Abstract
Background and purpose
Significant improvements in the treatment of anal cancer have produced a growing population of anal cancer survivors. These patients often experience late adverse effects related to their treatment. Research has revealed substantial unmet needs because of long-term symptoms and functional impairments after treatment that may negatively affect health-related quality of life. The purpose of the present guidelines is to review the scientific evidence for the management of late adverse effects after (chemo)radiotherapy ([C]RT) for anal cancer and to extrapolate knowledge from other pelvic malignancies treated with pelvic (C)RT so that they may guide the clinical management of late adverse effects.
Materials and methods
Relevant studies were systematically searched in four databases from their inception to June 2020 (no language limitation) and guidelines were searched in 16 databases, focussing on bowel dysfunction, psychosocial aspects, pain, and sexual and urinary dysfunction. The guidelines were developed by a panel of experts using the Oxford Centre for Evidence-based Medicine, levels of evidence, and grades of recommendations.
Scientific evidence
Late adverse effects after (C)RT for anal cancer are associated with a low overall quality of life among survivors. The most pronounced late adverse effects are bowel dysfunction (present in up to 78%), urinary dysfunction (present in up to 45%), and sexual dysfunction (present in up to 90% of men and up to 100% of women). Only indirect data on adequate treatment options of these late adverse effects for anal cancer are available.
Conclusion
Quality of life and late adverse effects should be monitored systematically following treatment for anal cancer to identify patients who require further specialist evaluation or support. Increased awareness of the extent of the problem may serve to stimulate and facilitate multidisciplinary collaboration, which is often required.
Background and purpose
Squamous cell carcinoma of the anal canal (anal cancer) is relatively rare, but the incidence has been increasing over the past two decades, whereas age at time of diagnosis has followed a decreasing trend [Citation1,Citation2]. The increasing incidence of anal cancer in men and women may be accounted for by an increase in the prevalence of exposures, such as cigarette smoking, anal intercourse, human papilloma virus (HPV) infection, and growth in the number of lifetime sexual partners [Citation1]. In Denmark, the incidence rate of HPV-associated anal cancers has increased significantly, whereas that of non-HPV-associated histological types has levelled out or even declined in the 30-year period during which observation has been in place indicating that vaccines against HPV may play an important role in the prevention of anal cancer and its precursor lesions [Citation3].
The standard of care for anal cancer is concomitant (chemo)radiotherapy ([C]RT). The purpose of CRT is to cure/control the tumour, preserve sphincter function and uphold the best possible quality of life (QoL). However, surgery and (C)RT have never been compared directly in a randomised study, but in addition to preserving sphincter function, (C)RT has shown to yield better local control and survival in several studies [Citation4]. In case of cancer recurrence, salvage surgery may be offered. CRT for anal cancer involves high-dose radiotherapy delivered to the anal tumour and pathological lymph nodes, and a lower dose delivered to the elective nodal areas. Organs that are often affected by radiotherapy include the small and large bowel, the bladder, the female internal genitalia, male genitalia, the skin, and the bony pelvic structures.
Significant improvements in anal cancer treatment have produced a growing population of anal cancer survivors. However, surviving anal cancer often comes at the price of treatment-related late adverse effects. Research has revealed substantial unmet needs because of long-term symptoms and functional impairments after (C)RT that may negatively affect health-related QoL [Citation5,Citation6].
The purpose of this work was to examine the scientific evidence for management of late adverse effects (>3–6 months after terminated treatment) after (C)RT for anal cancer and to extrapolate knowledge from other pelvic malignancies treated with pelvic (C)RT to guide clinical management of late adverse effects. Symptoms were divided into six categories (psychosocial, bowel, urinary, sexual, pain, and radiation dermatitis) and examined individually. Each of these categories are presented along with a literature review and evidence description, which is followed by recommendations and proposed action strategies.
Target population
These guidelines apply to anal cancer survivors after treatment with (C)RT. Patients who have undergone salvage surgery for anal cancer are not targeted in this guideline. The principles may also be applicable to patients with other pelvic organ cancers treated with CRT presenting with pelvic radiation disease [Citation7].
Target user
This guideline was developed to support clinical decision-making and facilitate quality improvement. Thus, the target users are healthcare professionals engaged in the treatment and follow-up for anal cancer and their affiliated clinical units providing care aiming to limit late adverse effects following anal cancer therapy.
Material and methods
Search strategy
We conducted a systematic search in the electronic databases Pubmed Central, the Cumulative Index of Nursing and Allied Health Literature (CINAHL), and Embase using medical subject headings (MeSH) with the word anal cancer including relevant subheadings and with the following limits: species (human), languages (no limitations). The search included studies from the date of inception to June 2020 (August 2020 for CINAHL). Further, a search in the Cochrane Library was conducted. The terms used for the search were anal cancer as population and radiotherapy as the medical intervention. Late toxicity and/or survivorship was added as comparative intervention. We defined one general category for the description of diagnosing and monitoring of late adverse effects and five organ-specific complaint/symptom categories (bowel, urinary, psychosocial, pain, and sexual) and conducted individual searches within all these categories. All the synonyms and associated sub-terms were combined using the “OR” operator and were combined with each of the five organ-specific complaint/symptom categories by the “AND” operator. One reviewer (SH) independently screened the titles and the abstracts of each reference. Among 242 identified articles, 57 were retained for full-text review and screened by two reviewers to assess their quality and evidence level. Among these articles, 34 were included in the finalised guidelines. During the development of the guidelines, we added a sixth organ-specific complaint – radiation dermatitis, as the magnitude of this complaint type became clearer whilst reviewing the literature.
The guideline content was approved by the disease-specific Multidisciplinary Cancer Group, whereas the format was approved by the Centre for Clinical Practice Guidelines on Cancer in Denmark.
During the development of the guidelines, another 63 articles were included by manual review of the reference lists of the previously included studies. As little data is available on anal cancer specifically, knowledge was extrapolated from other patient categories exposed to pelvic radiotherapy.
Evidence assessment and articulation of the recommendations
A minimum of two authors were assigned to review the literature covering each of the six symptom categories. The authors individually extracted data and graded the quality of evidence and the strength of recommendation using a shared internet-based platform. In case of discrepancies, the results were merged and discussed in plenum before the final description of the recommendation was made. As little evidence exists to support recommendations for anal cancer patients specifically, we chose to grade the level of evidence of relevant literature concerning pelvic radiation disease in general, but we graded all recommendations not based specifically on anal cancer studies as Grade D - no direct research evidence/expert opinion (Oxford Classification). Throughout the manuscript, the evidence level of the literature is stated in square brackets and the grade of recommendation in curved brackets.
Recommendations are based on disease-specific evidence. The recommendations are listed in , the recommendation quick guide. Action strategies are based on either indirect evidence or expert opinions and should be viewed as management option suggestions.
Scientific evidence
Diagnosing and monitoring late adverse effects
Symptom grading
According to the literature, the most frequently used toxicity assessment tools were the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) classification, the Radiation Therapy Oncology Group (RTOG/EORTC) radiation morbidity scoring scheme [Citation8], and the Late Effect on Normal Tissues- Subjective, Objective, Management and Analytic (LENT-SOMA) score [Citation9].
In the CTCAE, toxicity is graded as mild (Grade 1), moderate (Grade 2), severe (Grade 3), life-threatening (Grade 4), or death (Grade 5) with specific parameters according to the anal canal. The timing of adverse events is not defined in the CTCAE. This scale was adapted for patient self-reporting (NCI-PRO-CTCAE) [Citation10].
The LENT-SOMA score is obtained by completing a series of questions during a structured interview, which grade subjective and objective symptoms, and a score assessing the need for medical intervention. Scores are then summed and divided by the total number of questions to provide an overall score of between zero (no symptoms) and five (fatal toxicity).
The RTOG/EORTC morbidity criteria grade severity into four grades by symptoms. Grades are specifically described for most symptoms and roughly translate into: Grade 1 ‘slight bother’, Grade 2 ‘moderate bother’, Grade 3 ‘acute conditions, requiring surgery’, and Grade 4 ‘acute, potentially lethal conditions’. The late morbidity-scoring schemes grades toxicity occurring > 90 days after treatment initiation. None of these systems has been validated specifically for anal cancer patients.
Patient-reported outcomes (PROs)
Asking patients to report their symptoms via PROs has proven highly acceptable to patients in the clinical oncology setting. Reviews suggest that the use of PROs may lead to an improvement of symptom/function monitoring, communication, and clinical decision-making [Citation11]. Furthermore, investigator-led toxicity grading significantly underestimated morbidity compared with patient-administrated reporting by PROs [Citation12]. A prospective study of 100 anal cancer patients used both the CTCAE and PROs to evaluate acute toxicity and found that PROs were markedly higher with only slight to fair agreement with the CTCAE (C30/CR29) [Citation13].
A study including 142 patients (eight with anal cancer) who had completed pelvic radiotherapy a minimum of three months previously examined the use of self-administered questionnaires for late toxicity after pelvic radiation. Using the modified Inflammatory Bowel Disease Questionnaire and the Vaizey Incontinence Questionnaire, the study found highly significant correlations between the degree of gastrointestinal dysfunction recorded by the patients and scores recorded by the LENT-SOMA questionnaire [Citation14].
Using an internet-based platform, another study found that it was feasible for lower gastrointestinal (GI) cancer survivors (119 anal cancer patients) to obtain PROs from an internet-based survivorship tool. Survivors reported a wide spectrum of late and long-term effects, and these were used to inform counselling at the time of diagnosis and help anticipate and respond to disease-related and treatment-related sequelae during follow-up [Citation15].
Most studies have used the EORTC QLQ-C30 questionnaire (cancer-specific QoL) and the colorectal cancer module QLQ-CR38(/29) (site-specific QoL) that consists of 38 (29) items covering symptoms and side-effects related to different treatment modalities, body image, sexuality, and future perspectives. The RTOG/EORTC recently developed the so-called ANL27 module specifically for anal cancer, which is currently finalising international validation. The aim of the ANL27 is to establish common standards for morbidity reporting for both clinical and scientific use (https://qol.eortc.org/questionnaire/qlq-anl27/) [Citation16].
QoL is a multi-dimensional construct shaped by physical health, psychological state, level of independence, social relationships, personal beliefs, and their relationship to important features of their environment [Citation11]. Physical symptoms experienced due to disease and treatment affect QoL judgements [Citation17]. Furthermore, the patient’s response in terms of coping strategies, goals and expectations related to their treatment significantly affect their perception of QoL. Therefore, assumptions about QoL cannot be made from an inspection of toxicity grades; only the patient can provide an accurate QoL estimate [Citation18].
Psychosocial distress
In general, cancer survivors are at an elevated risk of psychosocial distress and mental health concerns; thus, a nationwide matched cohort study from Sweden found that psychosocial distress may persist for as long as ten years after the diagnosis [Citation19]. Furthermore, distress often related to a fear of recurrence is common among cancer survivors and can negatively affect QoL [Citation19]. QoL judgements are affected by the survivor’s physical health. Likewise, the survivors’ coping strategies, goals and expectations relating to treatment may significantly affect their perception of QoL [Citation17].
Generally, the literature on HRQOL in anal cancer survivors is of poor quality; limited by single-centre studies with low sample sizes and cross-sectional designs [Citation11].
Reduced overall or global QoL scores were reported in cross-sectional studies with long-term follow-up [Citation6,Citation20,Citation21]. However, others found that global QoL outcomes were acceptable as they were similar to those of normative data [Citation22–24]. Longitudinal studies found a significant decrease in QoL immediately following treatment, but substantial improvements were reported at one-year follow-up [Citation25–27].
Among anal cancer survivors with decreased long-term QoL, the cause seems to be multifactorial [Citation28]. Consistently, the studies point to bowel dysfunction such as faecal incontinence, faecal urgency, and faecal frequency as detrimental to QoL [Citation20–23]. Sexual dysfunction and urinary incontinence were also associated with a lower overall QoL [Citation20,Citation29].
Survivors report impairment of physical function and role and social function following treatment, but the latter two items improved significantly at one-year follow-up [Citation25,Citation27]. Survivors also frequently report disease-related symptoms such as fatigue, diarrhoea, appetite loss, buttock pain, flatulence, and faecal incontinence/diarrhoea [Citation20,Citation21,Citation23].
Pelvic organ symptoms such as diarrhoea, pain, faecal incontinence, and sexual problems are often perceived as private and embarrassing and may affect self-confidence and impact daily life. The nature and severity of symptoms seem to negatively affect a person’s ability to function and enjoy life, resulting in avoidance or isolation [Citation20]. The private and tabooed nature of pelvic symptoms may restrain survivors from mentioning this when not asked specifically about these issues. Recognition of the symptoms and dysfunctions may contribute to relieve and help survivors to cope with impaired function and pelvic symptoms. Increased awareness and acceptance of the extent of the problems will stimulate and facilitate multidisciplinary collaboration, which is often required.
Recommendations and proposed action strategies for management of psychosocial distress are listed in .
Figure 2. Recommendations and action strategies for management of psychosocial distress. The recommendations are based upon direct research evidence whereas action strategies are based on relevant literature concerning pelvic radiation disease in general. Recommendations marked A are the strongest, whereas recommendations marked D are the weakest according to the “Oxford Centre for Evidence-Based Medicine Levels of Evidence and Grades of Recommendations”. As action strategies are not based direct research evidence these are all marked D, however the quality of the associated literature is listed with evidence level.

Late gastrointestinal adverse effects
The overall incidence of any gastrointestinal (GI) late adverse effect after (C)RT for anal cancer has been reported to range from 7–64.5% [Citation5]. Late adverse effects tend to occur in tissues with a low cell turnover, such as subcutaneous tissue, fatty tissue, and muscle and within tissues that contain rapidly proliferating cells such as the intestinal wall [Citation30]. As anal cancer arises from squamous cells in the anal canal and in the anal margins, this area is the primary target of radiation therapy, and damage to the integrated and delicate function of anal continence is predictable.
Anal cancer survivors have been shown to have lower anal resting, squeeze and yield pressures, and lower resistance to flow than healthy volunteers, whereas rectal volume was found to be unaltered in comparison [Citation31]. Impaired peripheral and cortical processing of sensory anal stimuli in anal cancer patients after (C)RT may also contribute to various degrees of incontinence [Citation32].
Reviewing the literature, we found that the most common symptoms of late GI adverse effects in anal cancer survivors are faecal urgency (14–78%), faecal incontinence (7–60%), tenesmus (13–36%), diarrhoea (45–60%), excessive flatulence (38–55%), pain (13–27%), bloating (13–32%), and rectal bleeding (23–25%) [Citation5,Citation6,Citation20,Citation33–36].
Diarrhoea
The incidence of diarrhoea ranges from 0 to 26.7% among anal cancer survivors [Citation5], and the incidence of severe diarrhoea (Grade 3 CTCAE or a stool frequency > 8/day in the LENT-SOMA) ranges from 0.4 to 4.9% [Citation5]. Although psychological factors may contribute to episodes of loose stools after pelvic radiotherapy, specific physiological problems can commonly be identified, including small-bowel bacterial overgrowth, bile-acid malabsorption, carbohydrate malabsorption, changes in transit, development of small and/or large bowel strictures, neoplasia, or new-onset primary inflammatory bowel disease.
Management
Treatment of diarrhoea should follow gastrointestinal work-up to establish the cause of the diarrhoea. Studies suggest that 8–15% of diarrhoea cases following pelvic radiation are caused by small bowel bacterial overgrowth, though a reliable diagnosis is difficult. Optimal treatment strategies have not been defined. However, antibiotic treatment targeting gram-negative bacilli used for up to two weeks may abolish symptoms [Citation7,Citation37].
A chronic reduction in bile-acid absorption is common after pelvic radiation and may cause diarrhoea [Citation7,Citation38]. The condition is diagnosed by the selenium homocholic acid taurine (SeHCAT) test and responds to bile acid sequestrants. Data suggest that patients with radiation-induced bile-acid malabsorption benefit from regular use of bile-acid sequestrants (colestyramine; 4 g twice a day) [Citation7] or other bile-acid sequestrants. Dietary advice to reduce fat intake often adds to the effect of bile-acid sequestrants. New-onset lactose malabsorption persists after radiotherapy in about 5% of patients and frequently causes diarrhoea that requires qualified dietary advice [Citation7].
Other causes of diarrhoea include large-bowel strictures (3-15% of patients with diarrhoea after pelvic radiotherapy), small-bowel strictures (9%), disease relapse (4-10%), new neoplasia in the gastrointestinal tract (8%), new-onset inflammatory bowel disease (i.e., Crohn’s disease, or ulcerative, lymphocytic, or collagenous colitis; 4%), or radiation proctitis (33%) [Citation7].
If no cause for diarrhoea is found, dietary counselling, bulk-forming laxatives, or antidiarrheal drugs may be of help [Citation5].
Faecal incontinence
The reported rate of faecal incontinence following radiotherapy ranges from 0 to 45% [Citation5,Citation20], most likely overlapping with the incidence of diarrhoea. One study of 84 anal cancer patients specified incontinence and reported that incontinence for solid stools, liquid stools, and gas occurs at least monthly in 31%, 54%, and 79% of patients, respectively. Overall, 40% of patients reported great distress from incontinence for solid or liquid stools at least monthly. Faecal urgency occurring at least monthly was experienced by 87% of patients and caused great distress in 43% [Citation36]. No studies have explored treatment algorithms for faecal incontinence in anal cancer survivors specifically. However, algorithms for treatment of pelvic radiation disease have been investigated [Citation39].
Management
Several treatments exist that may alleviate symptoms. Treatment can be directed towards stool consistency (see above), improving anal sphincter function (pelvic floor muscle training, biofeedback, or plugs), improved rectal emptying (toileting training, enemas, or transanal irrigation), or towards neural co-ordination (sacral nerve modulation). A colostomy to divert the faecal flow has a role in the few patients (between 5 and 12%) who experience substantial loss of rectal volume and who have not responded to other interventions [Citation40].
A few studies have been published on interventions targeting management of patients with faecal incontinence after pelvic radiotherapy. In one retrospective study of 15 patients, the use of phenylephrine gel benefitted three quarters of all patients with faecal incontinence who had not responded to other treatments. A substantial benefit was observed in 25% of the treated patients [Citation41]. In another retrospective study of 13 patients with faecal incontinence after pelvic radiotherapy refractory to other treatment (including four anal cancer patients), seven patients (54%) had successful percutaneous nerve evaluation, and the number of incontinence episodes in the three-week bowel diary was reduced from a median of 24 (range, 4–56) to four (range, 3–6) [Citation42].
Radiation proctitis
Radiation proctitis includes a handful of symptoms including bleeding, pain, faecal urgency, and incontinence. Furthermore, radiation proctitis has a natural overlap with faecal incontinence. The incidence of radiation proctitis in anal cancer survivors ranges from 0 to 40% [Citation5]. Diagnosis is based on clinical history and endoscopic and histologic findings, also ruling out local recurrence. No studies have examined management of proctitis for anal cancer survivors specifically.
Management
The evidence for treatment of haemorrhagic radiation proctitis was summarised in a 2016 Cochrane review; sucralfate enemas are more effective than corticosteroid or mesalazine enemas [Citation43]. Oral metronidazole seems beneficial in patients with both diarrhoea and rectal bleeding, but with no pre-existing cytotoxic neuropathy as it may otherwise aggravate symptoms [Citation44]. Three endoscopic treatment options exist (argon plasma coagulation, laser therapy or applied formalin). However, none of these options have been examined in a randomised setting [Citation44].
Two studies reported on the use of formalin in the treatment of chronic radiation-induced haemorrhagic proctitis. One study (15 patients, including two anal cancer patients) showed that 87% of patients had complete cessation of bleeding [Citation45]. One prospective study (33 patients, including 11 anal cancer patients) also stated that formalin was an effective treatment for chronic radiation-induced haemorrhagic proctitis, but an unsuitable method for anal cancer survivors because of the increased morbidities of anal stricture and faecal incontinence [Citation46].
In a small randomised controlled study (RCT), daily self-administered colonic irrigation plus oral ciprofloxacin and metronidazole were superior to formalin application in terms of bleeding, urgency and diarrhoea [Citation47].
A randomised, double-blinded, sham-controlled, phase 3 trial studied the clinical benefits of hyperbaric oxygen in patients with chronic bowel dysfunction after radiotherapy for pelvic malignancies (84 cases, including eight anal cancer cases) and found no evidence that patients with radiation-induced chronic gastrointestinal symptoms, including patients with rectal bleeding, benefitted from hyperbaric oxygen therapy [Citation48]. However, specifically for radiation proctitis proven refractory to other interventions, a multicentre, randomised, controlled, double-blind trial with crossover and long-term follow-up evaluated the effect of hyperbaric oxygen therapy for these patients (n = 120, no anal cancer patients) found a significantly increased chance of improvement or cure following hyperbaric oxygen treatment (relative risk (RR), 1.72; 95% confidence interval (CI), 1.0–2.9, P = 0.04) [Citation49].
Recommendations and proposed action strategies for management of late GI adverse effects are listed in .
Figure 3. Recommendations and action strategies for management of late GI adverse effects. The recommendations are based upon direct research evidence whereas action strategies are based on relevant literature concerning pelvic radiation disease in general. Recommendations marked A are the strongest, whereas recommendations marked D are the weakest according to the “Oxford Centre for Evidence-Based Medicine Levels of Evidence and Grades of Recommendations”. As action strategies are not based direct research evidence these are all marked D, however the quality of the associated literature is listed with evidence level.

Late urological adverse effects
Urological complications following pelvic radiotherapy include lower urinary tract symptoms (LUTS), radiation cystitis, stricture disease, fistula formation, and the development of secondary urological cancers. Urological late adverse effects are reported in 3–45% of anal cancer survivors after CRT [Citation20,Citation34–36,Citation50,Citation51]. One cross-sectional study examining 84 anal cancer survivors found that 45% of patients experienced urinary incontinence at least once monthly and 48% experienced urinary urgency at least once monthly. Among these patients, 79% reported that urinary incontinence caused moderate or great distress; the same was true for 55% of patients experiencing urinary urgency. Morbidities because of dysuria, daytime urinary frequency and nocturia were, however, minor [Citation36].
Lower urinary tract symptoms
Cytotoxic drugs and radiotherapy to the bladder can lead to cystitis, fibrosis, and occasionally diminished bladder volume. This can cause symptoms of polyuria, dysuria, haematuria, and sphincter dysfunction [Citation52]. LUTS may be divided into irritative (storage), obstructive (voiding), and postmicturition symptoms. Symptoms such as urinary incontinence, frequency, urgency, and nocturia are often the most bothersome effects of LUTS [Citation53]. The symptoms of LUTS may develop from months to years after the treatment for pelvic cancers. Hence, regular assessment of LUTS in cancer survivors is necessary [Citation53].
General assessment of LUTS includes self-reported incontinence, questionnaires, and a three-day voiding dairy with registration of fluid intake, voiding episodes, voided volume, and a pad test. Moreover, dipstick urinalysis for leucocytes and nitrites is needed to rule out infection and haematuria. An additional ultrasound assessment of the bladder may be useful in identifying residual and structural issues [Citation53]. In men, it is important to keep in mind that the prevalence of LUTS increases with age, and new LUTS may be indicative of prostate hyperplasia or cancer. Therefore, any physical examination should include a prostate exam [Citation53]. In women, gynaecological examination is recommended to evaluate for pelvic organ prolapse and vaginal atrophy.
Management
The evidence‐base for conservative management of LUTS after treatment for pelvic cancers is limited and characterised by diversity of patient characteristics. Furthermore, although guidelines exist for treating both men and women with LUTS, these guidelines are not specific to cancer patients but are based on benign disease causality [Citation53].
Conservative management is the first-line treatment for LUTS and includes lifestyle interventions such as moderating fluid intake, avoiding known bladder irritants such as caffeine and alcohol, and smoking cessation. Use of, e.g., pads and collecting devices is an option for patients with fewer symptoms [Citation53,Citation54]. Pelvic floor muscle training (PFMT) with or without biofeedback seems beneficial and may be initiated prior to treatment commencement: two studies have assessed the effects of PFMT and multidisciplinary rehabilitation after pelvic radiotherapy, and both demonstrated significant improvements [Citation55,Citation56]. In postmenopausal women, vaginal oestrogen treatment has shown improvement of overactive bladder symptoms and is recommended as initial treatment [Citation57].
Oral medication is focussed on the use of alpha-blockers and antimuscarinics/mirabegron (beta-3 agonist). The sequencing of the medication should be tailored depending on what is the most bothersome symptom of LUTS identified on assessment. Alpha-blockers may be used to treat LUTS such as compromised bladder emptying. Antimuscarinics/mirabegron may be used to treat urgency and incontinence (overactive bladder) as they relax smooth muscles [Citation53,Citation58].
As third-line treatment for irritative urinary symptoms, intravesical installations are an option. Onabotulinum toxin A may be considered for patients with the ability to empty the bladder and uninhibited bladder contractions [Citation59]. For patients with irritative urinary symptoms in the absence of uninhibited contractions, percutaneous tibial nerve stimulation (PTNS), or sacral nerve stimulation (SNS) may be considered [Citation4].
Simple cystectomy is reserved for the treatment of intractable functional problems when all other management options have failed.
Haemorrhagic cystitis
Haemorrhagic cystitis is defined as a diffuse inflammatory condition of the urinary bladder resulting in bleeding from the bladder mucosa. When it occurs as a late toxicity, it is usually of non-infectious aetiology. The condition may be challenging to treat and is a source of substantial morbidity and occasionally even mortality.
Management
Systemic medical therapies for haemorrhagic cystitis are appealing as they are non-invasive and hospital admission is avoided [Citation60]. WF10 is an intravenous formulation (tetrachlorodecaoxygen) that reduces inflammation so that host-derived healing can commence. In one RCT, patients treated with WF10 had a significantly decreased rate of recurrent haematuria after 12 months [Citation61]. Sodium pentosan (100 mg) administration three times daily may reduce or eliminate symptoms [Citation62]. Tranexamic acid has been used to treat urological haemorrhagic emergencies. However, evidence of its efficacy in haemorrhagic radiation cystitis is lacking [Citation54].
Intravesical instillations of hyaluronic acid are used to upgrade the glycosaminoglycan (GAG) protective layer to reduce exposure of underlying epithelial cells to host urine. It has been safely administered with success for the treatment of chemical and radiation cystitis, producing improvements in urinary symptoms and reducing bladder pain [Citation53,Citation63]. In a pilot study, 30 symptomatic prostate cancer patients treated with (C)RT received bladder instillation therapy with hyaluronic acid and chondroitin sulphate weekly for the first month and then at weeks 6, 8, and 12 (one year in total), which significantly reduced overall symptoms and bother [Citation64].
Hyperbaric oxygen is used to treat severe haematuria refractory to conventional management with response rates ranging from 27 to 96% [Citation63]. RCTs with long-term follow-up are lacking and needed, as treatments are expensive and time-consuming.
Ablation and coagulation of ruptured submucosal vasculature with laser therapy, argon beam therapy or simple fulguration with a coagulation electrode are advantageous as these modalities can immediately control haemorrhage and are associated with a complete response in 75–97.5% of cases. The disadvantages associated with these modalities are the need for general or spinal anaesthesia. Small series seems promising [Citation54,Citation63,Citation65].
Urinary diversion with or without cystectomy may be performed if all other less invasive treatment modalities have failed [Citation63].
Stricture disease and management
Strictures may occur in the ureters or in the urethra. If unrecognised, partial, or total permanent loss of kidney function may ensue. Surgery (urethroplasty, dilation, urinary diversion, or reconstruction) remains the only definitive long-term option for managing these strictures [Citation54].
Fistula formation and management
Management of a fistula in the urinary tract or bladder is drainage. Good urinary drainage will keep a low pressure within the system and avoid urinary leakage through the fistula. In some cases, fistula surgery is an option [Citation54].
Recommendations and proposed action strategies for management of late urological adverse effects are listed in .
Figure 4. Recommendations and action strategies for management of late urological adverse effects. The recommendations are based upon direct research evidence whereas action strategies are based on relevant literature concerning pelvic radiation disease in general. Recommendations marked A are the strongest, whereas recommendations marked D are the weakest according to the “Oxford Centre for Evidence-Based Medicine Levels of Evidence and Grades of Recommendations”. As action strategies are not based direct research evidence these are all marked D, however the quality of the associated literature is listed with evidence level.

Sexual dysfunction as a late adverse effect of anal cancer
The prevalence of sexual difficulties associated with anal cancer and its treatment is sparsely investigated and much less so in male than in female survivors. The extant literature is mainly retrospective and characterised by small sample sizes [Citation66,Citation67].
Among female anal cancer survivors treated with (C)RT, vaginal stenosis has been reported in up to 79% [Citation68], vaginal dryness in up to 85% and dyspareunia in up to 100% [Citation6]. Maximum symptom prevalence for female-related sexual dysfunction was experienced between two and five years from diagnosis [Citation15].
In male anal cancer survivors, up to 90% have complaints of erectile dysfunction (difficulties getting and maintaining an erection), but also orgasmic dysfunction and pain [Citation6]. Even so, the sexual dysfunction of male anal cancer survivors has received very little attention.
In one study, female cancer patients indicated that sexual matters were never discussed with their healthcare providers, and 81% stated that it was extremely important to discuss the issue [Citation66]. For sexually active women, sexual dysfunction, most notably sexual/relationship satisfaction, was most consistently associated with specific measures of psychological well-being [Citation29]. Sexual well-being is acknowledged as a core aspect of QoL for people who are affected by cancer, particularly those receiving treatment for pelvic malignancies. Body image, anxiety, and cancer‐specific post-traumatic distress have been associated with subscales of sexual functioning, whereas a global QoL measure was largely unrelated [Citation29].
Management
No specific data exist on the treatment of sexual dysfunction in anal cancer survivors. Recommendations have been extrapolated from the existing literature on primarily pelvic malignancies.
Dysfunction of sexual organs
Both the testes and ovaries can be exposed to direct and/or scattered radiation. Systematic reviews of men with both rectal and prostate cancer treated with radiotherapy have found an increased risk of developing testicular dysfunction with decreased serum testosterone levels compared with both pre-treatment values and men treated with surgery alone. Testosterone levels < 8 nmol/L may precipitate specific symptoms caused by testosterone deficiency such as impaired physical, psychological, and sexual function after treatment [Citation69,Citation70].
In women, premature iatrogenic menopause secondary to chemo- and/or radiotherapy may cause infertility, mood disorders (depression, loss of self-esteem, and relational difficulties), disorders secondary to the oestrogenic loss (hot flashes, insomnia, memory difficulties, vaginal dryness, joint pain, or osteopenia/osteoporosis) and disorders secondary to the androgenic loss (loss of sexual interest, orgasmic difficulties, fatigue, or loss of assertiveness) [Citation71].
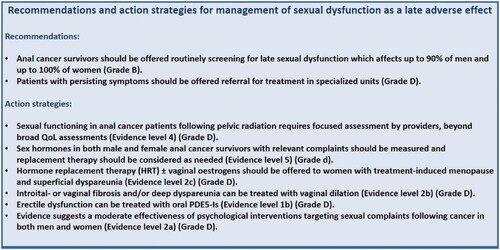
Sex hormones in both male and female anal cancer survivors with relevant complaints should be measured and replacement therapy considered as needed (5).
Psychosexual aspects
Whereas sexual dysfunction may result from physiological treatment effects, desire, orgasmic pleasure, and sexual satisfaction are also strongly related to psychological function (e.g., sexual performance anxiety). A systematic review identified 27 studies that compiled together showed moderate support for the effectiveness and feasibility of psychological interventions targeting sexual complaints following cancer in both men and women. However, a strong placebo response was observed [Citation72]. Approaches may be psychosexual therapy (sensate focus), psychological therapy (mindfulness, cognitive behavioural therapy), couple therapy (discrepant desire), alone or in combination with pharmacological or device-based (e.g., vibrators, constriction rings) interventions and targeting of affected individuals or couples [Citation72].
Sexual pain in women
Sexual pain difficulties (dyspareunia) in women are predominantly associated with radiation-induced vaginal dryness and vaginal stenosis. The most effective management for superficial dyspareunia in women with treatment-induced menopause is the offer of hormone replacement therapy (HRT) and, where appropriate, vaginal oestrogens [Citation73]. If contraindicated, then non-hormonal vaginal moisturisers can be used. Furthermore, most women will also need to use an intimate lubricant (water, oil, or silicone based) to decrease friction associated with penetrative sexual intercourse or vulvar contact (5).
For women with introital or vaginal fibrosis and/or deep dyspareunia following radiotherapy, vaginal dilation is recommended [Citation73,Citation74]. A systematic Cochrane review by Denton et al. [Citation74] from 2015 found that evidence is sufficient to endorse widespread recommendation of the use of vaginal dilators. However to disrupt the cycle that may arise from the repeated experience of sexual pain, couples may be asked to refrain from penetrative sexual activity while vaginal health strategies are introduced, with subsequent gradual introduction of sexual expression using a graduated exposure to vaginal dilation and penetration within a framework of sensate focus [Citation72].
Arousal
For men, penile erection is important objective feedback that reinforces subjective sexual feelings of arousal, whereas, for many women, awareness of the objective vaginal changes that accompany subjective sexual arousal are limited. Most evidence behind the management of treatment-induced erectile difficulties following pelvic radiotherapy stems from studies of prostate cancer survivors [Citation73,Citation75]. The efficacy of oral PDE5-Is has been established in RTCs of external beam radiotherapy for prostate cancer with significant improvement in assisted erectile function compared with placebo [Citation76]. Options for second-line therapies for erectile dysfunction that do not respond to PDE5-Is include vacuum erectile devices, intra-cavernosal injections and transurethral alprostadil. However, evidence of treatment following radiation-induced erectile difficulties is lacking [Citation68].
Recommendations and proposed action strategies for management of sexual dysfunction late are listed in .
Figure 5. Recommendations and action strategies for management of sexual dysfunction. The recommendations are based upon direct research evidence whereas action strategies are based on relevant literature concerning pelvic radiation disease in general. Recommendations marked A are the strongest, whereas recommendations marked D are the weakest according to the “Oxford Centre for Evidence-Based Medicine Levels of Evidence and Grades of Recommendations”. As action strategies are not based direct research evidence these are all marked D, however the quality of the associated literature is listed with evidence level.
Pain as a late adverse effect of anal cancer
Prolonged pelvic pain is defined as a pain that has lasted more than six months after (C)RT. It may have its origin in any pelvic organs and arise after cancer treatment. Prolonged pain may, via various mechanisms in the nervous system, lead to altered function and various symptoms/discomfort in the skin, bladder, muscles, intestines, and gynaecological organs [Citation77].
Treatment must be based on diagnostic work up to determine the mechanism of the pain and rule out recurrent disease. The analysis is performed in close consultation with the patient and followed by a treatment plan. Analgesics often have only limited effect, and especially opioids entail a risk of increased intestinal problems such as constipation and difficulty emptying, which may cause increased pain in the long term (5).
Evidence-based pain rehabilitation programmes, available through referral, focus on learning how to manage and live with pain as a long-term condition. Often several different mechanisms cause pain concurrently, none of which were described adequately in the literature. This guideline emphasises pelvic insufficiency fractures as this is a well-described late adverse effect following pelvic (C)RT.
Pelvic insufficiency fractures (PIFs)
PIF can be misinterpreted clinically as local recurrence (or vice versa) causing pain and decreased mobility [Citation78,Citation79]. PIFs are described in 1.4–14% of anal cancer patients following (C)RT [Citation80–82] but are best documented after radiation for gynaecological cancers [Citation83].
Studies on PIFs following (C)RT are mainly retrospective and characterised by heterogeneity with respect to definition, timing, imaging methods, RT techniques, and follow up. The imaging method is important as magnetic image resonance (MRI) is estimated to have a sensitivity of 98–100% and a specificity of 85% for stress fractures in general, and MRI was found to be superior to CT (sensitivity, 69%) in the pelvic/femoral area [Citation84,Citation85].
In anal cancer survivors, studies on PIFs are often smaller case series, and no systematic reviews or meta-analyses exist. Generally, fracture sites predominantly occur in weight-bearing areas and are related to higher radiation doses; moreover, their incidence rises with increasing age and postmenopausal status. Time to detection of PIF was 11 months after (C)RT (3–66 months) [Citation81,Citation86–92].
Two recent large studies (a systematic review and a meta-analysis) on PIF after RT for gynaecological cancers (n = 3929 and n = 6488) found incidences of PIF of 9.4% and 14%, detected a median of 8–39 and 7.1–19 months after RT [Citation83,Citation93]. The most frequently established risk factors across studies were advanced age, postmenopausal status, low body mass index, osteoporosis, older RT treatment techniques, and higher RT doses [Citation83]. The most frequent localisation was sacral body/near the sacroiliacal joint (60-73.6%) followed by the pubic bones (12-13%). The ratio of symptomatic patients differs but generally falls in the 50-60% range [Citation83]. These data seem comparable to data from anal cancer, but data are not directly applicable as radiation dose, techniques, and chemotherapy are different.
Management
Studies on the treatment and preventive measures for PIFs are lacking. In the 2020 systematic review on gynaecological patients, information on the treatment of PIFs was available for 456 patients. Conservative treatment was applied in 84.6% of cases (analgesics, bed rest, or observation), hospitalisation or surgery in 9.4% and bone-directed therapies were used in 6% (bisphosphonates, calcium, vitamin D, and hormone replacement therapy) [Citation83].
A Cochrane review on pharmacological interventions for prevention of PIF associated with pelvic RT has been conducted [Citation94]. Two RCTs were included, both in men undergoing pelvic RT and hormone replacement therapy for prostate cancer. The review concluded that there is insufficient evidence that zoledronic acid and other medicines are sufficient to prevent radiation-induced bone complications.
Hyperbaric oxygen treatment is used in some cases of osteoradionecrosis, but not in pelvic insufficiency fractures [Citation95].
The ESMO 2020 guidelines on Bone Health in cancer mainly focus on treatment-induced bone loss from systemic treatment and do not specifically address CRT-induced PIFs. However, the guidelines do state that, "All patients receiving treatments that are known to adversely affect bone health should be advised to consume a calcium enriched diet (or supplement), exercise moderately and take 1000–2000 IU vitamin D3 every day”.
Recommendations and proposed action strategies for management of pain as a late adverse effect are listed in .
Figure 6. Recommendations and action strategies for management of pain. The recommendations are based upon direct research evidence whereas action strategies are based on relevant literature concerning pelvic radiation disease in general. Recommendations marked A are the strongest, whereas recommendations marked D are the weakest according to the “Oxford Centre for Evidence-Based Medicine Levels of Evidence and Grades of Recommendations”. As action strategies are not based direct research evidence these are all marked D, however the quality of the associated literature is listed with evidence level.

Radiation dermatitis
Chronic radiation dermatitis is a late side effect of skin irradiation, which is mostly caused by the imbalance of proinflammatory and profibrotic cytokines [Citation96]. The incidence of chronic radiation dermatitis in anal cancer survivors remains unknown.
Clinical manifestations include changes in skin appearance, wounds, ulcerations, necrosis, fibrosis, and secondary cancers. The most severe complication of irradiation is extensive radiation-induced fibrosis (RIF). RIF can manifest in various ways including skin induration and retraction, lymphoedema, and restricted joint motion. The diagnosis of chronic radiation dermatitis is usually made by clinical examination [Citation96].
If the clinical presentation is unclear or suspicious, a biopsy and a histopathological examination are mandatory [Citation96].
The available literature on the management of chronic radiation dermatitis is inadequate. Most of the interventions are based exclusively on clinical practice and extrapolation of management used in similar conditions.
Recommendations and proposed action strategies for management radiation dermatitis are listed in .
Figure 7. Recommendations and action strategies for management of radiation dermatitis. The recommendations are based upon direct research evidence whereas action strategies are based on relevant literature concerning pelvic radiation disease in general. Recommendations marked A are the strongest, whereas recommendations marked D are the weakest according to the “Oxford Centre for Evidence-Based Medicine Levels of Evidence and Grades of Recommendations”. As action strategies are not based direct research evidence these are all marked D, however the quality of the associated literature is listed with evidence level.

Limitations
The chosen complaint/symptom categories were identified based on the available literature and the experience of the participating expert panel. Other late adverse effects of cancer treatment that are less specific to anal cancer survivors are not covered here.
Very little literature exists that focuses specifically on anal cancer survivors. Furthermore, most studies are limited by sample size (single centre) and design (cross-sectional, small retrospective cohort studies).
Conclusion
QoL and late adverse effects should be monitored systematically following treatment for anal cancer to identify patients who require further specialist evaluation or support. An increased awareness and acceptance of the extent of the problem may stimulate and facilitate multidisciplinary collaboration, which is often required to manage these problems and to generate much needed evidence-based treatment algorithms. Each cancer centre treating anal cancer patients should have a clear referral strategy for late adverse effects either in the form of a dedicated multidisciplinary late sequelae clinic or by using pre-established individual specialists at the local or regional level. Furthermore, an urgent need exists for a standardised assessment tool and for collaboration between centres to generate quality evidence within this field.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Daling JR, Madeleine MM, Johnson LG, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 2004;101(2):270–280.
- Chiao EY, Krown SE, Stier EA, et al. A population-based analysis of temporal trends in the incidence of squamous anal canal cancer in relation to the HIV epidemic. J Acquir Immune Defic Syndr. 2005;40(4):451–455.
- Nielsen A, Munk C, Kjaer SK. Trends in incidence of anal cancer and high-grade anal intraepithelial neoplasia in Denmark, 1978-2008 . Int J Cancer. 2012; 130(5):1168–1173.
- Glynne-Jones R, Saleem W, Harrison M, et al. Background and current treatment of squamous cell carcinoma of the anus. Oncol Ther. 2016;4(2):135–172.
- Pan Y, bin Maeda Y, Wilson A, et al. Late gastrointestinal toxicity after radiotherapy for anal cancer: a systematic literature review. Acta Oncol. 2018;57(11):1427–1437.
- Knowles G, Haigh R, McLean C, et al. Late effects and quality of life after chemo-radiation for the treatment of anal cancer. Eur J Oncol Nurs. 2015;19(5):479–485.
- Andreyev J. Gastrointestinal symptoms after pelvic radiotherapy: a new understanding to improve management of symptomatic patients. Lancet Oncol. 2007; 8(11):1007–1017.
- Cox JD, Stetz JA, Pajak TF. Toxicity criteria of the radiation therapy oncology group (RTOG) and the european organization for research and treatment of cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341–1346.
- Mornex F, Pavy JJ, Denekamp J, et al. Scoring system of late effects of radiations on normal tissues: the SOMA-LENT scale. Cancer Radiothe: J Soc Fr Radiother Oncol. 1997;1;1(6):622–668.
- Dueck AC, Mendoza TR, Mitchell SA, et al. Validity and reliability of the us national cancer institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). JAMA Oncol. 2015;1(8):1051–1059.
- Sodergren SC, Vassiliou V, Dennis K, et al. Systematic review of the quality of life issues associated with anal cancer and its treatment with radiochemotherapy. Support Care Cancer. 2015;23(12):3613–3623.
- Gilbert A, Francischetto EO, Blazeby J, et al. Choice of a patient-reported outcome measure for patients with anal cancer for use in cancer clinical trials and routine clinical practice: a mixed methods approach. Lancet. 2015;385 (Suppl 1):S38.
- Kronborg C, Serup-Hansen E, Lefevre A, et al. Prospective evaluation of acute toxicity and patient reported outcomes in anal cancer and plan optimization. Radiother Oncol. 2018;128(2):375–379.
- Olopade FA, Norman A, Blake P, et al. A modified inflammatory bowel disease questionnaire and the vaizey incontinence questionnaire are simple ways to identify patients with significant gastrointestinal symptoms after pelvic radiotherapy. Br J Cancer. 2005;92(9):1663–1670.
- Frick MA, Vachani CC, Hampshire MK, et al. Survivorship after lower gastrointestinal cancer: Patient-reported outcomes and planning for care. Cancer. 2017;123(10):1860–1868.
- Sodergren SC, Johnson CD, Gilbert A, et al. Phase I-III development of the EORTC QLQ-ANL27, a health-related quality of life questionnaire for anal cancer. Radiother Oncol. 2018;126(2):222–228. 1
- Schwartz CE, Rapkin BD. Integrating response shift into health-related quality of life research: a theoretical model. Health Qual Life Outcomes. 2004;2(11): 15–16.
- Fayers P, Aaronson NK, Bjordal K. EORTC QLQ–C30 scoring manual. Brussels: European Organisation for Research and Treatment of Cancer; 1995.
- Lu D, Andersson TM, Fall K, et al. Clinical diagnosis of mental disorders immediately before and after cancer diagnosis: a nationwide matched cohort study in Sweden. JAMA Oncol. 2016;2(9):1188–1196.
- Bentzen AG, Balteskard L, Wanderås EH, et al. Impaired health-related quality of life after chemoradiotherapy for anal cancer: late effects in a national cohort of 128 survivors. Acta Oncologica. 2013;52(4):736–744.
- Jephcott CR, Paltiel C, Hay J. Quality of life after non-surgical treatment of anal carcinoma: a case control study of long-term survivors. Clin Oncol (R Coll Radiol). 2004;16(8):530–535.
- Allal AS, Sprangers MAG, Laurencet F, et al. Assessment of long-term quality of life in patients with anal carcinomas treated by radiotherapy with or without chemotherapy. Br J Cancer. 1999;80(10):1588–1594.
- Welzel G, Hägele V, Wenz F, et al. Quality of life outcomes in patients with anal cancer after combined radiochemotherapy. Strahlenther Onkol. 2011;187(3):175–182.
- Vordermark D, Sailer M, Flentje M, et al. Curative-intent radiation therapy in anal carcinoma: Quality of life and sphincter function. Radiother Oncol. 1999;52(3):239–243.
- Han K, Cummings BJ, Lindsay P, et al. Prospective evaluation of acute toxicity and quality of life after IMRT and concurrent chemotherapy for anal canal and perianal cancer. Int J Radiat Oncol Biol Phys. 2014;90(3):587–594.
- Tournier-Rangeard L, Mercier M, Peiffert D, et al. Radiochemotherapy of locally advanced anal canal carcinoma: prospective assessment of early impact on the quality of life (randomized trial ACCORD 03). Radiotherapy and Oncology. 2008; 87(3):391–397.
- Joseph K, Vos LJ, Warkentin H, et al. Patient reported quality of life after helical IMRT based concurrent chemoradiation of locally advanced anal cancer. Radiother Oncol. 2016;120(2):228–233.
- Sterner A, Derwinger K, Staff C, et al. Quality of life in patients treated for anal carcinoma-a systematic literature review. Int J Colorectal Dis. 2019;34(9):1517–1528.
- Philip EJ, Nelson C, Temple L, et al. Psychological correlates of sexual dysfunction in female rectal and anal cancer survivors: analysis of baseline intervention data. Journal of Sexual Medicine. 2013;10(10):2539–2548.
- Barnett GC, West CML, Dunning AM, et al. Normal tissue reactions to radiotherapy: towards tailoring treatment dose by genotype. Nat Rev Cancer. 2009;9(2):134–142.
- Haas S, Faaborg P, Liao D, et al. Anal sphincter dysfunction in patients treated with primary radiotherapy for anal cancer: a study with the functional lumen imaging probe. Acta Oncol. 2018;57(4):465–472.
- Haas S, Faaborg P, Gram M, et al. Abnormal neuronal response to rectal and anal stimuli in patients treated with primary radiotherapy for anal cancer. Radiotherapy and Oncology. 2018;128(2):369–374.
- Das P, Cantor SB, Parker CL, et al. Long-term quality of life after radiotherapy for the treatment of anal cancer. Cancer. 2010;116(4):822–829.
- Ghareeb A, Paramasevon K, Mokool P, et al. Toxicity and survival of anal cancer patients treated with intensity-modulated radiation therapy. Ann R Coll Surg Engl. 2019;101(3):168–175.
- Dell'Acqua V, Surgo A, Arculeo S, et al. Intensity-modulated radiotherapy (IMRT) in the treatment of squamous cell anal canal cancer: acute and early-late toxicity, outcome, and efficacy. Int J Colorectal Dis. 2020;35(4):685–694.
- Sunesen KG, Nørgaard M, Lundby L, et al. Long-term anorectal, urinary and sexual dysfunction causing distress after radiotherapy for anal cancer: a Danish multicentre cross-sectional questionnaire study. Colorectal Dis. 2015;17(11):230–239.
- Jordan J, Gage H, Benton B, et al. Gastroenterologist and nurse management of symptoms after pelvic radiotherapy for cancer: an economic evaluation of a clinical randomized controlled trial (the ORBIT study). Clinicoecon Outcomes Res. 2017;9:241–249.
- Stryker JA, Hepner GW, Mortel R. The effect of pelvic irradiation on ileal function. Radiology. 1977;124(1):213–216.
- Benton B, Norton C, Lindsay JO, et al. Can nurses manage gastrointestinal symptoms arising from pelvic radiation disease? Clin Oncol (R Coll Radiol)). 2011;23(8):538–551.
- Sunesen KG, Nørgaard M, Lundby L, et al. Cause-specific colostomy rates after radiotherapy for anal cancer: a danish multicentre cohort study. J Clin Oncol. 2011;29(26):3535–3540.
- Badvie S, Andreyev HJN. Topical phenylephrine in the treatment of radiation-induced faecal incontinence. Clin Oncol (R Coll Radiol). 2005;17(2):122–126.
- Maeda Y, Høyer M, Lundby L, et al. Temporary sacral nerve stimulation for faecal incontinence following pelvic radiotherapy. Radiother Oncol. 2010;97(1):108–112.
- Kochhar R, Patel F, Sharma SC, et al. Radiation-induced proctosigmoiditis. Prospective, randomized, double-blind controlled trial of oral sulfasalazine plus rectal steroids versus rectal sucralfate. Dig Dis Sci. 1991;36(1):103–107.
- van de Wetering FT, Verleye L, Andreyev HJN, et al. Non-surgical interventions for late rectal problems (proctopathy) of radiotherapy in people who have received radiotherapy to the pelvis. Cochrane Database Syst Rev. 2016;4:CD003455.
- Pironi D, Panarese A, Vendettuoli M, et al. Chronic radiation-induced proctitis: the 4 % formalin application as non-surgical treatment. Int J Colorectal Dis. 2013;28(2):261–266.
- de Parades V, Etienney I, Bauer P, et al. Formalin application in the treatment of chronic radiation-induced hemorrhagic proctitis-an effective but not risk-free procedure: a prospective study of 33 patients. Dis Colon Rectum. 2005;48(8):1535–1541.
- Sahakitrungruang C, Patiwongpaisarn A, Kanjanasilp P, et al. A randomized controlled trial comparing colonic irrigation and oral antibiotics administration versus 4% formalin application for treatment of hemorrhagic radiation proctitis. Dis Colon Rectum. 2012;55(10):1053–1058.
- Glover M, Smerdon GR, Andreyev HJ, et al. Hyperbaric oxygen for patients with chronic bowel dysfunction after pelvic radiotherapy (HOT2): a randomised, double-blind, sham-controlled phase 3 trial. Lancet Oncol. 2016;17(2):224–233.
- Clarke RE, Tenorio LMC, Hussey JR, et al. Hyperbaric oxygen treatment of chronic refractory radiation proctitis: a randomized and controlled double-blind crossover trial with long-term follow-up. Int J Radiat Oncol Biol Phys. 2008;72(1):134–143.
- Di Santo S, Trignani M, Neri M, et al. Radiochemotherapy in anal cancer: cCR, clinical outcomes and quality of life using two different treatment schedules. Rep Pract Oncol Radiother. 2015;20(2):128–134.
- Abusaris H, Hoogeman M, Nuyttens JJ. Re-irradiation: outcome, cumulative dose and toxicity in patients retreated with stereotactic radiotherapy in the abdominal or pelvic region. Technol Cancer Res Treat. 2012;11(6):591–597.
- Payne H, Adamson A, Bahl A, et al. Chemical- and radiation-induced haemorrhagic cystitis: current treatments and challenges. BJU Int. 2013;112(7):885–897.
- Faithfull S, Lemanska A, Aslet P, et al. Integrative review on the non-invasive management of lower urinary tract symptoms in men following treatments for pelvic malignancies . Int J Clin Pract. 2015;69(10):1184–1208.
- Lobo N, Kulkarni M, Hughes S, et al. Urologic complications following pelvic radiotherapy. Urology. 2018;122:1–9.
- Faithfull S, Cockle‐Hearne J, Khoo V. Self‐management after prostate cancer treatment: evaluating the feasibility of providing a cognitive and behavioural programme for lower urinary tract symptoms. BJU International. 2011;107(5):783–790.
- Dieperink KB, Johansen C, Hansen S, et al. The effects of multidisciplinary rehabilitation: RePCa-a randomised study among primary prostate cancer patients. Br J Cancer. 2013;109(12):3005–3013.
- Selskab D, Obstetrik og Gynaekologi. Lokal vaginal østrogenbehandling til postmenopausale kvinder med vulvovaginalvaginal atrofi [Obstetrik og Gynaekologi. Lokal vaginal østrogenbehandling til postmenopausale kvinder med vulvovaginalvaginal atrofi]. Guideline. 2019. Available from: https://static1.squarespace.com/static/5467abcce4b056d72594db79/t/5e0491857abf7e54d64796c2/1577357730542/Guideline+051219.pdf.
- Thüroff JW, Abrams P, Andersson K-E, et al. EAU guidelines on urinary incontinence. Actas Urológicas Españolas (English Edition). 2011;35(7):373–388.
- Chuang YC, Yoshimura N, Huang CC, et al. Intravesical botulinum toxin a administration inhibits COX-2 and EP4 expression and suppresses bladder hyperactivity in cyclophosphamide-induced cystitis in rats. Eur Urol. 2009;56(1):159–167.
- Ribeiro de Oliveira TM, Carmelo Romão AJ, Gamito Guerreiro FM, et al. Hyperbaric oxygen therapy for refractory radiation-induced hemorrhagic cystitis. Int J Urol. 2015;22(10):962–966.
- Veerasarn V, Khorprasert C, Lorvidhaya V, et al. Reduced recurrence of late hemorrhagic radiation cystitis by WF10 therapy in cervical cancer patients: a multicenter, randomized, two-arm, open-label trial. Radiotherapy and Oncology. 2004;73(2):179–185.
- Sandhu SS, Goldstraw M, Woodhouse CRJ. The management of haemorrhagic cystitis with sodium pentosan polysulphate. BJU Int. 2004;94(6):845–847.
- Pascoe C, Duncan C, Lamb BW, et al. Current management of radiation cystitis: a review and practical guide to clinical management. BJU Int. 2019;123(4):585–594.
- Gacci M, Saleh O, Giannessi C, et al. Bladder instillation therapy with hyaluronic acid and chondroitin sulfate improves symptoms of postradiation cystitis: Prospective pilot study. Clinical Genitourinary Cancer. 2016;14(5):444–449.
- Ravi R. Endoscopic neodymium: YAG laser treatment of radiation‐induced hemorrhagic cystitis. Lasers Surg Med. 1994;14(1):83–87.
- Canty J, Stabile C, Milli L, et al. Sexual function in women with colorectal/anal cancer. Sex Med Rev. 2019;7(2):202–222.
- Arthur EK, Wills CE, Menon U. A systematic review of interventions for sexual well-being in women with gynecologic, anal, or rectal cancer. Oncol Nurs Forum. 2018;45(4):469–482.
- Savoie MB, Laffan A, Brickman C, et al. A multi-disciplinary model of survivorship care following definitive chemoradiation for anal cancer. BMC Cancer. 2019;19(1): 903–906.
- Farhood B, Mortezaee K, Haghi-Aminjan H, et al. A systematic review of radiation-induced testicular toxicities following radiotherapy for prostate cancer. J Cell Physiol. 2019;10: 14828–14837.
- Buchli C, Martling A, Arver S, et al. Testicular function after radiotherapy for rectal cancer–a review. J Sex Med. 2011;8(11):3220–3226.
- Graziottin A, Serafini A. Medical treatments for sexual problems in women. In: Mulhall J, Incrocci L, Goldstein I, Rosen R, editors. Cancer and sexual health. Current clinical urology. New York, NY: Humana Press (Springer Science+Business Media, LLC); 2011.
- Brotto LA, Yule M, Breckon E. Psychological interventions for the sexual sequelae of cancer: a review of the literature. J Cancer Surviv. 2010;4(4):346–360.
- White ID. Sexual difficulties after pelvic radiotherapy: improving clinical management. Clin Oncol (R Coll Radiol). 2015;27(11):647–655.
- Denton AS, Maher J. Interventions for the physical aspects of sexual dysfunction in women following pelvic radiotherapy. Cochrane Database Syst Rev. 2003;(1):CD003750.
- White ID, Wilson J, Aslet P, et al. Development of UK guidance on the management of erectile dysfunction resulting from radical radiotherapy and androgen deprivation therapy for prostate cancer. Int J Clin Pract. 2015;69(1):106–123.
- Watkins Bruner D, James JL, Bryan CJ, et al. Randomized, double-blinded, placebo-controlled crossover trial of treating erectile dysfunction with sildenafil after radiotherapy and short-term androgen deprivation therapy: results of RTOG 0215. J Sex Med. 2011;8(4):1228–1238.
- ACOG Committee on Practice Bulletins–Gynecology. ACOG practice bulletin no. 51. Chronic pelvic pain. Obstet Gynecol. 2004;103(3):589–605.
- Oh D, Huh SJ. Insufficiency fracture after radiation therapy. Radiat Oncol J. 2014;32(4):213–220.
- Higham CE, Faithfull S. Bone health and pelvic radiotherapy. Clin Oncol (R Coll Radiol)). 2015;27(11):668–678.
- Tomaszewski JM, Link E, Leong T, et al. Twenty-five-year experience with radical chemoradiation for anal cancer. Int J Radiat Oncol Biol Phys. 2012;83(2):552–558.
- Baxter NN, Habermann EB, Tepper JE, et al. Risk of pelvic fractures in older women following pelvic irradiation. J Am Med Assoc. 2005;294(20):2587–2593.
- Vitzthum LK, Park H, Zakeri K, et al. Risk of pelvic fracture with radiation therapy in older patients. Int J Radiat Oncol Biol Phys. 2020;106(3):485–492.
- Razavian N, Laucis A, Sun Y, et al. Radiation-induced insufficiency fractures after pelvic irradiation for gynecologic malignancies: a systematic review. Int J Rad Oncol Biol Phys. 2020;108(3):620–634.
- Cabarrus MC, Ambekar A, Lu Y, et al. MRI and CT of insufficiency fractures of the pelvis and the proximal femur. AJR Am J Roentgenol. 2008;191(4):995–1001.
- Matcuk GR, Mahanty SR, Skalski MR, et al. Stress fractures: pathophysiology, clinical presentation, imaging features, and treatment options. Emerg Radiol. 2016;23(4):365–375.
- Bazire L, Xu H, Foy J-P, et al. Pelvic insufficiency fracture (PIF) incidence in patients treated with intensity-modulated radiation therapy (IMRT) for gynaecological or anal cancer: single-institution experience and review of the literature. Brit J Radiol. 2017;90(1073);20160885.
- Okoukoni C, Randolph DM, McTyre ER, et al. Early dose-dependent cortical thinning of the femoral neck in anal cancer patients treated with pelvic radiation therapy. Bone. 2017;94:84–89.
- Chan S, Rowbottom L, McDonald R, et al. Pelvic insufficiency fractures in women following radiation treatment: a case series. Ann Palliat Med. 2016;5(3):233–237.
- Epps HR, Brinker MR, O'Connor DP. Bilateral femoral neck fractures after pelvic irradiation. Am J Orthop (Belle Mead NJ). 2004;33(9):457–460.
- Serkies K, Bednaruk-Mlynski E, Dziadziuszko R, et al. Conservative treatment for carcinoma of the anus-a report of 35 patients. Neoplasma. 2003;50(2):152–158.
- de Francesco I, Thomas K, Wedlake L, et al. Intensity-modulated radiotherapy and anal cancer: Clinical outcome and late toxicity assessment. Clin Oncol (R Coll Radiol). 2016;28(9):604–610.
- Myerson RJ, Outlaw ED, Chang A, et al. Radiotherapy for epidermoid carcinoma of the anus: thirty years' experience. Int J Radiat Oncol Biol Phys. 2009;75(2):428–435.
- Sapienza LG, Salcedo MP, Ning MS, et al. Pelvic insufficiency fractures after external beam radiation therapy for gynecologic cancers: a Meta-analysis and Meta-regression of 3929 patients. Int J Radiat Oncol Biol Phys. 2020;106(3):475–484.
- van den Blink QU, Garcez K, Henson CC, et al. Pharmacological interventions for the prevention of insufficiency fractures and avascular necrosis associated with pelvic radiotherapy in adults. Cochrane Database Syst Rev. 2018;4:CD010604.
- Mathieu D, Marroni A, Kot J. Tenth european consensus conference on hyperbaric medicine: recommendations for accepted and non-accepted clinical indications and practice of hyperbaric oxygen treatment. Diving Hyperb Med. 2017;47(1):24–32.
- Spałek M. Chronic radiation-induced dermatitis: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:473–482.

