Abstract
Background
Variations in symptom development among breast cancer (BC) survivors are understudied. We examined: (Q1) Symptom trajectories of pain, fatigue, insomnia, breast, and arm symptoms in BC survivors, (Q2) possible patterns or cluster-like associations between trajectory classification of different symptoms, and (Q3) characteristics of survivors assigned to high-burden symptom trajectories.
Material and methods
Participants were 968 women (mean age = 59.6 years) treated for early-stage BC and followed across a three-year postoperative period. As part of routine follow-up procedures, patients reported symptom burden and functioning levels at each hospital visit using the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ-C30) and the BC-specific module (QLQ-BR-23). Growth mixture modeling (GMM) analysis was used to differentiate potential subgroups of individuals with similar longitudinal symptom patterns, i.e., symptom trajectories (Q1). With this approach, groups experiencing persistent, highly distressing cancer- and treatment-related late effects (LEs) may be identified. Latent class analysis (LCA) was used for Q2 and logistic regression analysis for Q3.
Results
GMM identified two relatively parallel trajectories across the tested symptoms: The majority of the sample exhibited a low-burden symptom trajectory (74.4–89.2%) and a minority by a high-burden symptom trajectory (10.8–25.6%). LCA revealed that approximately one in five women (18.8%) were likely to be members of the high-burden symptom trajectory across all tested symptoms. In addition to a high probability of being burdened over time across multiple symptoms, these women were also characterized by poorer self-reported physical and social functioning.
Conclusion
A substantial minority followed a high-burden symptom trajectory for several years following BC treatment. Associations were found in trajectory classification across symptoms, indicating that cancer-related LEs appear in clusters of multiple concurrent symptoms.
Background
Breast cancer (BC) is the most common (non-skin) cancer, accounting for one-quarter of all cancers in women [Citation1]. Due to improved screening and intensified treatment, the current nine-year survival rate is 93% in approximately two-thirds of patients with early-stage BC who are diagnosed with small cancers without lymph node metastasis [Citation2]. Thus, the number of BC survivors is increasing [Citation3]. However, improved survival is often obtained at a cost, as more intensive treatments may result in persistent symptoms and late effects (LEs). Despite the high overall quality of life (QoL) in BC survivors, a range of adverse symptoms have been reported up to several years after primary diagnosis [Citation4]. Commonly identified LEs include BC treatment-specific physical symptoms, e.g., impaired arm functioning and breast fibrosis [Citation2,Citation5,Citation6]. Other more general LEs experienced across several cancer types include pain, fatigue, and insomnia [Citation7–10] together with functional and cognitive impairment, poor body image, and sexual dysfunction [Citation11–13].
Research in LEs most often presents average scores for entire samples, with longitudinal studies capturing overall increase or decrease in symptom severity. This approach may, however, hide the underlying heterogeneity in symptoms as they develop over time. In contrast, trajectory analysis identifying multiple latent subgroups of individuals presenting similar longitudinal patterns may provide a deeper understanding of the data [Citation14]. Most available trajectory studies with BC survivors have focused on depression [Citation15–19] or general psychological distress [Citation20–25]. For functional domains, trajectories have been established for cognitive and sexual functioning [Citation26–28] and more nonspecific mental and physical functioning [Citation29,Citation30]. The physical symptoms investigated include sleep disturbance [Citation31–33], fatigue [Citation33–39], and general and breast-specific pain [Citation33,Citation40]. Within BC trajectory studies, a diverse picture emerges regarding the number of identified symptom trajectories. For example, while most studies on fatigue have resulted in the identification of two symptom trajectories [Citation34–37], other studies have identified three to five separate trajectories [Citation33,Citation38,Citation39]. Variations across the literature may reflect different recruitment procedures and sample sizes, different study timeframes, and different instruments used to assess LEs. Given how certain subgroups (e.g., younger vs. older patients, early- vs. late-stage BC) may differ in symptom burden, underlying differences in study populations may also drive differences in the number of trajectories. Still, across the studied outcomes, studies repeatedly demonstrate that a majority of BC survivors follow a trajectory characterized by a relatively insignificant degree of LEs (i.e., low symptom burden/high functioning across time), while a substantial minority express a clinically significant degree of LEs (i.e., high symptom levels/impaired functioning across time). Such findings illustrate the importance of looking beyond the sample average when exploring the lasting effects of BC disease and treatment.
Although existing trajectory studies provide important insights into distinct symptom courses following BC treatment, the available research primarily focuses on the shorter-term post-diagnosis or post-treatment period [Citation15,Citation21,Citation31,Citation36]. Few studies included follow-ups beyond the initial two years post-surgery, limiting our knowledge of the long-term consequences of BC. Moreover, BC trajectory studies generally examined relatively small samples, thereby restricting the robustness of the results. Standing out are three US studies investigating cognitive functioning (n = 1280), fatigue (n = 191), and sexual functioning (n = 896), respectively, with 5–7-year follow-up, together with a Portuguese study examining sleep quality over 3 years (n = 458) [Citation26,Citation28,Citation32,Citation38]. The remaining studies relied on 6–24 months follow-up and investigated sample sizes with a median of around 300 participants. Lastly, most studies have investigated LEs in isolation, precluding examinations of potential associations across symptom trajectories. Associations between trajectory classification across symptoms may reveal whether certain LEs occur individually or in clusters of correlated symptom patterns over time.
Given these limitations in existing literature, the present study extends prior research by investigating data from a large cohort of BC survivors over a three-year postoperative period. It is among the first to investigate associations in symptom burden across different LEs through a trajectory framework. Our study expands the emerging knowledge on LE symptom clusters [Citation41,Citation42] while accounting for the symptoms’ development over time. From a clinical perspective, the study aims to identify patients with the highest probability of developing persistent LEs, thereby providing vital information on how follow-up services may be tailored to individual needs early following BC treatment.
Specifically, our research aims were to (1) identify possible symptom trajectories of common LEs through growth mixture modeling (GMM), (2) examine potential associations in trajectory membership across symptoms, and (3) explore demographic, clinical, and functional characteristics of individuals assigned membership to high-burden symptom trajectories.
Methods
Design
Since 2016, all BC patients receiving routine follow-up care at the Department of Oncology, Aarhus University Hospital, Denmark, have been invited to complete a questionnaire before each planned visit to understand symptoms better and inform the following consultation with the oncologist. Patients received a link and instructions on how to access the online questionnaire via e-mail. The present study uses data from questionnaires completed between April 2016 and February 2018.
Depending on the adjuvant BC treatment received (i.e., endocrine therapy [ET] only, chemotherapy [CT] only, or ET + CT), planned visits after primary treatment were: 3 weeks, 3 months, 6 months, 12 months, and at yearly intervals after that. Patients who received CT were seen at 3 weeks and 3 months. Patients who received CT but not ET were followed until 12 months. Adjuvant treatments were given according to the Danish Breast Cancer Group (DBCG) guidelines (for a detailed description of treatment modalities, see Supplementary Document 1).
In the present study, data were grouped into uniform postoperative 12-month time slots (i.e., time 1, T1 = 0–12 months; time 2, T2 = 13–24 months; time 3, T3 = 25–36 months). In cases with multiple observations within the same time slot (e.g., 3 and 6 months post-surgery), measures were averaged to create a single data point for each individual. Given the pragmatic nature of data collection, data organization relied on a floating baseline methodology where individual patient data were centered on the time of surgery. The sample thus includes individuals in their first, second, and third-year after surgery at the initiation of data collection (e.g., individuals receiving surgery in early 2016 provided T1–T3 data, while individuals who received surgery in early 2018 only provided T1 data in the present dataset). Maximum likelihood estimation was used to adjust for incomplete data by estimating parameters based on information contained in the available data. Questionnaires completed more than three years after surgery were not analyzed in the present study due to a limited number of data points.
Study population
Female early-stage BC survivors were included allowing bilateral BC and any histological type. Exclusion criteria were loco-regional recurrence, metastatic disease, ductal carcinoma in situ disease, other malignancy, and never received breast surgery. Low-risk patients (i.e., ≥60 years with tumor ≤10 mm, lymph-node negative, ER-positive, and HER2-negative) did not receive adjuvant systemic therapy. They were, therefore, neither followed at the Department of Oncology nor invited to complete questionnaires.
Initially, the cohort consisted of 2660 BC survivors. After excluding individuals with loco-regional, distant recurrent, or secondary cancer, or not treated according to DBCG guidelines (n = 271), patients with no follow-up visit within the first three years after surgery (n = 812), and finally, individuals who never completed questionnaires (n = 609), a total of 968 patients were eligible for analysis. Participants were on average 59.62 years old (range: 24–88), and most participants were postmenopausal (72.8%). Given the indicated 12-month time slots, data included in the GMM analyses were on average collected at 7.92 (T1), 17.52 (T2), and 29.52 (T3) months post-surgery, respectively. presents further details on demographic, clinical, functional, and symptomatic characteristics.
Table 1. Demographic, clinical, functional, and symptomatic characteristics at primary observation.
Patient-reported symptom burden and functioning
The questionnaire consisted of the entire European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ-C30, version 3) and the BC-specific module (BR23). The QLQ-C30 includes a measure of global health, five functional subscales (physical, role, emotional, social, and cognitive), eight symptom scales (fatigue, pain, nausea and vomiting, dyspnea, insomnia, appetite loss, constipation, and diarrhea), and one-item on cancer-related financial difficulties [Citation43]. The BR23 is a 23-item measure of functioning (body image, sexual functioning, sexual enjoyment, and future perspective) and symptoms (systemic therapy side effects, breast symptoms, arm symptoms, and hair loss-related distress) explicitly related to BC treatment [Citation44]. All domains of the EORTC questionnaires were transformed to 0–100 scales with higher scores on the functional scales indicating better function and higher symptom scale scores indicating worse symptomatology [Citation43]. The EORTC questionnaires were supplemented with three items on neuropathy, arthralgia, and myalgia from the PRO-CTCAE system [Citation45].
Patient-reported outcomes (PROs) were combined with data retrieved from the DBCG database and medical records on adjuvant clinical indicators, patient characteristics, and specific treatments.
Statistical analyses
To address our first research aim (identification of symptom trajectories), selected core symptoms in the follow-up setting after BC treatment from the QLQ-C30 (fatigue, pain, insomnia) and the BR23 (breast symptoms, arm symptoms) were explored in separate growth mixture modeling (GMM) analyses. GMM involves an iterative process of extracting different subgroups of participants who display the same symptomatic development over time and evaluating different model fit indices. The tested models differed on three aspects: (i) number of classes (with the most parsimonious model, a one-trajectory solution, tested followed by models with an increasing class count), (ii) time-specific effects (i.e., linear or quadratic trajectory shape), and (iii) fixed and random parameter estimates. Identification of the optimal trajectory solution with the best data fit included inspection of the following indices: Akaike Information Criteria (AIC), Bayesian Information Criteria (BIC), Sample-Size Adjusted BIC (SSA-BIC), entropy, and Lo-Mendell-Rubin Likelihood Ratio Test (LMR-LRT). Lower AIC, BIC, and SSA-BIC values, higher entropy, and significant LMR-LRT indicated improving fit. Final model selection was based on these fit indices, model parsimony, and interpretability [Citation14,Citation46]. This approach was repeated separately for each tested symptom.
Our second aim, uncovering associations in trajectory membership across symptoms, was addressed using a latent class analysis (LCA). LCA identifies latent groups (classes) of individuals with similar associations between tested inputs [Citation46]. The LCA identifies conditional item probabilities for being classified within different classes. Values ≥0.60 were considered a high probability (i.e., 60% likelihood), values ≤0.59 and ≥0.20 as representing moderate, and values ≤0.19 as representing low probability. For our purpose, membership within the five symptom trajectories, as identified by the GMM models, was used as input data to explore whether individuals assigned membership to a high-burden symptom trajectory differed across separate LEs. As in the GMM analyses, fit indices (i.e., AIC, BIC, SSA-BIC, entropy, LMR-LRT), parsimony, and model interpretability were explored and evaluated for final model selection. GMM analyses and LCA were conducted in Mplus, version 8.3 [Citation47].
Addressing our third research aim (assessing characteristics of individuals assigned membership to high-burden symptom trajectories), we conducted logistic regression analyses in SPSS, version 26 [Citation48], with optimal class solution from the LCA as the outcome. In the initial univariate logistic regression analyses, age was included as a demographic variable. Clinical factors included menopausal status, surgery type (lumpectomy, mastectomy), auxiliary lymph node dissection (ALND; Sentinel lymph node biopsy, partial ALND, complete ALND), chemotherapy (CT; yes/no), HER2-targeted treatment (yes/no), endocrine therapy (ET; yes/no), and radiotherapy (RT; no RT, local, or loco-regional RT). Functioning was measured with the respective subscales of the QLQ-C30 and the QLQ-BR23, and symptomatic factors included neuropathy, arthralgia, and myalgia pain severity. For the logistic regression, we used the participants’ primary self-report score on the functional and symptomatic measures (i.e., data from their first completed questionnaire). Significant factors (p < .05) were retained and tested simultaneously in a multivariate logistic regression model to determine the individual contribution of each predictor while accounting for their shared variance.
Ethics
The study was approved by the Danish Patient Safety Authority (3-3013-2975/1) and by the Danish Data Protection Agency (1-16-02-894-17).
Results
Research aim 1: identification of symptom trajectories
Statistical and visual inspections revealed that model convergence improved when using linear rather than quadratic effect by fixing the slope and testing random intercept. Based on fit indices and interpretability of the class solutions, models with two trajectories were retained as the optimal solution for all tested LEs (see ).
Table 2. Fit indices for one- to three-class growth mixture models of symptom trajectories.
illustrates separate panels for the two-class trajectories across symptoms. Across tested LEs, majorities (74.4–89.2%) showed a low-burden symptom trajectory with a decreasing slope, while minorities (10.8–25.6%) followed a high-burden symptom trajectory. Although not statistically significant, the high-burden fatigue and breast symptom trajectories showed decreasing slopes, while pain, insomnia, and arm symptom trajectories exhibited increasing slopes. provides parameter estimates for each outcome.
Figure 1. Symptom trajectories of (A) fatigue, (B) pain, (C) insomnia, (D) breast symptoms, and (E) arm symptoms. Fatigue, pain, and insomnia were measured by the European Organisation for Research and Treatment of Cancer (EORTC) general Quality of Life Questionnaire (QLQ-C30, version 3); breast symptoms and arm symptoms were measured by the breast cancer module (QLQ-BR23). All symptom domains of the EORTC questionnaires were transformed to 0–100 scales with higher scores representing a larger symptom burden. T1: time 1 (7.92 months post-surgery); T2: time 2 (17.52 months post-surgery); T3: time 3 (29.52 months post-surgery).
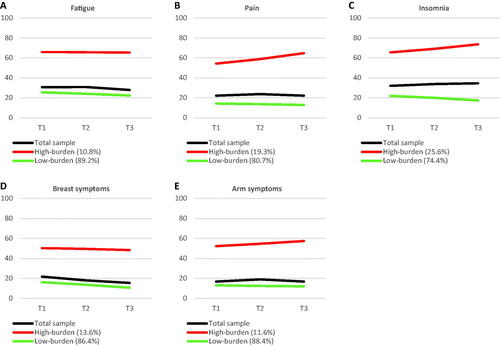
Table 3. Parameter estimates for growth mixture models of two-class symptom trajectories.
Research aim 2: associations in trajectory membership across symptoms
In an LCA, we used trajectory membership from separate GMM models as input variables. Members with low-burden symptom trajectories received a score of ‘0’, and members with high-burden symptom trajectories a score of ‘1’ to explore potential associations in trajectory classification across the investigated symptoms. A two-class solution was selected as the optimal solution with an entropy of .80, corresponding to ‘high’ classification accuracy.
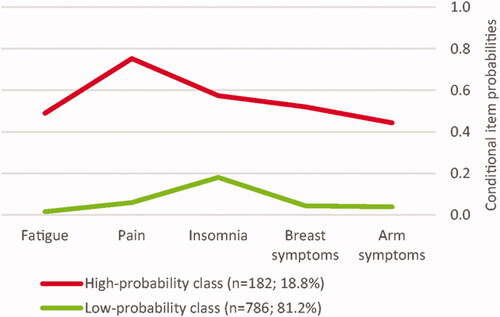
The final model indicated substantial associations in trajectory membership across the five tested LEs. The two-class solution indicated how the majority (81.2%) of the total sample reported low probabilities (1.7–18.1%) of being classified as belonging to a high-burden symptom trajectory in the separate GMM models. The remaining minority (18.8%) showed moderate-high probabilities (44.5–75.3%) of being classified as belonging to a high-burden symptom trajectory. depicts the two classes with conditional item probabilities for being classified as a high-burden symptom trajectory for each of the five LEs, evaluated by GMM analyses. The separation between classes was generally high across symptoms (odds ratios of item probabilities between classes >5) [Citation46], indicating that the LEs performed well in differentiating the two classes. Notably, the insomnia item was only marginally above this value (6.1), suggesting that this symptom only contributed little to the overall class separation.
Figure 2. Latent class analysis of associations across symptom trajectory classification. Conditional item probabilities refer to the probability of membership within a high-burden symptom trajectory across separate LEs. Values of ≥0.60 were considered as the high probability (i.e., 60% likelihood of high-burden classification), values ≤0.59 and ≥0.20 as representing moderate, and values of ≤0.19 as representing low probability.
Research aim 3: members of the high-burden symptom trajectories
Univariate (i.e., unadjusted) analyses identified (a) younger age; (b) complete ALND and loco-regional RT; (c) lower general functioning (global health, physical, role, emotional, cognitive, social), (d) BC-specific functioning (body image, sexual functioning, sexual enjoyment, future perspective), and (e) higher neuropathy, arthralgia, and myalgia severity as significant characteristics of high-class membership (see Supplementary Table 1).
A final multivariate logistic model including only univariately significant covariates (p < .05) revealed that women in the high symptom class reported poorer physical (p = .003) and social functioning (p = .018) compared with women in the low symptom class. The complete model, which included and adjusted for all covariates, was statistically significant, ꭕ2(16) = 102.54, p < .001, and explained 46.3% (Cox & Snell R Square) to 73.2% (Nagelkerke R Square) of the variance in class membership.
Discussion
The general aim of the study was to expand knowledge on symptomatic development and symptom associations in BC survivors through a trajectory framework. Across tested symptoms, GMM analyses revealed how a substantial minority followed trajectories characterized by a high symptom burden. Findings suggest associations in trajectory membership, indicating how cancer-related LEs present in clusters of multiple concurrent symptoms. Furthermore, women with high probabilities of being burdened over time across multiple symptoms reported lower physical and social functioning.
Across all tested LEs, a two-class GMM provided the best and most interpretable fit of the data. Consistent with the literature [Citation34,Citation37,Citation40], our study revealed how most experienced a low symptom burden, while a minority followed trajectories characterized by a substantial symptom burden up to three years after surgery. Individual data points indicated how the mean between-trajectory difference in symptomatology levels increased across time for all five outcomes. The total sample repeatedly scored below a previously proposed arbitrary threshold (≥40) for clinically significant symptom-level on the QLQ-C30 [Citation33] and below recently established anchor-based Thresholds for Clinical Importance (TCI) on each item on the QLQ-C30 [Citation49], indicating low symptom burden at the cohort level. This finding mirrors a recent study conducted with postmenopausal BC survivors in the present patient sample, showing how mean levels of self-reported symptoms were below these cut-points for more than five years after primary treatment [Citation50].
In contrast, the high-burden symptom trajectories demonstrated a clinically significant symptom level across LEs until the three-year follow-up. Beyond statistical significance, Minimal Important Differences (MIDs) is another approach to investigate group differences. MIDs reflect the smallest change or difference that a patient or clinician would interpret as ‘important,’ indicating an adjustment of the patient’s symptom management. A recent study has identified MIDs for between-group differences for EORTC-QLQ-C30 scores of patients with advanced BC [Citation51]. In the present study, the fatigue trajectories differed by more than 40 points across all data waves, by far surpassing the identified MIDs, ranging from 6 to 8 points [Citation51]. No MIDs have yet been established for the other examined symptoms among BC survivors. However, anchors from samples of mixed cancer groups [Citation52] suggest that the low- and high-burden symptom trajectories differ to a clinically significant extent. Applying TCIs and the ≥40 cutoff value [Citation33,Citation49], all high-burden symptom trajectories were above these cutoff values, whereas the corresponding low-burden trajectories were below. Identifying those who continue to show high burdened symptom levels over several years may be an essential step in improving the management of LEs in BC survivors.
We conducted an LCA with the trajectory solutions from the separate GMM models as input data to investigate whether the highly distressed minorities overlapped individual symptoms. This analysis identified a class with low probabilities of membership within the high-burden symptom trajectories across tested LEs and a class with moderate-high probabilities of following the high-burden symptom trajectories. This finding indicates that LEs do not present in isolation but rather in clusters of multiple concurrent symptoms, as suggested by earlier findings [Citation41,Citation42]. Individuals struggling with high pain levels are, for example, likely to experience concurrent insomnia and breast pain. This finding highlights the importance of identifying this highly distressed minority in order to provide proper support. Our findings also suggest the relevance of studying combinations of LEs in BC survivors to reveal potential clustering effects.
In the multivariate model, no demographic or clinical variables emerged as significant characteristics of individuals with large probabilities of belonging to high-burden symptom trajectories. Conversely, these individuals reported lower physical and social functioning, outcomes that are potentially amenable through psychosocial interventions in follow-up settings. For example, meta-analyses suggest how exercise interventions can improve physical functioning and reduce fatigue, pain, and insomnia in BC patients [Citation53,Citation54]. An individual patient data (IPD) meta-analysis with half of the sample studied diagnosed with BC revealed how psychosocial interventions significantly improved social function [Citation55]. Thus, interventions tailored to improve functional levels among BC survivors may ameliorate the derivative distress caused by symptomatic LEs. Future studies should investigate the effects of screening and intervention for poor functioning in BC survivors.
Our study has several strengths, including the large sample, a long follow-up period, cross-symptom analysis, and multi-source data (PROs combined with information from clinical databases). Only slight deviations were evident between our QLQ-C30 scores and BC population-based reference values [Citation56], supporting the representativeness of our sample of women treated for BC. Some limitations should also be noted. First, the study may not capture changes in the acute postoperative period with sufficient sensitivity by relying on one-year data time slots. Studies have shown how BC survivors and reference groups only differed in health-related QoL in the first year after diagnosis with a declining effect over time [Citation30,Citation49,Citation57,Citation58], and symptoms might stabilize over time following a period of more pronounced change immediately following BC diagnosis. We may not have sufficiently captured such changes with only one assessment in the first year following diagnosis. Second, the EORTC questionnaire may not be the most sensitive instrument to capture specific LE symptoms. Larger questionnaire batteries with specific symptom measures, e.g., the Insomnia Severity Index [Citation59] or numerical rating scales for measuring different aspects of pain [Citation60], could result in model solutions with greater symptom change across time. Third, as our pragmatic data set was collected as part of routine follow-up, primarily to inform clinicians of symptom burden and functioning to tailor procedures per patient needs, it did not contain information on demographic characteristics (e.g., marital status, educational attainment, level of income). These factors could be relevant predictors of long-term symptom development. Fourth, the study population did not include low-risk patients who did not receive treatment at the Department of Oncology. These patients receive less intensive treatment than the study participants, why the current data may overestimate the severity and persistence of LEs among BC survivors. Finally, a considerable number of individuals never completed the questionnaires. The lack of PROs on these women challenges detailed non-responder analyses. Still, a recent study on the postmenopausal women from the same overall sample revealed how especially older patients failed to respond to the questionnaire [Citation49]. This finding reflects an inherent limitation in requesting PROs in a real-life clinical setting, potentially biasing the results.
In conclusion, our study illustrates how BC survivors form two distinct subgroups characterized by relatively stable high and low symptom burden over a three-year postoperative period. Approximately one in five BC survivors had a high probability of belonging to a high-burden symptom trajectory across all tested LEs (fatigue, pain, insomnia, breast symptoms, and arm symptoms). These highly burdened women reported poorer physical and social functioning, representing a patient group in need of timely targeted support.
Author contributions
AWMN contributed to data collection and the interpretation of the data and drafted and revised the paper. ML contributed to the study conception, the analysis, and interpretation of the data, and drafted and revised the paper. HMN contributed to the study design, data collection and revised the paper. JA contributed to the study design, interpretation of the data and revised the paper. BVO contributed to the study design, data collection and revised the paper. MHK contributed to data collection and revised the paper. RZ contributed to the study conception and design, the interpretation of the data, and revisions of the various versions of the paper. All authors read and approved the final paper.
Supplemental Material
Download MS Word (23.8 KB)Acknowledgments
We thank the many women participating in the Ambuflex survey.
Disclosure statement
The authors have no conflicts of interest to declare.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. 2021;71:209–249.
- Offersen BV, Alsner J, Nielsen HM, et al. Hypofractionated versus standard fractionated radiotherapy in patients with early breast cancer or ductal carcinoma in situ in a randomized phase III trial: the DBCG HYPO trial. J Clin Oncol. 2020;38(31):3615–3625.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Ganz PA, Desmond KA, Leedham B, et al. Quality of life in long-term, disease-free survivors of breast cancer: a follow-up study. J Natl Cancer Inst. 2002;94(1):39–49.
- DiSipio T, Rye S, Newman B, et al. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14(6):500–515.
- Levangie PK, Drouin J. Magnitude of late effects of breast cancer treatments on shoulder function: a systematic review. Breast Cancer Res Treat. 2009;116(1):1–15.
- Bao T, Basal C, Seluzicki C, et al. Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: prevalence, risk factors, and fall risk. Breast Cancer Res Treat. 2016;159(2):327–333.
- Hong D, Bi L, Zhou J, et al. Incidence of menopausal symptoms in postmenopausal breast cancer patients treated with aromatase inhibitors. Oncotarget. 2017;8(25):40558–40567.
- Bardwell WA, Profant J, Casden DR, et al. The relative importance of specific risk factors for insomnia in women treated for early-stage breast cancer. Psycho-Oncology. 2008;17(1):9–18.
- Johannsen M, Christensen S, Zachariae R, et al. Socio-demographic, treatment-related, and health behavioral predictors of persistent pain 15 months and 7-9 years after surgery: a nationwide prospective study of women treated for primary breast cancer. Breast Cancer Res Treat. 2015;152(3):645–658.
- Carreira H, Williams R, Müller M, et al. Associations between breast cancer survivorship and adverse mental health outcomes: a systematic review. J Natl Cancer Inst. 2018;110(12):1311–1327.
- Lyngholm CD, Christiansen PM, Damsgaard TE, et al. Long-term follow-up of late morbidity, cosmetic outcome and body image after breast conserving therapy. A study from the Danish breast cancer cooperative group (DBCG). Acta Oncol. 2013;52(2):259–269.
- Fogh M, Højgaard A, Rotbøl CB, et al. The majority of Danish breast cancer survivors on adjuvant endocrine therapy have clinically relevant sexual dysfunction: a cross-sectional study. Acta Oncol. 2021;60(1):61–68.
- Muthén B. Statistical and substantive checking in growth mixture modeling: Comment on Bauer and Curran (2003). Psychol Methods. 2003;8(3):369.
- Dunn LB, Cooper BA, Neuhaus J, et al. Identification of distinct depressive symptom trajectories in women following surgery for breast cancer. Health Psychol. 2011;30(6):683–692.
- Stanton AL, Wiley JF, Krull JL, et al. Depressive episodes, symptoms, and trajectories in women recently diagnosed with breast cancer. Breast Cancer Res Treat. 2015;154(1):105–115.
- Donovan KA, Gonzalez BD, Small BJ, et al. Depressive symptom trajectories during and after adjuvant treatment for breast cancer. Ann Behav Med. 2014;47(3):292–302.
- Avis NE, Levine BJ, Case LD, et al. Trajectories of depressive symptoms following breast cancer diagnosis. Cancer Epidemiol Biomarkers Prev. 2015;24(11):1789–1795.
- Rottmann N, Hansen DG, Hagedoorn M, et al. Depressive symptom trajectories in women affected by breast cancer and their male partners: a nationwide prospective cohort study. J Cancer Surviv. 2016;10(5):915–926.
- Lam WWT, Bonanno GA, Mancini AD, et al. Trajectories of psychological distress among Chinese women diagnosed with breast cancer. Psychooncology. 2010;19(10):1044–1051.
- Kant J, Czisch A, Schott S, et al. Identifying and predicting distinct distress trajectories following a breast cancer diagnosis – from treatment into early survival. J Psychosom Res. 2018;115:6–13.
- Henselmans I, Helgeson VS, Seltman H, et al. Identification and prediction of distress trajectories in the first year after a breast cancer diagnosis. Health Psychol. 2010;29(2):160–168.
- Park J-H, Chun M, Jung Y-S, et al. Predictors of psychological distress trajectories in the first year after a breast cancer diagnosis. Asian Nurs Res. 2017;11(4):268–275.
- Pérez S, Conchado A, Andreu Y, et al. Acute stress trajectories 1 year after a breast cancer diagnosis. Support Care Cancer. 2016;24(4):1671–1678.
- Bidstrup PE, Christensen J, Mertz BG, et al. Trajectories of distress, anxiety, and depression among women with breast cancer: looking beyond the mean. Acta Oncol. 2015;54(5):789–796.
- Mandelblatt JS, Clapp JD, Luta G, et al. Long-term trajectories of self-reported cognitive function in a cohort of older survivors of breast cancer: CALGB 369901 (Alliance). Cancer. 2016;122(22):3555–3563.
- Lam WWT, Li WWY, Bonanno GA, et al. Trajectories of body image and sexuality during the first year following diagnosis of breast cancer and their relationship to 6 years psychosocial outcomes. Breast Cancer Res Treat. 2012;131(3):957–967.
- von Hippel C, Rosenberg SM, Austin SB, et al. Identifying distinct trajectories of change in young breast cancer survivors’ sexual functioning. Psychooncology. 2019;28(5):1033–1040.
- Helgeson VS, Snyder P, Seltman H. Psychological and physical adjustment to breast cancer over 4 years: identifying distinct trajectories of change. Health Psychol. 2004;23(1):3–15.
- Avis NE, Levine B, Goyal N, et al. Health-related quality of life among breast cancer survivors and noncancer controls over 10 years: pink SWAN. Cancer. 2020;126(10):2296–2304.
- Van Onselen C, Cooper BA, Lee K, et al. Identification of distinct subgroups of breast cancer patients based on self-reported changes in sleep disturbance. Support Care Cancer. 2012;20(10):2611–2619.
- Fontes F, Severo M, Gonçalves M, et al. Trajectories of sleep quality during the first three years after breast cancer diagnosis. Sleep Med. 2017;34:193–199.
- Lazarewicz MA, Wlodarczyk D, Lundgren S, et al. Diversity in changes of HRQoL over a 1-year period after radiotherapy in Norwegian breast cancer patients: results of cluster analyses. Qual Life Res. 2019;28(6):1521–1530.
- Donovan KA, Small BJ, Andrykowski MA, et al. Utility of a cognitive-behavioral model to predict fatigue following breast cancer treatment. Health Psychol. 2007;26(4):464–472.
- Bødtcher H, Bidstrup PE, Andersen I, et al. Fatigue trajectories during the first 8 months after breast cancer diagnosis. Qual Life Res. 2015;24(11):2671–2679.
- Kober KM, Smoot B, Paul SM, et al. Polymorphisms in cytokine genes are associated with higher levels of fatigue and lower levels of energy in women after breast cancer surgery. J Pain Symptom Manage. 2016;52(5):695–708.e4.
- Li H, Marsland AL, Conley YP, et al. Genes involved in the HPA axis and the symptom cluster of fatigue, depressive symptoms, and anxiety in women with breast cancer during 18 months of adjuvant therapy. Biol Res Nurs. 2020;22(2):277–286.
- Bower JE, Wiley J, Petersen L, et al. Fatigue after breast cancer treatment: biobehavioral predictors of fatigue trajectories. Health Psychol. 2018;37(11):1025–1034.
- Person H, Guillemin F, Conroy T, et al. Factors of the evolution of fatigue dimensions in patients with breast cancer during the 2 years after surgery. Int J Cancer. 2020;146(7):1827–1835.
- Miaskowski C, Cooper B, Paul SM, et al. Identification of patient subgroups and risk factors for persistent breast pain following breast cancer surgery. J Pain. 2012;13(12):1172–1187.
- Zucca AC, Boyes AW, Linden W, et al. All's well that ends well? Quality of life and physical symptom clusters in long-term cancer survivors across cancer types . J Pain Symptom Manage. 2012;43(4):720–731.
- Sanford SD, Beaumont JL, Butt Z, et al. Prospective longitudinal evaluation of a symptom cluster in breast cancer. J Pain Symptom Manage. 2014;47(4):721–730.
- Fayers P, Aaronson N, Bjordal K. EORTC QLQ-C30 scoring manual. 3rd ed. EORTC quality of life group. Brussels: European Organisation for Research and Treatment of Cancer; 2001.
- Sprangers MA, Groenvold M, Arraras JI, et al. The European organization for research and treatment of cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol. 1996;14(10):2756–2768.
- Basch E, Reeve BB, Mitchell SA, et al. Development of the national cancer institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst. 2014;106(9):dju244.
- Nylund-Gibson K, Choi AY. Ten frequently asked questions about latent class analysis. Transl Issues Psychol Sci. 2018;4(4):440–461.
- Muthén LK, Muthén BO. Mplus user’s guide. 8th ed. Los Angeles (CA): Muthén & Muthén; 1998–2017.
- IBM Corporation. IBM SPSS statistics for windows. Armonk, NY: IBM Corporation; 2017.
- Giesinger JM, Kuijpers W, Young T, et al. Thresholds for clinical importance for four key domains of the EORTC QLQ-C30: physical functioning, emotional functioning, fatigue and pain. Health Qual Life Outcomes. 2016;14:87.
- Nielsen AWM, Kristensen MH, Offersen BV, et al. Patient-reported outcomes in postmenopausal breast cancer survivors – comparisons with normative data. Acta Oncol. 2021;60(1):78–86.
- Musoro JZ, Coens C, Fiteni F, et al. Minimally important differences for interpreting EORTC QLQ-C30 scores in patients with advanced breast cancer. JNCI Cancer Spectr. 2019;3(3):pkz037.
- Cocks K, King MT, Velikova G, et al. Evidence-based guidelines for interpreting change scores for the European Organisation for the research and treatment of cancer quality of life questionnaire core 30. Eur J Cancer. 2012;48(11):1713–1721.
- Nakano J, Hashizume K, Fukushima T, et al. Effects of aerobic and resistance exercises on physical symptoms in cancer patients: a meta-analysis. Integr Cancer Ther. 2018;17(4):1048–1058.
- Juvet LK, Thune I, Elvsaas IKØ, et al. The effect of exercise on fatigue and physical functioning in breast cancer patients during and after treatment and at 6 months follow-up: a meta-analysis. Breast. 2017;33:166–177.
- Kalter J, Verdonck‐de Leeuw IM, Sweegers MG, et al. Effects and moderators of psychosocial interventions on quality of life, and emotional and social function in patients with cancer: an individual patient data meta-analysis of 22 RCTs. Psychooncology. 2018;27(4):1150–1161.
- Scott NW, Fayers PM, Aaronson NK, et al. EORTC QLQ-C30 reference values. Brussels: European Organisation for Research and Treatment of Cancer; 2008.
- Stover AM, Mayer DK, Muss H, et al. Quality of life changes during the pre- to postdiagnosis period and treatment-related recovery time in older women with breast cancer. Cancer. 2014;120(12):1881–1889.
- Karlsen RV, Frederiksen K, Larsen MB, et al. The impact of a breast cancer diagnosis on health-related quality of life. A prospective comparison among Middle-aged to elderly women with and without breast cancer. Acta Oncol. 2016;55(6):720–727.
- Bastien CH, Vallières A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307.
- Price DD, McGrath PA, Rafii A, et al. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17(1):45–56.
