Abstract
Background
This study aimed to evaluate the association of body composition with toxicity to first-line chemotherapy and three-year survival in women with ovarian adenocarcinoma.
Methods
We enrolled, in a retrospective cohort, 239 women treated with carboplatin and paclitaxel between 2008 and 2017. Pretreatment computed tomography scans were used to quantify skeletal muscle index (SMI), skeletal muscle radiodensity (SMD), and subcutaneous adipose tissue index (SATI). Chemotherapy doses, related toxicities, potential drug-drug interactions (DDI), and clinical variables were collected from medical records. Outcomes were the number of adverse events ≥ grade 3 toxicity, toxicity-induced modification of treatment (TIMT), and three-year survival.
Results
Average age was 56.3 years and 35.1% had myopenia. Almost 33% had TIMT and 51.3% presented any grade 3 toxicity. Potential severe DDI occurred in 48.1% of the patients and 65.1% died three years after the first treatment. The SMD and SATI below the median were independent predictors for the number of adverse events ≥ grade 3 and TIMT. Also, SMD was the only body composition parameter able to predict reduced three-year survival. The SMI was not associated with any of the outcomes.
Conclusion
Fewer amounts of SATI and low SMD were associated with the occurrence of toxicity to chemotherapy, and the low SMD increased the risk of death in the three years after oncologic treatment.
Introduction
Ovarian cancer is the eighth most common type of cancer worldwide and the seventh most incident in Brazil among women [Citation1,Citation2]. It represents approximately 2.3% of all cancer deaths, with a high lethality rate, since four out of five patients are diagnosed with advanced disease [Citation3]. The combination of carboplatin and paclitaxel administered every 3 weeks is the standard chemotherapeutic regimen for the treatment of epithelial ovarian cancer [Citation4]. The most common adverse events of this protocol include neurotoxicity and hematopoiesis suppression, triggering anemia, thrombocytopenia, and leucopenia [Citation5,Citation6].
Patients with advanced age, comorbidities, poor performance status, and changes in nutritional status are known to be more likely to have adverse events during chemotherapy [Citation7,Citation8]. Toxicity to chemotherapy may lead to modifications in the planned regimen, including delay, dose reduction, or permanent discontinuation of treatment [Citation9] and its determinants must be identified as early as possible.
It has been well described in the literature that changes in body composition, such as reduced skeletal muscle (SM) and fat mass increase the risk of toxicity and may reduce response to the treatment [Citation10,Citation11]. Other studies suggest that myosteatosis, i.e., an increase of intra- and intermuscular fat, that can be measured radiologically by muscle radiodensity, is also associated with a lower tolerance to antineoplastic agents [Citation12–14].
Methodological differences are observed in studies of body composition and cancer chemotherapy toxicity, such as the concomitant analysis of different chemotherapy regimens [Citation15] and the inclusion of patients with different types of cancer, leading to heterogeneous results [Citation16]. There are few studies on toxicity for chemotherapy that assess the presence of comorbidities, the concomitant use of supportive drugs (palliative, analgesic, and antiemetic therapies that help control adverse events), or those used for comorbidities control [Citation17–19], which may increase the number of drug interactions [Citation20].
Finally, studies that associate body composition with the toxic effects of chemotherapy, especially in ovarian cancer patients, are still scarce, and, so far, only one study has been found that considers the carboplatin and paclitaxel protocol [Citation19]. Besides, most existing studies evaluated relapsed cancer patients and/or used body mass index as a parameter of analysis [Citation21,Citation22], which is unable to differentiate the SM content and the fat mass content [Citation23]. Our hypothesis is that body composition parameters when adjusted for confounding variables, may explain severe chemotherapy toxicity events and short-term survival in patients with adenocarcinoma ovarian cancer.
This study aims to evaluate the association between body composition parameters and the outcomes toxicity to carboplatin and paclitaxel chemotherapy protocol and three-year survival in women with ovarian adenocarcinoma who have undergone cancer treatment at a referral center in Brazil.
Material and methods
Study design
In this retrospective cohort, we enrolled patients with histopathological confirmation of adenocarcinoma-type epithelial ovarian cancer who received carboplatin and paclitaxel as the first-line chemotherapy and had computed tomography (CT) images at the level of the third lumbar vertebra (L3) available up to 90 days before or 15 days after the first cycle of chemotherapy. The study was conducted at a Gynecological Cancer Reference Institution in Rio de Janeiro – RJ, Brazil, between January 1, 2008, and December 31, 2017.
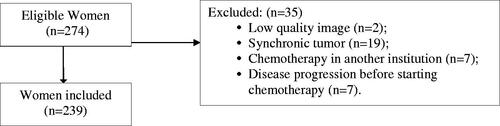
Exclusion criteria were women with a synchronous tumor, those who underwent chemotherapy at another institution, with low-quality CT images, and those who had disease progression before starting chemotherapy. The patient selection flowchart for the study is described in .
Data collection
Information regarding age, race, histological subtype, tumor grade, tumor staging, comorbidities, and performance status were collected from medical charts. The International Federation of Gynecology and Obstetrics (FIGO) criteria for gynecological cancers was used for staging, considering the current version when the cancer diagnosis occurred [Citation24–26]. Nonmalignant comorbidities were classified according to the Charlson Comorbidity Index (CCI) [Citation27]. Performance status was classified according to the Eastern Cooperative Oncology Group [Citation28].
Chemotherapy treatment data included the number of cycles, potential severe drug-drug interaction (DDI), treatment delay (at least 7-days delay, associated with a medical report in the medical chart with the reason for the delay due to toxicity), dose reduction (when temporary or permanent administration of a lower carboplatin dose than the target area under the curve (AUC) at the beginning of treatment or a 20% reduction in paclitaxel from the starting dose), monotherapy (discontinuation of any given drug, carboplatin or paclitaxel) and permanent treatment discontinuation due to toxicity.
Chemotherapy toxicity
The standard chemotherapy scheme adopted by the Institution's Clinical Oncology Service for ovarian cancer is based on intravenous infusions on the first day (D1) of pre-chemotherapy drugs (dexamethasone: 20 mg; ondansetron: 8 mg; diphenhydramine: 50 mg; and ranitidine: 50 mg) and intravenous infusion of carboplatin, AUC 5–6, and paclitaxel at 175 mg/m2 also at D1, repeatedly every 21 days for six cycles [Citation29]. AUC was calculated using Calvert Equation [Carboplatin Dose (mg) = Target area under the curve (mg/mL/min) × (Glomerular filtration rate (GFR) + 25)] [Citation30]. Variations in the number of cycles, administration interval, carboplatin, and paclitaxel dose occurred due to the need to adjust to the clinical condition of the patients.
Chemotherapy toxicity data were recorded for each cycle and up to 30 days after the last cycle. Severe toxicity caused by chemotherapy is traditionally evaluated during the first cycle and defined as dose-limiting toxicity in phase one studies [Citation31]. However, studies assessing body composition in cancer patients, in general, defined as dose-limiting toxicity the presence of severe adverse events leading to temporary or permanent chemotherapy dose reduction, permanent discontinuation, treatment delay, treatment-related hospitalization, or death, which might not be the most appropriate term [Citation32–35].
Given the controversies about the use of this term in observational studies, toxicity-induced modification of treatment (TIMT) has been used to refer to any toxicity that resulted in delayed treatment, dose reduction, discontinuation of any given chemotherapy and/or permanent discontinuation due to toxicity, when related to the effects of chemotherapy toxicity, as recommended by Kok et al. [Citation36].
Laboratory tests were collected in an electronic medical record, within the period from 30 days before the first cycle to 30 days after each cycle, for the evaluation of the following toxicities: hematologic (hemoglobin, leukocytes, platelets, and neutrophils), hepatic, and renal. Glomerular filtration rate was calculated using the simplified CKD-EPI formula, according to a specific race, gender, and serum creatinine in mg/dL. GFR was considered low when < 60 ml/min/1.73 m2, as recommended by Kidney Disease: Improving Global Outcomes (KDIGO) [Citation37].
Chemotherapy-related toxicity was assessed by gathering the grade ranging from 0 (absence of event) up to 5 (in cases of death) reported on medical charts for each adverse event throughout the first-line treatment, according to the Common Toxicity Criteria for adverse events of the National Cancer Institute (CTCAE/NCI). The grade of the hematological, hepatic, and renal toxicities was classified by the researchers, using the CTCAE/NCI version 5.0 [Citation38]. For all adverse events, grade ≥ 3 was defined as severe toxicity. Alopecia was not considered as severe toxicity, since it is not a life-threatening condition.
Platinum sensitivity was classified as refractory (relapse during or within 4 weeks following platinum-based chemotherapy), resistant (relapse under 6 months from last platinum therapy as platinum-resistant), partially platinum-sensitive (relapse between 6 and 12 months), or platinum-sensitive (relapse after more than 12 months) [Citation39].
Survival
Overall survival was defined as the time from the first treatment (surgery or chemotherapy) to death by any cause. Patients who were alive after a three-year follow-up date were censored.
Pharmacotherapy and evaluation of drug interaction
We assessed all recipes prescribed before each chemotherapy cycle. All drugs used for comorbidities treatment or symptoms management, that was prescribed or dispensed in the outpatient pharmacy section during chemotherapy treatment were considered for DDI evaluation. The supportive medication and regular medications used for comorbidities control were classified according to the Anatomical Therapeutic Chemical system, considering the main anatomical/pharmacological groups or 1st level and pharmacological or therapeutic groups or 2nd level [Citation40]. Potentially nephrotoxic drugs were identified using the UptoDate® database [Citation41] (Supplementary Table 2).
Finally, the database Drugs.com Statistics [Citation42] was used to identify if any medication interacted pharmacologically. Data sources included IBM Watson Micromedex and Cerner Multum™. All drugs with severe interaction (highly clinically significant) with carboplatin and paclitaxel chemotherapeutic drugs and/or with the supportive drugs used in the protocol (ondansetron, dexamethasone, diphenhydramine, and ranitidine) were collected (). Patients who received at least one potentially severe interactive drug were considered as exposed to drug interaction.
Figure 2. Chemotherapy protocol drugs with potential severe drug-drug interaction with regular and support medications waived at Institution during the treatment of patients. SOURCE: Drugs.com Statistics (2020) [Citation42].
![Figure 2. Chemotherapy protocol drugs with potential severe drug-drug interaction with regular and support medications waived at Institution during the treatment of patients. SOURCE: Drugs.com Statistics (2020) [Citation42].](/cms/asset/73d67552-1190-4bb3-8f84-f7ce91e7319f/ionc_a_1983210_f0002_b.jpg)
Body composition assessment by computed tomography
For the body composition evaluation, an image at the L3 level was selected for each patient. A single trained researcher (K.A.B.), who was blinded to outcome assessment, selected the L3 and analyzed the SM and subcutaneous adipose tissue areas of the cross-sectional images according to tissue-specific Hounsfield Units Mitsiopoulos et al. [Citation43], with the aid of Slice O Matic software version 5.0 (Tomovision, Canada). All images were subsequently reviewed by an experienced researcher (G.V.C). Approximately 10% of those images were also analyzed by the reviewer and the inter-observer variance of SM was below 1.0% (inter-observer intraclass correlation coefficient, ICC of 0.98; 95% CI= 0.97–0.99).
The skeletal muscle area included the psoas, paraspinal, lumbar square, transverse abdominal, internal and external oblique, and rectus abdominis muscles. Skeletal muscle index (SMI, cm2/m2), after normalized by squared height, and was used to classify myopenia, according to the cutoff point established for women: SMI ≤38.9 cm2/m2 [Citation44]. Skeletal muscle radiodensity (SMD) was assessed as the mean HU of the skeletal muscle area [Citation45,Citation46].
The subcutaneous adipose tissue index (SATI, cm2/m2), normalized by the squared height, was the only adipose tissue compartment included in the analysis. Because the pelvic masses are in most cases large, the visceral adipose compartment and, thus, the total fat mass, could not be quantified. Given the lack of cutoff point established in the literature for the other body composition parameters, SMD (HU) and SATI (cm2/m2) were presented according to their median distribution (50th percentile) for comparison between groups (21.24 HU and 61.76 cm2/m2, respectively).
Data analysis
The collected data were analyzed using the statistical program, Statistical Package for Social Sciences, version 25.0, SPSS (Chicago-USA). The mixed-effects Poisson Regression model was analyzed using R, version 04.2 (package lme4).
Kolmogorov-Smirnov test was used to determine whether the continuous variables deviate from a normal distribution. Associations between categorical variables were analyzed by Chi-square test (χ2) or Fisher's exact test.
Multivariable Mixed-effects Poisson Regression models were performed to identify possible factors contributing to the outcome number of adverse events ≥ grade 3 with the chemotherapy cycles as the clustering variable. The clinical variables that made up the models were selected according to their significance in the univariable analysis, when p < .20: performance status (PS) ≥ 2 (vs. <2), first cycle monotherapy (vs. no), potential severe DDI (vs. no), SMD below the median – HU (vs. above the median) and SATI below the median – cm2/m2 (vs. above the median). Those considered as clinically relevant or as exposure variables of interest, such as age (continuous), CCI 0 point (vs. ≥1 point), first cycle dose reduction (vs. no), and SMI <38.9 cm2/m2 (vs. ≥38.9 cm2/m2) were also included in the model.
Multivariable logistic regression models were performed for the outcome TIMT, considering the following variables selected according to their significance in the univariable analysis, when p < .20, except for SMI <38.9 cm2/m2 (vs. ≥38.9 cm2/m2) and age (each year increase), which presented p > .4 but were included as exposure variables of interest: PS ≥ 2 (vs. <2), CCI 0 point (vs. ≥1 point), potential severe DDI (vs. no), SMD below the median – HU (vs. above the median) and SATI below the median – cm2/m2 (vs. above the median). The number of cycles was not considered as an independent variable for the TIMT outcome since patients who discontinued treatment due to toxicity performed fewer cycles and it could generate a reverse causality bias.
Multivariable Cox regression models assessed the association of the independent variables with the outcome three-year survival, considering the following variables selected according to their significance in the univariable analysis, when p < .20: age (each year increase), PS ≥ 2 (vs. <2), chemotherapy sensitivity (platinum sensitivity and partially sensitive vs. platinum-resistant and platinum-refractory), tumor staging (continuous), SMI <38.9 cm2/m2 (vs. ≥38.9 cm2/m2), SMD below the median – HU (vs. above the median) and SATI below the median – cm2/m2 (vs. above the median). For all analyzes, a significance level of 5% was adopted.
Ethical aspects
The project was approved by the Research Ethics Committee of the Institution under number 466.070/2013.
Results
The study included 239 women with ovarian adenocarcinoma, mostly aged less than 65 years (75.7%), with an average age of 56.3 (± 11.4) years. Roughly 87% of the patients had advanced disease (stage III-IV) before starting chemotherapy treatment and the prevalence of myopenia was 35.1%. The other clinical-pathological characteristics are described in . Nearly 14% and 16% of patients have already started chemotherapy with dose adjustment and monotherapy, respectively, while 10.5% of the women experienced dose reduction of carboplatin and/or paclitaxel and only 4.6% changed the protocol to monotherapy during treatment.
Table 1. Clinical, pathological, and treatment-related characteristics of women with ovarian adenocarcinoma (n = 239).
Treatment was delayed or permanently discontinued due to toxicity in approximately 22% and 5% of patients, respectively. Also, 51.3% of the patients had at least one adverse event ≥ grade 3, disregarding the alopecia, 32.6% had TIMT and 61.5% died within three years after starting cancer treatment, surgery or chemotherapy, with a median survival time of 23.3 months ().
The chemotherapy-related adverse events with the highest incidence were nausea (65.7%), anemia (81.7%), leukopenia (60.5%), neutropenia (55.5%), and hepatic toxicity (52.2%) when we considered all toxicity grades, except alopecia. The least frequent adverse events were hypoacusis (1.7%), renal toxicity (5.4%), dysgeusia (6.7%), and hyporexia (6.7%). Considering only events with toxicity ≥3, except alopecia, neutropenia occurred in 35.9%, anemia 13.8%, leucopenia 10.1%, hepatic toxicity 4,2%, and nausea 3.0% (Supplementary Table 1). Potentially nephrotoxic drugs were not associated with grade 3 toxicity events or renal toxicity (data not shown).
In the adjusted models for the outcome number of adverse events ≥ grade 3 (), SMD below the median was an independent predictor (RR 1.67, CI 1.25–2.25) whereas SMI <38.9 cm2/m2 did not show significant influence. In both models, SATI below the median increased the risk of the outcome by 42% and 53% (models 1 and 2, respectively). Besides, a potential severe DDI was associated with at least a 50% greater risk of experiencing adverse events ≥ grade 3 in both models.
Table 2. Regression models for adverse events ≥ grade 3 and toxicity-induced modification of treatment.
The presence of potential severe DDI was also associated with a higher risk of TIMT, and SMD below the median (OR 2.25, CI 1.17–4.31), but not SMI <38.9 cm2/m2 was able to predict the risk of TIMT. In both models, SATI below the median increased the risk of the outcome ().
shows the association of the independent variables with three-year overall survival. PS, resistance to chemotherapy, and tumor staging were significantly related to worse survival. The only body composition parameter that independently predicted this outcome was SMD below the median (HR 2.66, CI 1.68–4.20).
Table 3. Multivariable Cox regression models for three-year overall survival.
Discussion
The goal of chemotherapy dose determination is to strike a balance between optimal efficacy and severe toxicity, which may lead to delays or dose reductions of the administered drug. In this sense, researchers have been interested in developing new strategies that consider body composition in determining chemotherapy dose [Citation11].
However, before considering an individual's body composition in chemotherapy planning, observational studies describing the association between body composition and toxicity outcomes need to be refined. Our study took a step further in this research area, considering as confounding variables the potential presence of drug interaction, performance status, and comorbidities, as well as enrolling a relatively homogeneous population, regarding the tumor site, histology, and systemic treatment received.
A total of 13.8% and 15.9% of the patients have already started chemotherapy with dose reduction and monotherapy, respectively, similar to the described previously for solid tumors treated with chemotherapy (15%), including gynecological cancer [Citation47]. Also, 13.8% required dose adjustments during treatment. The literature reports dose reductions between 8% and 53% in the carboplatin and paclitaxel protocol for gynecological cancer patients [Citation6,Citation9,Citation48–50].
Only for four patients, there was no record of adverse events during treatment. Regardless of the toxicity grade, nausea occurred in 65.7%, being the most incident symptom, higher than the 57.3% reported by Lhommé et al. (2008) for 377 ovarian cancer patients [Citation51].
Except for the incidence of nausea, hematologic toxicities were the most frequent, especially anemia, leukopenia, and neutropenia. Considering only the incidence of hematological toxicities ≥ grade 3, the incidence found for neutropenia (35.9%) and anemia (13.8%) were much higher than the reported by Du Bois et al. (2003) for ovarian cancer patients treated with the same protocol, 17.4% and 1.4%, respectively [Citation52].
The prevalence of myopenia in our study was 35.1%. In the literature, this prevalence varies from 11% to 50.4% among ovarian cancer patients [Citation53–56], depending on the cutoff point used for the SMI classification. Despite the need for a greater consensus on the most appropriate cutoff point, myopenia has been associated with increased chemotherapy toxicity in patients with different types of solid tumors [Citation57–59].
On the other hand, in our study, myopenia was not associated with the occurrence of the number of adverse events ≥ grade 3, TIMT, and three-year survival, even after adjustments for confounding variables. To date, only two studies evaluated myopenia as a predictor of toxicity to carboplatin and paclitaxel chemotherapy in women with ovarian cancer. The median area of the psoas muscle was reported as independently associated with peripheral neuropathy in one of them [Citation19]. However, the isolated evaluation of the psoas is not recommended, since it is not a representative measure of total skeletal muscle [Citation60]. Another recent study, although using a slightly different cutoff, did not find any differences in the frequency of dose reduction, dose delay, changes in chemotherapy regimen, or toxicity among ovarian cancer patients stratified by the presence of myopenia. Furthermore, myopenia was not associated with worse survival [Citation61].
Myopenia does not necessarily indicate reduced muscle quality, as skeletal muscle mass may have high or low radiodensity [Citation62,Citation63]. In contrast, SMD, generally interpreted as myosteatosis, is moderately correlated with biopsy-proven triglyceride content in healthy and diabetic individuals [Citation64], as well as in cancer [Citation65]. Low muscle radiodensity has been associated with systemic inflammation and worse functional status [Citation66], higher risk of chemotherapy toxicity [Citation15,Citation67,Citation68], and shorter survival [Citation69].
In the present study, SMD below the median was an independent predictor of the number of adverse events ≥ grade 3, TIMT, and three-year survival. Dijksterhuis et al. [Citation68] evaluated 88 patients with advanced esophagogastric cancer treated with standard first-line palliative chemotherapy and identified that pretreatment SMD was independently associated with grade 3–4 toxicity (OR 0.94, 95% CI 0.89–1.00). Conversely, da Rocha et al. [Citation70] did not identify low SMD as a predictive factor for reduced drug dose, delay, or definitively discontinue the protocol. Patients with low SMD may have a lower metabolically active skeletal muscle and, therefore, a higher risk of toxicity since hydrophilic drugs are mainly metabolized in this tissue. However, the mechanisms by which SMD relates to these outcomes remain unknown.
Regarding survival outcome, a meta-analysis of six studies found an unfavorable association among low SMD and 3 (OR 3.0, CI 2.0–4.5) and 5-year survival (OR 2.3, CI 1.6–3.4) in 1226 women with epithelial ovarian cancer [Citation71].
Adipose tissue compartment, represented in this study by SATI, was also associated with the number of adverse events ≥ grade 3 and TIMT, but not with three-year survival. Only one previous study evaluated adiposity in patients with relapsed ovarian cancer treated with trabectedin and pegylated liposomal doxorubicin in a randomized, multicenter, open, phase III study context. Authors observed that, among overweight and obese patients, those who discontinued chemotherapy or reduced the dose due to the presence of adverse events ≥ grade 3, had a significantly lower average fat mass than those who did not (24.0 Kg vs. 27.3 Kg; p = .03) [Citation22]. Hydrophobic drugs, such as paclitaxel, are distributed in adipose tissue and may increase toxicity to this drug in patients with reduced fat mass as there is a smaller volume of distribution to adipose tissue, resulting in higher plasma levels of systemic drugs [Citation72].
The presence of potential severe DDI independently predicted the risk of grade ≥ 3 toxicity events, TIMT, and three-year mortality. Cancer patients are particularly at higher risk of drug interactions because they generally take many drugs during treatment, including supportive care medications and drugs to treat comorbidities, beyond cytotoxic and molecularly targeted agents [Citation73], requiring dose reduction and/or discontinuation of chemotherapy. DDI may interfere with response to treatment, by decreasing response or increasing toxicity of a regimen [Citation74].
Patients with platinum-refractory and the platinum-resistant disease typically have low response rates to subsequent chemotherapy (20%), a median progression-free survival of 3–4 months, and median overall survival in the range of 12–15 months [Citation75]. In our study, patients who were refractory or resistant to platinum had an increased risk of death within three years after starting treatment. Worse PS and tumor staging were also associated with three-year survival, as expected.
Finally, the presence of any comorbidity was associated with TIMT. These results highlight that considering these clinical characteristics in the adjusted models when assessing body composition parameters as predictors of chemotherapy outcomes is of great importance.
The limitations of the study are mostly related to its retrospective nature, which prevented the inclusion of other important variables for toxicity studies, such as the systemic inflammation profile and albumin [Citation76]. The analysis of the medications was also made through outpatient dispensation records, and it cannot be ensured that the patients adequately consumed the prescribed medication. Toxicity events may be underreported, which may partly explain the most frequent observation of hematological events, as recording this information is less susceptible to error, as the results of the laboratory tests and are available electronically in the institution.
Only patients who had pretreatment CT images could be included, which greatly restricted the study sample size compared to the population of patients with similar characteristics seen at the institution during the study period. Also, the high prevalence of patients with PS ≥2 who have undergone chemotherapy is probably one of the reasons for the high rates of TIMT and mortality found. Moreover, due to the occurrence of large pelvic masses, it was not possible to appraise visceral adipose tissue and, consequently, the calculation of body fat mass, which is may also be associated with ovarian cancer chemotherapy toxicity [Citation22]. Altogether, these limitations should be considered when interpreting our results, as they could reduce the external validity of the study.
In conclusion, subcutaneous adipose tissue and SMD below the median were associated with a greater occurrence of adverse events ≥ grade 3 and TIMT, and only SMD was an independent predictor of three-year survival. Further clinical studies with a prospective design and in a larger set of patients are needed to clarify the mechanisms underlying the association with myosteatosis and adipose tissue to chemotherapy-related toxicities. To date, there is no evidence supporting that body composition should be considered to define drug dosing or as a guide for therapy decisions. Until studies testing the impact of such intervention on therapeutic efficacy, including survival, are available, early identification of body composition disorders will ensure specialized intervention based on nutritional counseling, nutritional supplements, and physical exercise before treatment.
Author contributions
The authors’ responsibilities were as follows: GVC: conceptualization, methodology, formal analysis, resources, writing – original draft and supervision. KAB: formal analysis, investigation, data curation, writing – original draft and project administration. MJSS: investigation and writing – review & editing. All authors: read and approved the final version submitted.
Ethics approval
The project was approved by the Ethics Committee [removed for blind peer view].
Supplemental Material
Download MS Word (31 KB)Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- World Health Organization – WHO. International Agency for Research on Cancer. GLOBOCAN 2020: Cancer Today – Estimated number of new cases in 2020, worldwide, female, all ages; 2020. [cited 2021 Jan 1]. Available from: https://gco.iarc.fr/today/
- Brazil. Ministry of Health. National Cancer Institute José Alencar Gomes da Silva (INCA). Estimates 2020: Cancer Incidence in Brazil, Rio de Janeiro, 122. p. 2019. [cited 2021 Jan 1]. Available from: https://www.inca.gov.br/
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2017, National Cancer Institute. Bethesda, MD. 2020. [cited 2021 Jan 1]. Available from: https://seer.cancer.gov/csr/1975_2017//
- Armstrong DK, Alvarez RD, Bakkum-Gamez JN, et al. NCCN guidelines insights: ovarian cancer, version 1.2019. J Natl Compr Cancer Netw. 2019;17(8):896–909.
- Katsumata N, Yasuda M, Takahashi F, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374(9698):1331–1338.
- Takaya H, Nakai H, Murakami K, et al. Efficacy of weekly administration of paclitaxel and carboplatin for advanced ovarian cancer patients with poor performance status. Int J Clin Oncol. 2018;23(4):698–706.
- Chen RC, Royce TJ, Extermann M, et al. Impact of age and comorbidity on treatment and outcomes in elderly cancer patients. Semin Radiat Oncol. 2012;22(4):265–271.
- Gérard S, Bréchemier D, Lefort A, et al. Body composition and anti-neoplastic treatment in adult and older subjects – a systematic review. J Nutr Health Aging. 2016;20(8):878–888.
- Liutkauskiene S, Janciauskiene R, Jureniene K, et al. Retrospective analysis of the impact of platinum dose reduction and chemotherapy delays on the outcomes of stage III ovarian cancer patients. BMC Cancer. 2015;15:105.
- Baracos VE, Arribas L. Sarcopenic obesity: hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann Oncol. 2018;29(suppl_2):ii1–ii9.
- Purcell SA, Elliott SA, Kroenke CH, et al. Impact of body weight and body composition on ovarian cancer prognosis. Curr Oncol Rep. 2016;18(2):8.
- Daly LE, Power DG, O'Reilly Á, et al. The impact of body composition parameters on ipilimumab toxicity and survival in patients with metastatic melanoma. Br J Cancer. 2017;116(3):310–317.
- Kroenke CH, Prado CM, Meyerhardt J, et al. Muscle radiodensity and mortality in patients with colorectal cancer. Cancer. 2018;124(14):3008–3015.
- Shachar SS, Deal AM, Weinberg M, et al. Body composition as a predictor of toxicity in patients receiving anthracycline and taxane-based chemotherapy for earlystage breast cancer. Clin Cancer Res. 2017;23(14):3537–3543.
- Shachar SS, Deal AM, Weinberg M, et al. Skeletal muscle measures as predictors of toxicity, hospitalization, and survival in patients with metastatic breast cancer receiving Taxane-Based chemotherapy. Clin Cancer Res. 2017;23(3):658–665.
- Cousin S, Hollebecque A, Koscielny S, et al. Low skeletal muscle is associated with toxicity in patients included in phase I trials. Invest New Drugs. 2014;32(2):382–387.
- Sjøblom B, Grønberg BH, Benth JŠ, et al. Low muscle mass is associated with chemotherapy-induced haematological toxicity in advanced non-small cell lung cancer. Lung Cancer. 2015;90(1):85–91.
- Srdic D, Plestina S, Sverko-Peternac A, et al. Cancer cachexia, sarcopenia and biochemical markers in patients with advanced non-small cell lung cancer-chemotherapy toxicity and prognostic value. Support Care Cancer. 2016;24(11):4495–4502.
- Yoshikawa T, Takano M, Miyamoto M, et al. Psoas muscle volume as a predictor of peripheral neurotoxicity induced by primary chemotherapy in ovarian cancers. Cancer Chemother Pharmacol. 2017;80(3):555–561.
- Bertholee D, Maring JG, Van Kuilenburg ABP. Genotypes affecting the pharmacokinetics of anticancer drugs. Clin Pharmacokinet. 2017;56(4):317–337.
- Grabowski JP, Richter R, Rittmeister H, et al. Impact of body mass index (BMI) on chemotherapy-associated toxicity in ovarian cancer patients. A pooled analysis of the North-Eastern german society of gynecological oncology (NOGGO) Databank on 1,213 patients. Anticancer Res. 2018;38(10):5853–5858.
- Prado CM, Baracos VE, Xiao J, et al. The association between body composition and toxicities from the combination of doxil and trabectedin in patients with advanced relapsed ovarian cancer. Appl Physiol Nutr Metab. 2014;39(6):693–698.
- Laky B, Janda M, Cleghorn G, et al. Comparison of different nutritional assessments and body-composition measurements in detecting malnutrition among gynecologic cancer patients. Am J Clin Nutr. 2008;87(6):1678–1685.
- Benedet JL, Bender H, 3rd JH, et al. FIGO committee on gynecologic oncology. FIGO staging classifications and clinical practice gudelines in the management of gynecologic cancers. Int J Gynecol Obstet. 2000;70:209–262.
- Heintz APM, Odicino F, Maisonneuve P, et al. Carcinoma of the ovary. FIGO 26th annual report on the results of treatment in gynecological cancer. Int J Gynecol Obstet. 2006;95:S161–S92.
- Prat J. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynecol& Obstetr. 2014;124(1):1–5.
- Charlson ME, Pompei P, Ales K, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Oken M, Creech R, Tormey D, et al. Toxicity and response criteria of the Eastern cooperative oncology group. Am J Clin Oncol. 1982;5(6):649–655.
- Brazil Ministry of Health. Clinical oncology service: Internal routines of INCA. Brazilian institute of cancer. Rio De Janeiro, RJ. 2011;241–245.
- Calvert AH, Newell DR, Gumbrell LA, et al. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol. 1989;7(11):1748–1756.
- Postel-Vinay S Redefining dose-limiting toxicity. Clin Adv Hematol Oncol. 2015;13:87–89.
- Anandavadivelan P, Brismar TB, Nilsson M, et al. Sarcopenic obesity: a probable risk factor for dose limiting toxicity during neo-adjuvant chemotherapy in oesophageal cancer patients. Clin Nutr. 2016;35(3):724–730.
- Huillard O, Mir O, Peyromaure M, et al. Sarcopenia and body mass index predict sunitinib-induced early dose-limiting toxicities in renal cancer patients. Br J Cancer. 2013;108(5):1034–1041.
- Kazemi-Bajestani SMR, Mazurak VC, Baracos V. Computed tomography-defined muscle and fat wasting are associated with cancer clinical outcomes. Semin Cell Dev Biol. 2016;54:2–10.
- Tan BHL, Brammer K, Randhawa N, et al. Sarcopenia is associated with toxicity in patients undergoing neo-adjuvant chemotherapy for oesophago-gastric cancer. Eur J Surg Oncol. 2015;41(3):333–338.
- Kok DE, Winkels RM, van Herpen CM, et al. Toxicity-induced modification of treatment: what is in a name? Eur. J.Cancer. 2018;104:145–150.
- Levin A, Stevens PE, Bilous RW, et al. Kidney disease: improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150.
- National Cancer Institute – NIH. U.S. department of health and human services. Common Terminology Criteria for Adverse Events (CTCAE) – Version 5.0; 2017.
- Thigpen JT, Blessing JA, Ball H, et al. Phase II trial of paclitaxel in patients with progressive ovarian carcinoma after platinum-based chemotherapy: a gynecologic oncology group study. J Clin Oncol. 1994;12(9):1748–1753.
- World Health Organization – WHO. Guidelines for ATC classification and DDD assignment. ATC/DDD Index, 2021. [cited 2021 Jun 7]. Available from: https://www.whocc.no/atc_ddd_index//
- UpToDate database. [cited 2021. Jun 7]. Available from: https://www.uptodate.com/contents/search
- Drugs.com. Drug Interaction Checker; c1996-2018; 2020. [cited 2020 Dec 1]. Available from: https://www.drugs.com/drug_interactions.html
- Mitsiopoulos N, Baumgartner RN, Heymsfield SB, et al. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85(1):115–122.
- Mourtzakis M, Prado CM, Lieffers JR, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33(5):997–1006.
- Aubrey J, Esfandiari N, Baracos VE, et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol. 2014;210(3):489–497.
- Miljkovic I, Zmuda JM. Epidemiology of myosteatosis. Curr Opin Clin Nutr Metab Care. 2010;13(3):260–264.
- Gajra A, Klepin HD, Feng T, et al. Predictors of chemotherapy dose reduction at first cycle in patients age 65 years and older with solid tumors. J Geriatr Oncol. 2015;6(2):133–140.
- Au-Yeung G, Webb PM, DeFazio A, et al. Impact of obesity on chemotherapy dosing for women with advanced stage serous ovarian cancer in the Australian Ovarian Cancer Study (AOCS). Gynecol Oncol. 2014;133(1):16–22.
- Gutierrez F, Gonzalez-de-la-Fuente GA, Nazco GJ, et al. Hematological toxicity of carboplatin for gynecological cancer according to body mass index. Eur J Clin Pharmacol. 2016;72(9):1083–1089.
- Nagel CI, Backes FJ, Hade EM, et al. Effect of chemotherapy delays and dose reductions on progression free and overall survival in the treatment of epithelial ovarian cancer. Gynecol Oncol. 2012;124(2):221–224.
- Lhommé C, Joly F, Walker JL, et al. Phase III study of valspodar (PSC 833). combined with paclitaxel and carboplatin compared with paclitaxel and carboplatin alone in patients with stage IV or suboptimally debulked stage III epithelial ovarian cancer or primary peritoneal cancer. J Clin Oncol. 2008;26(16):2674–2682.
- Du bois A. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as First-Line treatment of ovarian cancer. J Natl Cancer Inst. 2003;95:1320–1329.
- Bronger H, Hederich P, Hapfelmeier A, et al. Sarcopenia in advanced serous ovarian cancer. Int J Gynecol Cancer. 2017;27(2):223–232.
- De Paula NS, Rodrigues CS, Chaves GV. Comparison of the prognostic value of different skeletal muscle radiodensity parameters in endometrial cancer. Eur J Clin Nutr. 2019;73(4):524–530.
- Kumar A, Moynagh MR, Multinu F, et al. Muscle composition measured by CT scan is a measurable predictor of overall survival in advanced ovarian cancer. Gynecol Oncol. 2016;142(2):311–316.
- Rutten IJ, van Dijk DP, Kruitwagen RF, et al. Loss of skeletal muscle during neoadjuvant chemotherapy is related to decreased survival in ovarian cancer patients: Loss of skeletal muscle in ovarian cancer. J Cachexia Sarcopenia Muscle. 2016;7(4):458–466.
- Bozzetti F. Forcing the vicious circle: sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann Oncol. 2017;28(9):2107–2118.
- Hilmi M, Jouinot A, Burns R, et al. Body composition and sarcopenia: the next-generation of personalized oncology and pharmacology? Pharmacol Ther. 2019;196:135–159.
- Kurk S, Peeters P, Stellato R, et al. Skeletal muscle mass loss and dose-limiting toxicities in metastatic colorectal cancer patients. J Cachexia Sarcopenia Muscle. 2019;10(4):803–813.
- Rutten IJG, Ubachs J, Kruitwagen RFPM, et al. Psoas muscle area is not representative of total skeletal muscle area in the assessment of sarcopenia in ovarian cancer: psoas muscle for assessment of sarcopenia in ovarian cancer. J Cachexia Sarcopenia Muscle. 2017;8(4):630–638.
- Staley A, Tucker K, Newton M, et al. Sarcopenia as a predictor of survival and chemotoxicity in patients with epithelial ovarian cancer receiving platinum and taxane-based chemotherapy. Gynecol Oncol. 2020;156(3):695–700.
- Antoun S, Lanoy E, Iacovelli R, et al. Skeletal muscle density predicts prognosis in patients with metastatic renal cell carcinoma treated with targeted therapies: prognostic role of muscle density. Cancer. 2013;119(18):3377–3384.
- Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31(12):1539–1547.
- Goodpaster BH, Kelley DE, Thaete FL, et al. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. 2000;89(1):104–110.
- Bhullar AS, Anoveros-Barrera A, Dunichand-Hoedl A, et al. Lipid is heterogeneously distributed in muscle and associates with low radiodensity in cancer patients. J. Cachexia Sarcopenia Muscle. 2020;11(3):735–747.
- Rollins KE, Tewari N, Ackner A, et al. The impact of sarcopenia and myosteatosis on outcomes of unresectable pancreatic cancer or distal cholangiocarcinoma. Clin Nutr. 2016;35(5):1103–1109.
- Daly LE, Ní Bhuachalla ÉB, Power DG, et al. Loss of skeletal muscle during systemic chemotherapy is prognostic of poor survival in patients with foregut cancer: Muscle loss during chemotherapy is prognostic of poor survival. J Cachexia Sarcopenia Muscle. 2018;9(2):315–325.
- Dijksterhuis WPM, Pruijt MJ, van der Woude SO, et al. Association between body composition, survival, and toxicity in advanced esophagogastric cancer patients receiving palliative chemotherapy. J Cachexia Sarcopenia Muscle. 2019;10(1):199–206.
- Aleixo GFP, Shachar SS, Nyrop KA, et al. Myosteatosis and prognosis in cancer: systematic review and meta-analysis. Crit Rev Oncol Hematol. 2020;145:102839.
- Da Rocha IMG, Marcadenti A, Medeiros GOC, et al. Is cachexia associated with chemotherapy toxicities in gastrointestinal cancer patients? A prospective study. J Cachexia Sarcopenia Muscle. 2019;10(2):445–454.
- McSharry V, Mullee A, McCann L, et al. The impact of sarcopenia and low muscle attenuation on overall survival in epithelial ovarian cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2020;27(9):3553–3356.
- Rier HN, Jager A, Sleijfer S, et al. Changes in body composition and muscle attenuation during taxane-based chemotherapy in patients with metastatic breast cancer. Breast Cancer Res Treat. 2018;168(1):95–105.
- Riechelmann RP, Krzyzanowska MK. Drug interactions and oncological outcomes: a hidden adversary. Ecancermedicalscience. 2019;13:ed88.
- Buchner P, Buchler W, Wagenbauer E, et al. Dose reduction and delay or discontinuation of chemotherapy in cancer patients experiencing potential drug-drug interactions. Eur J Hosp Pharm. 2016;23(Suppl 1):A29.1–A29.
- Pujade-Lauraine E, Banerjee S, Pignata S. Management of platinum-resistant, relapsed epithelial ovarian cancer and new drug perspectives. J Clin Oncol. 2019;37(27):2437–2448.
- Mir O, Coriat R, Blanchet B, et al. Sarcopenia predicts early dose-limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. PLOS One. 2012;7(5):e37563.