy [Citation3–5], albeit at the cost of a higher rate of wound complications (35% vs. 17%). This combined treatment approach has resulted in high local control rates, but has limited, if any, effect on the prevention of distant metastases and survival parameters [Citation6], hence the importance of examining other (combinations of) treatment modalities.
For this purpose, peri-operative chemotherapy for localized STS has been examined in multiple trials. Although some studies showed promising results in terms of survival and disease control, others have not shown an added benefit of chemotherapy, which is why peri-operative chemotherapy is not standard therapy for STS [Citation2].
Angiogenesis is an important factor in the growth of tumors, providing the tumor with new blood vessels that deliver nutrients and oxygen needed for rapid expansion [Citation7]. Multiple studies showed increased expression of angiogenic factors like vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGFR) for soft tissue neoplasms, and their correlation to clinical features and prognosis [Citation8], suggesting an important role of angiogenesis in the soft tissue sarcoma disease course.
Pazopanib is an oral multitarget tyrosine kinase inhibitor (TKI), and its mode of action is suggested to be an interference with angiogenesis by blocking the VEGF-axis. Additionally, it provides a direct anti-growth effect through the blockade of various other receptor tyrosine kinases. Pazopanib has demonstrated activity in metastatic soft tissue sarcoma, after the phase III PALETTE trial [Citation9], randomizing pazopanib to placebo, showed a significant improvement in progression-free survival. Furthermore, pazopanib has demonstrated promising results when combined with radiotherapy for several tumor types, with a potential synergistic, radiosensitizing effect [Citation10,Citation11]. This has led to the investigation of the neo-adjuvant combination of pazopanib and radiotherapy for localized, high-risk soft tissue sarcoma in our phase I trial in 2015 [Citation12] (NCT01985295, acronym PASART-1). A 27% rate of grade 3+ hepatotoxicity (3 out of 11 patients) was observed, which was transient in all 3 patients after a maximum of 3 weeks. Apart from hepatotoxicity, the combination of 50 Gy radiotherapy and 800 mg daily pazopanib appeared tolerable and showed promising efficacy, with 4 out of 10 evaluable patients showing a complete pathological response.
This paper reports the results of the subsequent phase II trial (NCT02575066, acronym PASART-2), in which the efficacy, feasibility, and safety of the neo-adjuvant combination of radiotherapy and pazopanib for localized soft tissue sarcoma were further investigated, exploring the recommended pazopanib dose derived from PASART-1.
Patients and methods
Study design
PASART-2 was a phase II, multi-center, prospective clinical trial investigating the efficacy of neo-adjuvant pazopanib and concurrent external beam radiotherapy for non-metastatic soft tissue sarcoma patients. The use of peri-operative chemotherapy was not allowed in this trial. Patients were enrolled from 2 hospitals in the Netherlands and 1 in the United Kingdom. The study protocol and all amendments were approved by the local institutional review boards and the study was conducted in accordance with the declaration of Helsinki.
Inclusion- and exclusion criteria
Eligible patients were aged ≥18 years, WHO performance status ≤1, with a histologically confirmed newly diagnosed soft tissue sarcoma localized in the extremities, trunk and chest wall or the head and neck area, for which the standard treatment is a combination of radiotherapy and surgery (either deep-seated and/or >5 cm and/or anticipated close resection margin and/or Fédération Nationale des Centers de Lutte Contre Le Cancer (FNCLCC) grade II/III). Patients were evaluated for adequate organ function before study entry.
Exclusion criteria were treatment with chemotherapy or radiotherapy within 2 weeks before, or treatment with biologicals within 28 days or five half-lives before the first pazopanib dose. Additionally, patients with prior malignancies (except if the patient was disease-free for at least 5 years) and females being pregnant or breastfeeding were excluded. Effective methods of birth control were required for all patients of reproductive potential. Written informed consent was obtained from each patient.
Treatment plan
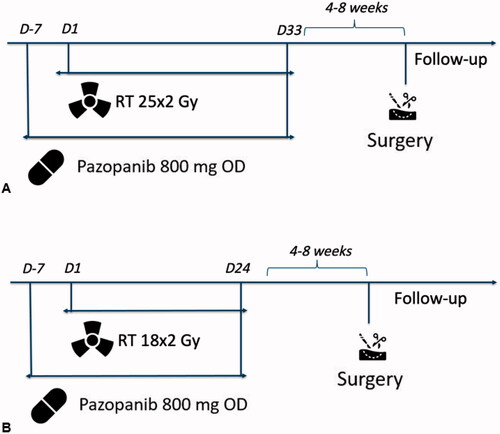
PASART-2A: external beam radiotherapy started at day 1 and was administered in 25 fractions of once-daily 2 Gy for 5 days a week, to a total dose of 50 Gy. On day −7, one week before the start of radiotherapy, pazopanib treatment commenced with a once-daily dose of 800 mg. Pazopanib treatment was continued until radiotherapy completion (day 33).
PASART-2B: After an interim analysis, which showed the supposed high effectiveness of the treatment regimen of PASART-2A, the study was amended, with the radiotherapy dose reduced to 36 Gy, in 18 fractions of once-daily 2 Gy for 5 days a week. The aim of this amendment was to reduce the risk of acute post-operative wound complications, which have been shown to correlate with the total RT dose administered [Citation13]. Again, once-daily 800 mg pazopanib intake started one week before radiotherapy (day −7) and finished simultaneously with radiotherapy completion (day 24).
Radiotherapy technique: the clinical target volume (CTV) was obtained by expanding the gross target volume (GTV) with a 4 cm margin craniocaudally and a 1,5 cm margin transversally. EBRT was delivered with intensity-modulated radiotherapy (IMRT) or 3-dimensional conformal radiotherapy (3 D-CRT).
Surgery: surgery was performed after an interval of 4–8 weeks post combined pazopanib and radiotherapy treatment. An overview of study procedures is shown in .
Figure 1. Overview of (A) study procedures of PASART-2A and (B) study procedures of PASART-2B. Abbreviations: D: day; RT: radiotherapy; Gy: Gray; OD: once daily.
Translational assessments: multiparametric MRI was performed at baseline, day 1, day 22 and pre-surgery. For pharmacokinetic analyses, plasma samples were taken on day 1 and day 22 and a tumor tissue sample on day 22. The results of these will be reported elsewhere.
Endpoints
The primary endpoint of this study was the proportion of patients with a pathological complete response (pCR) on central pathological examination of the resection specimen. Pathological examination of the resection specimen was first performed by pathologists from the local treatment centers, following STS pathology guidelines described by Wardelmann et al. [Citation14]. Subsequently, slides were centrally assessed by a reference pathologist (JVMG.B.) in the Slidescore software (www.slidescore.com) for the percentage of viable cells, necrosis, and hyalinization/fibrosis. Patients with the biggest discrepancies between local and central pathology were reviewed a second time by the same central pathologist. In line with PASART-1, a pathological complete response was defined as ≤5% viable cells, as at the time of trial design (2015), this was the most accepted surrogate for survival parameters and studies indicated a good correlation with survival outcomes [Citation15–17]. The outcomes from the central pathology review were designated as the definitive pathology outcomes for this trial.
Secondary endpoints included response rate as measured by the Response Evaluation Criteria In Solid Tumors (RECIST) version 1.1 at 4 weeks after completing radiotherapy, the incidence of acute and late toxicities measured by the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) v 4.0 and the proportion of patients with a major (post-operative) wound complication, for which we used the definition put forth by O’Sullivan in 2002 [Citation3]. Other secondary endpoints included the proportion of patients with R0 resections, assessed locally on pathological examination of the resection specimen, and overall survival (OS) at the time of data report.
Statistical analysis
To estimate the number of patients Simon’s optimal two-stage design was used. A total of 35 patients will be included in the study to test the null hypothesis that pCR p ≤ 0.10 versus the alternative that p ≥ 0.50 with an alpha level of 5% and power 90%. A pCR rate of 30% or higher should be observed for the treatment regimen to be declared effective. After 18 patients completed part 2 A of the study, a preplanned interim analysis was performed, at which the continuation of the trial would be decided by the number of pCRs observed in these 18 patients by the local pathologist. With 2 or less pCRs, the trial would be terminated. With 3 to 6 pCRs, 17 additional patients would be added. With 7 or more pCRs, the trial would proceed to part 2B. When the trial would proceed to part 2B, an additional 4 patients would be treated with the amended protocol. If among these 4 patients, 0 pCRs would be observed, the trial would be terminated, and if at least 1 pCR would be observed, an additional 9 patients would be added to stage II of the trial. An overview of the termination/continuation rules of PASART-2A and PASART-2B can be found in Supplementary Materials Figure 1.
All statistical analyses were performed with IBM SPSS Statistics 25. Descriptive statistics were employed to report the primary and secondary outcomes and Kaplan–Meier’s methodology was used to estimate overall survival (OS) for all patients from the time of study inclusion. p ≤ 0.05 was considered statistically significant.
Results
Baseline characteristics
Between March 2016 and November 2018, 25 patients were registered in the study (21 in PASART-2A, 4 in PASART-2B). The majority was male (60%). The median age was 57 years (range 24–79) and the most common histological subtypes, by the WHO 2013 classification [Citation18], were undifferentiated pleomorphic sarcoma (UPS) (36%) and myxofibrosarcoma (32%). Most tumors were located in the lower extremity (64%), followed by the trunk (24%). 84% of tumors were located deep within myofascial compartments. FNCLCC histological grade II and III tumors were equally represented (both 44%). The three grade I tumors were all located deep and larger than 50 mm. The median tumor size was 79 mm (range 28–211 mm). Baseline patient and tumor characteristics are described in .
Table 1. Baseline characteristics of the PASART study.
Local pathology results and trial decision
While the interim analysis took place, after 18 patients were treated in PASART-2A, 3 more patients were included in PASART-2A. Local pathology results indicated 9 pCRs in the first 18 patients, which meant continuation to PASART-2B, and 1 additional pCR in the subsequent 3 patients of PASART-2A, yielding a total of 10 pCRs out of 21 patients in PASART-2A. Four patients were subsequently treated with the amended protocol of PASART-2B. Local pathology indicated no pCRs and the trial was terminated after 25 patients were included. Local and central pathology results, sorted on the chronological order of trial inclusion, are provided in Supplementary Table 1.
Central pathological and radiological response
After central pathology review of all resection specimens, 5 out of the 25 patients (20%) with a pCR (≤5% viable tumor cells) remained. According to the European Organization for Research and Treatment of Cancer STS pathological response score [Citation14], no patients showed score A (no stainable tumor cells) or B (single stainable tumor cells, <1% of the whole specimen), 5 patients score C (≥1%-<10% stainable tumor cells), 12 patients score D (≥10%-<50% stainable tumor cells) and 8 patients score E (≥50% stainable tumor cells). When subdivided into the two parts of this study, 5 out of 21 patients (23,8%) treated with 50 Gy RT in PASART-2A showed a pCR after central review, while in PASART-2B, no pathological complete response was observed in the 4 patients treated with 36 Gy RT. An overview of pathological and radiological responses is provided in .
Table 2. Pathological and radiological response of patients with soft-tissue sarcoma treated with neo-adjuvant radiotherapy and pazopanib.
On radiological examination, 22/25 (88%) patients had stable disease (SD) according to RECIST criteria, 2/25 (8%) had a partial response (PR) and 1/25 (4%) had progressive disease (PD).
There were no delays in surgery. R0 resection margins (microscopically negative) were obtained in 24 out of 25 patients (96%), 1 patient had a positive surgical margin (R1).
Toxicities
An overview of toxicities is presented in . Grade 3+ toxicities during neo-adjuvant treatment occurred in 17 out of 25 patients (68%). Only 2 grade 4 toxicities occurred, both of them transient alanine aminotransferase (ALT) increases. Of the grade 3+ AE’s, the most common were increased ALT in 9 patients, and increased aspartate aminotransferase (AST) and hypertension in 8 patients both. Nine patients (36%) discontinued pazopanib treatment before completion, due to ALT and/or AST elevations, in the majority of cases after 4–5 full weeks (D22-D29 of the study) of pazopanib treatment (8/9). Normalization of measured liver enzyme levels occurred within 1–2 weeks after pazopanib discontinuation in all patients. Two patients (8%) had an interruption (of 10 days and 4 days, respectively) of pazopanib treatment due to grade 3 hypertension. One of those patients was also in the group of 9 patients that discontinued pazopanib treatment, so a total of 10/25 (40%) patients did not fully complete the pazopanib schedule.
Table 3. Toxicities of patients with soft-tissue sarcoma treated with neo-adjuvant radiotherapy and pazopanib.
Grade 3 acute post-operative toxicities occurred in 5 patients (20%). The most common was wound infection, which was registered in 5 patients. As of late toxicities, fibrosis, localized edema, and decreased joint range of motion were recorded, of which only fibrosis was grade 3, in one patient (4%). In 6 patients (24%), a major wound complication according to the O’Sullivan definition occurred.
Follow-up
At the time of the data report, after a median follow-up of 39 months (range 19–57 months), 6 patients have deceased. For 4 patients cause of death was metastatic disease in the lungs (+pelvis for one patient). For 1 patient cause of death was an untreated pneumothorax, this patient had developed metastatic disease in the lungs as well. For the 1 remaining patient that passed away, the cause of death was unknown. However, a local recurrence was recorded for this patient 15.5 months after surgery.
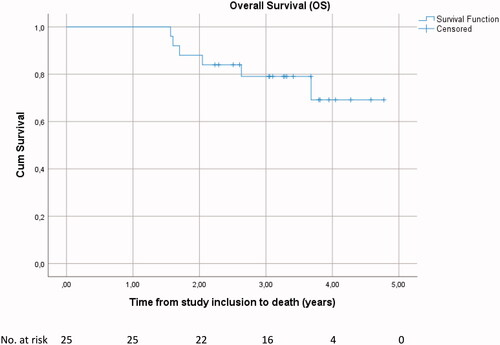
Of the 19 patients that are currently alive, 3 have developed metastatic disease (2 in the lungs, 1 in the bones) and 1 had a local recurrence 4 months after surgery (this was the patient with R1 resection) that was excised, this patient is currently alive without any evidence of disease. The remaining 15 patients are alive without any evidence of disease. Overall survival at 1-year, 2-year and 3-year was 100.0%, 88.0% (95%CI 75.3–100.0) and 79.1% (95%CI 62.6–95.6), respectively ().
Discussion
Overall, neo-adjuvant combined oral once-daily 800 mg pazopanib and 18–25 × 2 Gy external beam radiotherapy resulted in a pathological complete response (pCR) in 20% of patients. Hereby, the study did not meet its primary endpoint set at a rate of 30% pCR.
In this PASART-2 study design, the definition of pCR was set at ≤5% viable cells. However, defining pCR on the basis of the amount of viable cells is currently also subject of debate, as recent studies have shown a better correlation of other histopathological features to oncologic outcomes, such as hyalinization/fibrosis [Citation19]. These results need validation in larger cohorts to become new surrogate endpoints.
After pre-operative radiotherapy alone, pCR rates of 8–10% are observed [Citation16,Citation17]. The recent NCT02379845 study [Citation20] randomized 25 × 2 Gy external beam radiotherapy to the same radiotherapy dose plus intratumoral nanoparticles. This randomized phase III trial used the same pCR definition as our study and was blinded and centrally reviewed; the pathology review panel included the same pathologist as in our study (JVMG.B.). They reported a pCR rate of 7.9% in patients treated with neo-adjuvant 50 Gy radiotherapy alone. Although our study did not meet the predefined efficacy criteria, still a more than doubling of the pCR rate by the addition of pazopanib to RT alone was observed.
The investigated treatment regimen was generally well-tolerated, as the majority of grade 3+ acute toxicities were asymptomatic, transient ALT or AST increases or hypertension, and the rate of wound complications (24%) was relatively low compared to other studies investigating neo-adjuvant RT for STS [Citation6]. The high proportion of patients experiencing grade 3+ ALT or AST elevations (10/25, 40%), which was also reported in the previous PASART-1 phase I study [Citation12] is a remarkable finding, as pazopanib monotherapy causes substantially lower incidences of grade 3+ ALT and AST elevations [Citation21] (11% and 8%, respectively). Strikingly, when the combination of pazopanib and radiotherapy was used in other malignancies [Citation22,Citation23], or in conjunction with chemotherapy as neo-adjuvant treatment for STS [Citation24], no increased grade 3+ liver toxicity was reported. A reasonable explanation for the increased rate of ALT and AST elevations in PASART-1 and 2 is lacking, further research into this issue is needed. Whereas ALT is predominantly present in liver tissue, AST is also found in substantial concentrations in heart tissue, skeletal muscle, the kidneys, the brain, and red blood cells [Citation25]. AST elevations could potentially have been caused by irradiation to skeletal muscle, however, in all but one patient grade, 3 AST elevations occurred together with grade 3+ ALT elevations, which are more liver-specific. Elevations of other liver enzymes (gamma-glutamyl transferase, alkaline phosphatase, bilirubin) were relatively uncommon and of low grade. Interestingly, the 5 patients with pCR all fully completed the pazopanib regimen, while in the 9 patients that prematurely discontinued pazopanib due to ALT or AST elevations no pCRs were observed. All grade 3+ ALT or AST elevations were asymptomatic and the majority occurred late in the treatment course, with 2 weeks or less of pazopanib treatment remaining. Future trials could consider completing the 2 remaining weeks of pazopanib treatment in patients with asymptomatic liver enzyme elevations, possibly with corticosteroid administration, as these are able to decrease ALT levels with minimal risk of additional toxicities [Citation26,Citation27].
Anti-angiogenic drugs like pazopanib were originally developed to cause the destruction of tumor vasculature, thereby blocking the delivery of nutrients and oxygen needed for tumor expansion and growth. However, subsequent studies have shown an opposite effect: in certain, lower doses anti-angiogenics cause tumor vessel normalization, by normalizing the pro- and anti-angiogenic balance in the tumor micro-environment, and thus lead to enhanced tumor perfusion and oxygenation [Citation28,Citation29]. This effect is thought to be transient and is therefore described by the term ‘normalization window’ [Citation30,Citation31]. As the effect of radiotherapy is improved in better-oxygenated tumors, anti-angiogenic drugs might therefore serve as radiosensitizers if RT is administered during this normalization window [Citation11]. Other combinations of neo-adjuvant anti-angiogenic therapy combined with RT have also been investigated for STS, in a non-randomized, prospective fashion, such as the study by Yoon published in 2011 [Citation32], investigating neo-adjuvant bevacizumab plus 50.4 Gy RT. This study also reported a pCR rate of 20%. Other phases I studies suggested higher pCR rates with the combination of anti-angiogenics and (chemo)RT for STS [Citation33–35], but these studies, as our own PASART-1 trial [Citation12], were mainly designed for dose-finding and performed in smaller patient populations.
The transient tumor vasculature normalization seen after anti-angiogenic treatment also provides opportunities for synergy with systemic chemotherapy. The NCT02180867 trial [Citation24] combined neo-adjuvant pazopanib with chemotherapy (doxorubicin and ifosfamide) and RT in children and adults with soft-tissue sarcoma and found a pathological response in 58% of the patients. Of note, pathological response in this study was defined as 90% or more non-viable tumors.
We also reported response on the basis of RECIST in this study, although the value of these criteria in sarcoma is questionable [Citation36], as histopathological changes in response to therapy often precede tumor size changes. This is highlighted in our study, where the one patient with progressive disease on RECIST was identified as a pathological complete responder on central pathology review.
Our study has some limitations. Central pathology review was not foreseen at study initiation and was performed after all patients completed the treatment and the study was closed. If at the preplanned interim analysis after 18 patients, the central pathology review would have already been performed, then only 4/18 patients would have had a pCR and the trial would not have continued to part 2B. Instead, an additional 17 patients would have been treated with the original RT dose of 50 Gy. This observation, together with a mean difference of 13.1% between the viable cells identified at local and central pathology examination, underlines the importance of performing an early central pathology review in clinical (sarcoma) trials [Citation37].
Another limitation of this study is the inclusion of different histological subtypes. In recent years, these STS subtypes are increasingly viewed as distinct disease entities with their own specific clinical behavior and underlying biology. While ideal trials would study new therapies in separate subtypes, this is not always feasible, due to the rarity of STS.
This trial showed that neo-adjuvant pazopanib and RT for STS are well tolerated and is able to induce a pathological complete response in 20% of the patients. Although the primary endpoint was not met, this pCR rate is still twice as high as observed after 50 Gy external beam radiotherapy alone. As currently there are no drugs available for the neo-adjuvant treatment of STS with the aim to increase the pCR rate, this would justify further investigation in patients being marginally resectable, while the long-term results of PASART-2 are eagerly awaited.
Supplemental Material
Download MS Word (34.2 KB)Acknowledgments
Hester van Boven (Department of Pathology, The Netherlands Cancer Institute, Amsterdam, the Netherlands), Gert-Jan Liefers and Henk Hartgrink (Department of Surgery, Leiden University Medical Center, Leiden, The Netherlands) for their participation in the conduct of the study and their critical review of the manuscript
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data used to support the findings of this study and the complete study protocol are available from the corresponding author upon request.
Additional information
Funding
References
- WHO Classification of Tumours Editorial Board. WHO classification of tumours of soft tissue and bone. 5th ed. Lyon: IARC Press; 2020.
- PDQ Adult Treatment Editorial Board. Adult soft tissue sarcoma treatment (PDQ®): health professional version. In: PDQ cancer information summaries. Bethesda (MD): National Cancer Institute (US); 2002.
- O'Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359(9325):2235–2241.
- O'Sullivan B, Davis A, Turcotte R, et al. Five-year results of a randomized phase III trial of pre-operative vs post-operative radiotherapy in extremity soft tissue sarcoma. J Clin Oncol. 2004;22(14_suppl):9007–819s.
- Davis A, Osullivan B, Turcotte R, et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol. 2005;75(1):48–53.
- Haas RL, Delaney TF, O'Sullivan B, et al. Radiotherapy for management of extremity soft tissue sarcomas: why, when, and where? Int J Radiat Oncol Biol Phys. 2012;84(3):572–580.
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86(3):353–364.
- Rocchi L, Caraffi S, Perris R, et al. The angiogenic asset of soft tissue sarcomas: a new tool to discover new therapeutic targets. Biosci Rep. 2014;34(6):e00147.
- van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379(9829):1879–1886.
- Bitterman D, Du K. Safety and efficacy of combination targeted therapy and radiotherapy. Am J Hematol Oncol. 2016;12:23–31.
- Goedegebuure RSA, de Klerk LK, Bass AJ, et al. Combining radiotherapy with anti-angiogenic therapy and immunotherapy; a therapeutic triad for cancer? Front Immunol. 2018;9:3107.
- Haas RL, Gelderblom H, Sleijfer S, et al. A phase I study on the combination of neoadjuvant radiotherapy plus pazopanib in patients with locally advanced soft tissue sarcoma of the extremities. Acta Oncol. 2015;54(8):1195–1201.
- Haas RL, Miah AB, LePechoux C, et al. Preoperative radiotherapy for extremity soft tissue sarcoma; past, present and future perspectives on dose fractionation regimens and combined modality strategies. Radiother Oncol. 2016;119(1):14–21.
- Wardelmann E, Haas RL, Bovée JV, et al. Evaluation of response after neoadjuvant treatment in soft tissue sarcomas; the European organization for research and treatment of Cancer-Soft tissue and bone sarcoma group (EORTC-STBSG) recommendations for pathological examination and reporting. Eur J Cancer. 2016;53:84–95.
- Eilber FC, Rosen G, Eckardt J, et al. Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol. 2001;19(13):3203–3209.
- Canter RJ, Martinez SR, Tamurian RM, et al. Radiographic and histologic response to neoadjuvant radiotherapy in patients with soft tissue sarcoma. Ann Surg Oncol. 2010;17(10):2578–2584.
- Shah D, Borys D, Martinez SR, et al. Complete pathologic response to neoadjuvant radiotherapy is predictive of oncological outcome in patients with soft tissue sarcoma. Anticancer Res. 2012;32(9):3911–3915.
- Fletcher CD, Hogendoorn P, Mertens F, et al. WHO classification of tumours of soft tissue and bone. 4th ed. Lyon: IARC Press; 2013.
- Schaefer IM, Hornick JL, Barysauskas CM, et al. Histologic appearance after preoperative radiation therapy for soft tissue sarcoma: assessment of the European organization for research and treatment of Cancer-Soft tissue and bone sarcoma group response score. Int J Radiat Oncol Biol Phys. 2017;98(2):375–383.
- Bonvalot S, Rutkowski PL, Thariat J, et al. NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (act.In.Sarc): a multicentre, phase 2-3, randomised, controlled trial [published correction appears in lancet oncol. 2019 sep;20(9):e468]. Lancet Oncol. 2019;20(8):1148–1159.
- Powles T, Bracarda S, Chen M, et al. Characterisation of liver chemistry abnormalities associated with pazopanib monotherapy: a systematic review and meta-analysis of clinical trials in advanced cancer patients. Eur J Cancer. 2015;51(10):1293–1302.
- Goyal S, Shah S, Khan AJ, et al. Evaluation of acute locoregional toxicity in patients with breast cancer treated with adjuvant radiotherapy in combination with pazopanib. ISRN Oncol. 2012;2012:896202.
- De Wolf K, Rottey S, Vermaelen K, et al. Combined high dose radiation and pazopanib in metastatic renal cell carcinoma: a phase I dose escalation trial. Radiat Oncol. 2017;12(1):157.
- Weiss AR, Chen YL, Scharschmidt TJ, et al. Pathological response in children and adults with large unresected intermediate-grade or high-grade soft tissue sarcoma receiving preoperative chemoradiotherapy with or without pazopanib (ARST1321): a multicentre, randomised, open-label, phase 2 trial. Lancet Oncol. 2020;21(8):1110–1122.
- Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ. 2005;172(3):367–379.
- Hu PF, Xie WF. Corticosteroid therapy in drug-induced liver injury: pros and cons. J Dig Dis. 2019;20(3):122–126.
- Weber S, Benesic A, Rotter I, et al. Early ALT response to corticosteroid treatment distinguishes idiosyncratic drug-induced liver injury from autoimmune hepatitis. Liver Int. 2019;39(10):1906–1917.
- Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol. 2013;31(17):2205–2218.
- Viallard C, Larrivée B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. 2017;20(4):409–426.
- Dings RPM, Loren M, Heun H, et al. Scheduling of radiation with angiogenesis inhibitors anginex and avastin improves therapeutic outcome via vessel normalization. Clin Cancer Res. 2007;13(11):3395–3402.
- Matsumoto S, Batra S, Saito K, et al. Antiangiogenic agent sunitinib transiently increases tumor oxygenation and suppresses cycling hypoxia. Cancer Res. 2011;71(20):6350–6359.
- Yoon SS, Duda DG, Karl DL, et al. Phase II study of neoadjuvant bevacizumab and radiotherapy for resectable soft tissue sarcomas. Int J Radiat Oncol Biol Phys. 2011;81(4):1081–1090.
- Meyer JM, Perlewitz KS, Hayden JB, et al. Phase I trial of preoperative chemoradiation plus sorafenib for high-risk extremity soft tissue sarcomas with dynamic contrast-enhanced MRI correlates. Clin Cancer Res. 2013;19(24):6902–6911.
- Canter RJ, Borys D, Olusanya A, et al. Phase I trial of neoadjuvant conformal radiotherapy plus sorafenib for patients with locally advanced soft tissue sarcoma of the extremity. Ann Surg Oncol. 2014;21(5):1616–1623.
- Jakob J, Simeonova A, Kasper B, et al. Combined sunitinib and radiation therapy for preoperative treatment of soft tissue sarcoma: results of a phase I trial of the German interdisciplinary sarcoma group (GISG-03). Radiat Oncol. 2016;11:77.
- Stacchiotti S, Verderio P, Messina A, et al. Tumor response assessment by modified Choi criteria in localized high-risk soft tissue sarcoma treated with chemotherapy. Cancer. 2012;118(23):5857–5866.
- Vujanić GM, Sandstedt B, Kelsey A, et al. Central pathology review in multicenter trials and studies: lessons from the nephroblastoma trials. Cancer. 2009;115(9):1977–1983.