Abstract
Background
Childhood acute lymphoblastic leukemia (ALL) is associated with cognitive impairment in adulthood. Cognitive interference processing and its correlated functional magnetic resonance imaging (fMRI) activity in the brain have not yet been studied in this patient group.
Material
Twenty-six adult childhood ALL survivors (median [interquartile range {IQR}] age, 40.0 [37.0–42.3] years) were investigated at median age (IQR), 35.0 (32.0–37.0) years after treatment with intrathecal and intravenous chemotherapy as well as cranial radiotherapy (24 Gy) and compared with 26 matched controls (median [IQR] age, 37.5 [33.0–41.5] years).
Methods
Cognitive interference processing was investigated in terms of behavioral performance (response times [ms] and accuracy performance [%]) and fMRI activity in the cingulo-fronto-parietal (CFP) attention network as well as other parts of the brain using the multisource interference task (MSIT).
Results
ALL survivors had longer response times and reduced accuracy performance during cognitive interference processing (median [IQR] interference effect, 371.9 [314.7–453.3] ms and 6.7 [4.2–14.7]%, respectively) comparedwith controls (303.7 [275.0–376.7] ms and 2.3 [1.6–4.3]%, respectively), but did not exhibit altered fMRI activity in the CFP attention network or elsewhere in the brain.
Conclusion
Adult childhood ALL survivors demonstrated impaired behavioral performance but no altered fMRI activity when performing cognitive interference processing when compared with controls. The results can be used to better characterize this patient group and to optimize follow-up care and support for these individuals.
Background
Acute lymphoblastic leukemia (ALL) constitutes the largest group of childhood cancer and with improved treatment regimens almost 90% of the patients have a life expectancy longer than 5 years after diagnosis [Citation1]. However, ALL survivors exhibit cognitive impairment such as deficits in working memory capacity, inhibition, cognitive flexibility, executive visuomotor control, and sustained attention [Citation2,Citation3]. The cognitive outcomes for patients treated with cranial radiotherapy (CRT) and chemotherapy are somewhat worse compared with chemotherapy only [Citation4,Citation5]. Diagnosis and treatment at a younger age is associated with adverse neurocognitive outcomes since the immature brain is more vulnerable to treatment related neurotoxic effects, and because these effects may also interfere with the ongoing maturation of the brain [Citation4,Citation6]. It is important to understand the nature of cognitive impairment in ALL survivors since it has large impact on patients’ ability of independent living, education, and employment [Citation7–9]. Additionally, some studies have reported that female ALL survivors are at somewhat greater risk for cognitive impairment compared with males [Citation3,Citation8].
In recent years, magnetic resonance imaging (MRI) has been used to study structural brain alterations in ALL survivors to better understand the pathophysiology of the cognitive deficits. ALL survivors have been shown to have reduced white matter volumes compared with controls [Citation10]. White matter volume reductions correlated with decline in attention and were more pronounced in ALL survivors that had received CRT [Citation10]. It has also been reported an association between decline in immediate memory and smaller temporal lobes, as well as an association between impaired delayed memory and thinner parietal and frontal cortices in ALL survivors [Citation11]. Using diffusion tensor imaging (DTI), it has been shown that that microstructural white matter alterations were associated with neuropsychological dysfunction 25 years after treatment [Citation12]. A recent study used both DTI and diffusional kurtosis imaging in ALL survivors and reported an association between microstructural white matter alterations in several major white matter tracts, such as the fornix, the uncinate fasciculus, and the ventral cingulum, and a decline in episodic verbal and visual memory [Citation13].
Blood-oxygen level dependent functional MRI (fMRI) exploits the magnetic susceptibility of blood to detect alterations in the MRI signal because of local changes in blood oxygenation, flow, and volume from the metabolism associated with neuronal activity. Studies using task-based fMRI to evaluate cognitive function in ALL survivors are hitherto rather scarce and report somewhat varying results. To our knowledge, five previous studies have used task-based fMRI to investigate functional alterations related to cognitive function in ALL survivors [Citation11,Citation14–17].
Increased fMRI activity in the dorsal anterior cingulate cortex (dACC) and the dorsolateral prefrontal cortex (DLPFC) has been reported during the visual N-back task to assess working memory [Citation14]. Another study reported increased fMRI activity in the hippocampus during an auditory cued recall memory task [Citation11]. A third study reported increased fMRI activity in several areas, including the claustrum during a paradigm used to test episodic visual memory [Citation15]. A positive correlation between intravenous methotrexate concentration during treatment and fMRI activity in the frontal and anterior cingulate cortices, the caudate nuclei, and the putamen has also been reported during the attention network test [Citation16]. Another study reported a correlation between diagnosis at a younger age, as well as higher intravenous methotrexate concentration during treatment, and decreased fMRI activity in the parietal and temporal lobes during the continuous performance task and the attention network task to assess sustained attention, alerting, orienting, and conflict [Citation17].
Cognitive interference processing, which is the ability to be attentive to goal-relevant information, and at the same time to be able to reject goal-irrelevant information, can be tested using the multisource interference task (MSIT), which has been shown to reliably activate the cingulo-fronto-parietal (CFP) attention network. This includes the dACC, the dorsal anterior midcingulate cortex (daMCC), and the DLPFC. These are all examples of structures involved in target detection, novelty detection, error detection, decision-making, response selection, and stimulus/response competition [Citation18–19]. These areas are also partially connected through the white matter tracts previously investigated in ALL survivors [Citation13].
Our aim was to evaluate cognitive interference processing in terms of behavioral performance and fMRI activity using the MSIT and whole-brain fMRI analysis in adult childhood ALL survivors. We hypothesized that ALL survivors would exhibit increased response times, reduced accuracy performance, and altered fMRI activity in the CFP attention network as compared with controls. In addition, we also hypothesized that the ALL survivors could display compensatory activity and/or decreased activity in other parts of the brain.
Material and methods
Study population
Fifty ALL survivors from the Southern Region of Sweden (population, 2.5 million), who were treated with CRT (24 Gy) and chemotherapy at Lund University Hospital in Sweden between 1971 and 1992, and had undergone clinical pituitary hormone evaluation were invited to participate in the study. Data from the study participants were acquired between 2013 and 2014. ALL survivors treated with CRT (18 Gy) and chemotherapy or chemotherapy only were excluded. Thirty-eight (76%) agreed to participate and 12 declined to participate. Ten of the participating 38 subjects did not complete MRI examination either because of claustrophobia or technical difficulties. Another two subjects were excluded because they did not understand how to perform the MSIT. Thus, 26 ALL survivors (median [interquartile range {IQR}] age, 40.0 [37.0–42.3] years; 14 females) were included in the study (). All subjects had received CRT (24 Gy) and chemotherapy (intrathecal and intravenous) according to the common protocols of the Nordic countries at that time [Citation20]. The ALL survivors were investigated at median (IQR) 35.0 (32.0–37.0) years after treatment (). All were growth hormone deficient and were supplemented with growth hormone (median [IQR], 0.4 (0.3–0.5) mg/day). Four subjects were on thyroxin and one subject was treated with hydrocortisone for adrenal insufficiency. Ten of the females ALL survivors had spontaneous regular menstrual cycles, and the remaining four used oral contraceptives. Six of the 12 male ALL survivors had received radiation to the testes and were substituted with testosterone as intramuscular injections or gel. None were smokers. Comparisons were made with 26 control subjects (median [IQR] age, 37.5 [33.0–41.5] years; 15 females) that were matched for age, gender, and smoking habits. Controls were randomly selected from a computerized register as previously described [Citation2].
Table 1. Characteristics of the study participants.a
Compliance with ethical standards
All subjects gave written informed consent. The study was approved by the local ethics committee (DNR 2011/770 and 2012/596).
fMRI acquisition
A 3 T MRI scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) equipped with a 20-channel head/neck receiver coil was used to acquire MRI data. fMRI data were acquired using a gradient-echo planar sequence (TR/TE 1500/30 ms/ms, 25 slices, 64 dynamic scans, voxel size = 3 × 3 × 4 mm3). Additionally, a T1-weighted three-dimensional magnetization prepared gradient echo (MP-RAGE) sequence (TR/TE 1900/2.54 ms/ms), with 1 mm3 isotropic resolution, was also acquired to be able to perform the fMRI analyses described below.
fMRI task
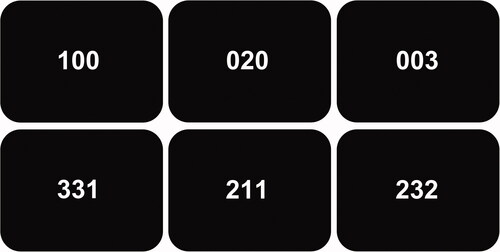
The MSIT was performed according to Bush et al. [Citation19]. Briefly, the subjects were given an MRI-compatible three-button keypad and told that the keypad buttons represented the numbers 1, 2, and 3, from left to right. The participants were then instructed to use the right index, middle, and ring finger to respond. All subjects were right-handed and had or were corrected to normal vision. Subjects were informed that three numbers would appear in the center of the screen every few seconds. The objective was to report, via button-press, the identity of the displayed number that differed from the other two distractor numbers (). During the ‘control tasks’, the distractors were zeros, and the target numbers (either 1, 2, or 3) were always placed congruently with their position. During the ‘interference tasks’, the distractors were either 1, 2, or 3, and the target numbers (either 1, 2, or 3) were never placed congruently with their position. After receiving instructions, each subject performed the MSIT once to make sure that they could perform the task correctly. Next, each subject performed the MSIT during fMRI acquisition. The subjects completed two scans each with a few minutes long break between the scans. Each scan was 396 s long, including a 30 s fixation dot at the start and at the end. Between the fixation dots, four 42 s blocks of the control tasks alternated with four 42 s of the interference tasks without any interruption between blocks. Each block consisted of 24 three digit-number combinations, which means that the subjects had 1.75 s per task. Response times (ms), accuracy performance (%), and missing responses were recorded using E-prime 2.0 (Psychology Software Tools, Pittsburgh, PA, USA).
Figure 1. The objective during the multisource interference task was to report, via button-press, the identity of the displayed number that differed from the other two numbers. During the control tasks (upper row), the distractors were zeros, and the target numbers (either 1, 2, or 3) were always placed congruently with their position. During the interference tasks (lower row), the distractors were either 1, 2, or 3, and the target numbers were never placed congruently with their position. The correct answer for the first column is hence ‘1’, for the second ‘2’, and for the third ‘3’.
Statistical analysis of behavioral performance
Median response times (ms) and median accuracy performance (%) for the interference and control tasks as well as missing responses were calculated. The interference effect was defined as the difference in response time and accuracy performance between the interference and control tasks. The Mann-Whitney U test was used to make comparisons between ALL survivors and controls. The Wilcoxon signed-rank test was used to compare differences in response time and accuracy performance between interference and control tasks within the groups. Additionally, the statistical analyses described above were performed on a matched subsets consisting of only males and females, respectively. Non-parametric tests were chosen since the groups were relatively small and thus could not be assumed to be normally distributed. Calculations were made using SPSS (SPSS, Chicago, IL, USA, version 26) and results were regarded as statistically significant if p < .05.
fMRI activity analysis
An exploratory whole-brain fMRI analysis approach was used. fMRI contrasts of the interference effect for lower-level analyses were generated through the subtraction of fMRI signal during the control tasks from the interference tasks for each subject. The contrasts from the lower-level analysis for each subject were used to make group contrasts in an additional higher-level analysis to compare the group mean interference effect between the ALL survivors and the controls. Additionally, the higher-level analysis described above was also performed on a matched subsets consisting of only males and females, respectively. fMRI data were processed and analyzed using FEAT (FMRI Expert Analysis Tool) Version 6.00, part of FSL (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). Registration to standard space images was carried out using FLIRT (FMRIB's Linear Image Registration Tool), followed by registration using FNIRT (FMRIB's Nonlinear Image Registration Tool) [Citation21–24]. The following prestatistics processing was applied: motion correction using MCFLIRT (Motion Correction using FMRIB's Linear Image Registration Tool) [Citation22]; slice-timing correction using Fourier-space time-series phase-shifting; non-brain removal using BET (Brain Extraction Tool) [Citation25]; spatial smoothing using a Gaussian kernel of full width of half maximum 5 mm; grand-mean intensity normalization of the entire four-dimensional dataset by a single multiplicative factor; and high-pass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma = 45.0 s). Time-series statistical analysis was carried out using FILM with local autocorrelation correction [Citation26]. Z statistic images were nonparametrically thresholded using clusters determined by Z > 3.1 and a (corrected) cluster significance threshold of p = .05 [Citation27]. The general linear model for both lower and higher-level analyses was created using FLAME (FMRIB’s Local Analysis of Mixed Effects) stage 1 [Citation28–30].
Results
Behavioral performance
Results of the analyses of behavioral performance in terms of response times (ms) and accuracy performance (%) during the MSIT are summarized in . The interference effect, regarded as the difference in reaction time and accuracy performance between the control and interference tasks was statistically significantly increased (p = .003) and reduced (p = .0001), respectively, in ALL survivors (median [IQR], 371.9 [314.7–453.3] ms and 6.7 [4.2–14.7]%, respectively), as compared with controls (median [IQR], 303.7 [275.0–376.7] ms and 2.3 [1.6–4.3]%, respectively, see Supplementary Fig. 1). The ALL survivors had statistically significantly increased reaction time during both interference (p = .0002) and control tasks (p = .002) as compared with controls. The ALL survivors also had statistically significantly reduced accuracy performance during interference tasks (p = .0001), but not during control tasks (p = .9) as compared with controls. The reaction time was statistically significantly increased during interference tasks as compared with the control tasks for both ALL survivors (median [IQR], 949.9 [897.5 –1038.6] ms vs. 568.9 [520.0–630.7] ms; p = .000008) and controls (median [IQR], 821.0 [769.8–884.7] ms vs. 504.3 [454.0–538.8] ms; p = .000008). The accuracy performance was also statistically significantly reduced during interference tasks as compared with the control tasks for both ALL survivors (median [IQR], 92.7 [84.9–95.8] % vs. 100.0 [99.5–100.0] %; p = .000008) and controls (median [IQR] 97.1 [95.6–97.9] % vs. 100.0 [99.5–100.0] %; p = .00001). Additionally, all comparisons presented above were also performed for subgroups of males and females, respectively, with similar results. The missing response rate was <5% for both groups and there were no differences in missing response rate between the groups after exclusion of two ALL survivors who were not able to adequately perform the MSIT.
Table 2. Reaction time and accuracy performance during the interference and control tasks in the multisource interference task, as well as the interference effect, i.e., the difference in reaction time and accuracy performance between the interference and control tasks, for both adult survivors of childhood ALL and controls.a
fMRI activity
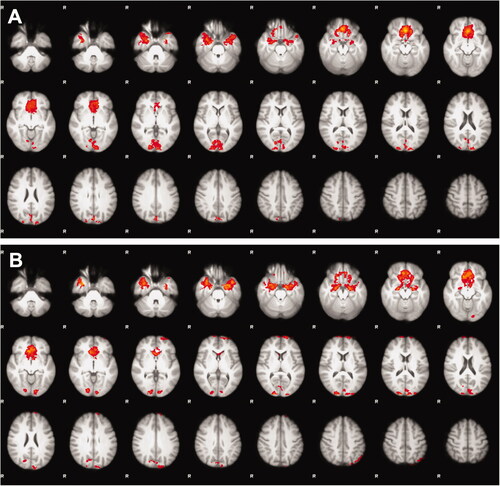
Results of the analyses of fMRI activity during the MSIT are presented in . The difference in fMRI activity between interference and control tasks, i.e., the difference in neuronal activity required to perform the more cognitively demanding interference tasks as compared with the control tasks, revealed fMRI activity in the CFP attention network in both the ALL survivors and in the control group. Comparisons between ALL survivors and controls showed no significant differences in fMRI activity in neither the CFP attention network nor in any other part of the brain between the two groups. No significant differences between subgroups of males and females were present.
Figure 2. Mean differences in whole-brain functional magnetic resonance imaging activity between the interference and control tasks in the multisource interference task, i.e., the difference in neuronal activity required to perform the additionally more cognitively demanding interference tasks compared with the control tasks, revealed fMRI activity pattern in the cingulo-fronto-parietal attention network as excepted, but showed no significant differences between the adult survivors of (A) childhood acute lymphoblastic leukemia and (B) controls.
Discussion
The aim of the study was to evaluate cognitive interference processing in adult childhood ALL survivors in terms of behavioral performance and fMRI activity using the MSIT. Our results show that the ALL survivors had longer response times to perform the more cognitively demanding interference tasks, as compared with the control group, and their accuracy performance was also lower than the control group. Nonetheless, there were no differences in fMRI activity between the groups in performing the interference tasks.
As expected, a significant difference in reaction time as well as in accuracy performance was recorded between the more cognitively demanding interference tasks and the control tasks for both the ALL survivors and the controls. This indicates that the MSIT was performed according to instructions described in the literature [Citation18,Citation19]. However, the ALL survivors needed longer time to complete both tasks as compared with controls, and performed less accurately during the more cognitively demanding interference tasks compared with the control group. The interference effect, i.e., the difference in reaction time was also longer, and the accuracy performance was reduced between the interference and control tasks in ALL survivors as compared with controls. Our results suggest that the effort needed to solve the more cognitively demanding interference tasks was greater in the ALL survivors than in controls.
The interference effect was also studied regarding fMRI activity and revealed activation in the CFP attention network as expected in both groups, again demonstrating that the results are reliable. Although there were differences in reaction time and accuracy performance between the investigated groups, the difference in fMRI activity that was needed to solve the more cognitively demanding interference tasks did not differ between the groups in neither the CFP attention network nor in any other areas of the brain. This suggests that the mechanism for the difference in cognitive performance between the ALL survivors and the control group was either too small to detect using this method, or that the ALL survivors did not have altered fMRI activity during cognitive interference processing.
The MSIT has previously been used to study alterations in behavioral performance and fMRI activation in different conditions, mostly within the field of psychiatry [Citation31–35], but also in patients with heart disease [Citation36]. The results have been highly varying and not all studies performed fMRI acquisition during the MSIT [Citation31,Citation32] whereas the rest of the studies have only reported behavioral performance results [Citation33–36]. In studies using fMRI, decreased fMRI activity in the rostral anterior cingulate/medial prefrontal cortex and the precuneus/posterior cingulate cortex were seen in patients with schizophrenia [Citation31]. The same research group found increased fMRI activity in the medial frontal cortex in patients with obsessive-compulsive disorder but no differences in behavioral performance [Citation32]. These somewhat ambiguous results might be because of different conditions affecting different parts of the brain. Further, the structures within the investigated area are responsible for different functions, so that decreased activity of one area could result in the same altered behavioral performance as increased activity in another area.
Results in neuroimaging studies on ALL survivors are highly variable, largely because of methodological variations and limitations such as small samples, different treatment protocols, and different follow-up times [Citation37]. This is the first study that has used the MSIT in combination with fMRI to study cognitive interference processing in ALL survivors. Reaction times, accuracy performance, and fMRI activity indicate that the MSIT was performed correctly and that the results are reliable. To our knowledge, only five previous studies have used task-based fMRI to evaluate cognitive function in childhood ALL () [Citation11,Citation14–17]. These previous studies have all used different fMRI paradigms from the one used in this study and therefore comparisons are challenging.
Table 3. Previous fMRI studies on adult survivors of childhood ALL.
Our findings are, however, in conjunction with a few previous studies e.g., demonstrating similar findings in regard of similar behavioral performance in ALL survivors and controls in a study using the N-back task to assess working memory in eight teenage ALL survivors treated with intrathecal and intravenous chemotherapy [Citation14]. Contradictory to our findings, the same study reported increased fMRI activity in the dACC and DLPFC. Even though the visual N-back task has been shown to activate partially the same regions as the MSIT (e.g., the dACC and the DLPFC) the task is different than the MSIT and hence comparisons of the results are difficult to interpret [Citation18,Citation19,Citation38]. Another study reported an increased fMRI activity in the hippocampus during an auditory cued-recall memory task in 85 adult ALL survivors treated with chemotherapy (intrathecal and intravenous) and radiotherapy [Citation11]. Increased fMRI activity in several areas throughout the brain including the claustrum during encoding of visual memories has also been reported in 10 adult ALL survivors treated with chemotherapy (intrathecal and intravenous) and radiotherapy [Citation15]. The same study also found that ALL survivors had differences in behavioral performance as compared with controls during the task, with lower recognition memory accuracy [Citation15]. Two studies performed the attention network test, which activates emotion and executive function networks [Citation16–17]. The first study found that higher plasma methotrexate during treatment was associated with higher fMRI activity in the frontal and anterior cingulate cortices, and in the caudate nuclei and the putamen in 142 teenage ALL survivors treated with intrathecal and intravenous chemotherapy [Citation16]. The other study found, somewhat contradictory, an association between decreased fMRI activity in the parietal and temporal lobes and the hippocampus during the attention network task and higher serum methotrexate exposure in 165 teenage ALL survivors treated with intravenous methotrexate [Citation17].
The present study set out to investigate late treatment effect on cognition in adult childhood ALL survivors treated with CRT and chemotherapy. However, treatment regimens have changed during the relatively long follow-up time, and current Nordic standard protocols for treating childhood ALL do not include prophylactic CRT anymore. Therefore, it can be suspected that late treatment effects on children treated for ALL today may not be as severe compared with the ALL survivors in the present study, even though some studies have reported some degree of cognitive impairment in subjects only treated with chemotherapy [Citation4,Citation5]. Nevertheless, it is still of great importance to be able to optimize proper hormone substitution and follow-up care for ALL survivors treated with CRT. Thus, the results of the present study could have impact on the life-long follow-up care in this population.
To our knowledge, this is the first study that has used the MSIT in combination with fMRI to study cognitive interference processing in ALL survivors. Reaction time, accuracy performance, and fMRI activity indicate that the MSIT was performed correctly and that the results are reliable. Nonetheless, there are some limitations to this study. Most important, even though the MSIT has been shown to robustly activate the CFP attention network, we chose an exploratory whole-brain fMRI analysis approach instead of a region of interest (ROI)-based approach focusing on the area of the CFP attention network. This was done because we wanted to see that the MSIT activated the CFP attention network in this cohort, which had not been tested before. We also wanted to study potential differences in fMRI activity in other areas of the brain that could indicate compensatory increased or decreased activity due to neuronal damage. The disadvantage of a whole-brain analysis compared with a ROI-based approach is, however, that the sensitivity for potential differences in activity in a specific area decreases. On the other hand, both behavioral and fMRI activity results of this study indicate that MSIT can be used in this patient group and further studies focusing directly on the area of the CFP attention network using a ROI-based approach could therefore be a feasible next step toward better understanding the late-effect cognitive impairment in adult childhood ALL survivors. Another limitation is that the study groups were relatively small, mostly because of the long follow-up time, which may have underpowered the study, leading to discarded true differences between the investigated groups. Further, a potential selection bias might have been introduced when subjects that were unable to perform the task were excluded. A more general limitation in task-based fMRI studies is that it is difficult to interpret results because of the abundance of undeliberate inconsistency regarding different parameter settings between different studies.
In conclusion, adult childhood ALL survivors treated with intravenous chemotherapy and cranial radiotherapy performed cognitive interference processing somewhat worse compared with controls, but exhibited no altered fMRI activity in the CFP attention network or elsewhere in the brain. The findings of the present study are valuable to better characterize this patient group and to optimize follow-up care and support for these individuals. Additionally, the results can be used to justify a further ROI-based approach analysis to be able to probe for differences in fMRI activity with higher sensitivity. Another interesting approach would be to correlate the fMRI activity with co-variates such as chemotherapy dosage. Further studies on cognition in general in these subjects are also needed to fully understand the degree of cognitive impairment and its correlated functional and structural alterations in ALL survivors.
Supplemental Material
Download PNG Image (31 KB)Supplemental Material
Download PNG Image (34.9 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975–2017. Bethesda: National Cancer Institute; 2020.
- Link K, Moëll C, Österberg K, et al. Adult survivors of childhood acute lymphoblastic leukaemia with GH deficiency have normal self-rated quality of life but impaired neuropsychological performance 20 years after cranial irradiation. Clin Endocrinol. 2006;65(5):617–625.
- Schuitema I, de Sonneville L, Kaspers G, et al. Executive dysfunction 25 years after treatment with cranial radiotherapy for pediatric lymphoid malignancies. J Int Neuropsychol Soc. 2015;21(9):657–669.
- Halsey C, Buck G, Richards S, et al. The impact of therapy for childhood acute lymphoblastic leukaemia on intelligence quotients; results of the risk-stratified randomized Central nervous system treatment trial MRC UKALL XI. J Hematol Oncol. 2011;4(1):12.
- Cheung YT, Krull KR. Neurocognitive outcomes in long-term survivors of childhood acute lymphoblastic leukemia treated on contemporary treatment protocols: a systematic review. Neurosci Biobehav Rev. 2015;53:108–120.
- Edelstein K, D’Agostino N, Bernstein LJ, et al. Long-term neurocognitive outcomes in young adult survivors of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2011;33(6):450–458.
- Mody R, Li S, Dover DC, et al. Twenty-five-year follow-up among survivors of childhood acute lymphoblastic leukemia: a report from the childhood cancer survivor study. Blood. 2008;111(12):5515–5523.
- Kirchhoff AC, Krull KR, Ness KK, et al. Physical, mental, and neurocognitive status and employment outcomes in the childhood cancer survivor study cohort. Cancer Epidemiol Biomarkers Prev. 2011;20(9):1838–1849.
- Kunin‐Batson A, Kadan‐Lottick N, Zhu L, et al. Predictors of independent living status in adult survivors of childhood cancer: a report from the childhood cancer survivor study. Pediatr Blood Cancer. 2011;57(7):1197–1203.
- Reddick WE, Shan ZY, Glass JO, et al. Smaller white-matter volumes are associated with larger deficits in attention and learning among long-term survivors of acute lymphoblastic leukemia. Cancer. 2006;106(4):941–949.
- Armstrong GT, Reddick WE, Petersen RC, et al. Evaluation of memory impairment in aging adult survivors of childhood acute lymphoblastic leukemia treated with cranial radiotherapy. J Natl Cancer Inst. 2013;105(12):899–907.
- Schuitema I, Deprez S, Van Hecke W, et al. Accelerated aging, decreased white matter integrity, and associated neuropsychological dysfunction 25 years after pediatric lymphoid malignancies. J Clin Oncol. 2013;31(27):3378–3388.
- Follin C, Svärd D, van Westen D, et al. Microstructural white matter alterations associated to neurocognitive deficits in childhood leukemia survivors treated with cranial radiotherapy – a diffusional kurtosis study. Acta Oncol. 2019;58(7):1021–1028.
- Robinson KE, Livesay KL, Campbell LK, et al. Working memory in survivors of childhood acute lymphocytic leukemia: functional neuroimaging analyses. Pediatr Blood Cancer. 2010;54(4):585–590.
- Monje M, Thomason ME, Rigolo L, et al. Functional and structural differences in the hippocampus associated with memory deficits in adult survivors of acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60(2):293–300.
- Krull KR, Cheung YT, Liu W, et al. Chemotherapy pharmacodynamics and neuroimaging and neurocognitive outcomes in long-term survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 2016;34(22):2644–2653.
- Fellah S, Cheung YT, Scoggins MA, et al. Brain activity associated with attention deficits following chemotherapy for childhood acute lymphoblastic leukemia. J Natl Cancer Inst. 2019;111(2):201–209.
- Bush G, Shin LM, Holmes J, et al. The multi-source interference task: validation study with fMRI in individual subjects. Mol Psychiatry. 2003;8(1):60–70.
- Bush G, Shin LM. The multi-source interference task: an fMRI task that reliably activates the cingulo-frontal-parietal cognitive/attention network. Nat Protoc. 2006;1(1):308–313.
- Gustafsson G, Kreuger A, Dohlwitz A. Acute lymphoblastic leukemia in Swedish children 1973-1978. Acta Paediatr Scand. 1981;70(5):609–614.
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–156.
- Jenkinson M, Bannister P, Brady M, et al. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841.
- Andersson JL, Jenkinson M, Smith S. Non-linear optimisation. FMRIB technical report TR07JA1. FMRIB Analysis Group of the University of Oxford; 2007. Available from: https://www.fmrib.ox.ac.uk/datasets/techrep/
- Andersson JL, Jenkinson M, Smith S. Non-linear registration, aka spatial normalisation FMRIB technical report TR07JA2. FMRIB Analysis Group of the University of Oxford; 2007. Available from: https://www.fmrib.ox.ac.uk/datasets/techrep/
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155.
- Woolrich MW, Ripley BD, Brady M, et al. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14(6):1370–1386.
- Worsley KJ. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: an introduction to methods. Oxford University Press; 2001. p. 251–270.
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20(2):1052–1063.
- Woolrich MW, Behrens TE, Beckmann CF, et al. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21(4):1732–1747.
- Woolrich MW. Robust group analysis using outlier inference. Neuroimage. 2008;41(2):286–301.
- Harrison BJ, Yücel M, Pujol J, et al. Task-induced deactivation of midline cortical regions in schizophrenia assessed with fMRI. Schizophr Res. 2007;91(1–3):82–86.
- Yücel M, Harrison BJ, Wood SJ, et al. Functional and biochemical alterations of the medial frontal cortex in obsessive-compulsive disorder. Arch Gen Psychiatry. 2007;64(8):946–955.
- Allen KJ, Hooley JM. Negative mood and interference control in nonsuicidal self-injury. Compr Psychiatry. 2017;73:35–42.
- Capri T, Santoddi E, Fabio RA. Multi-source interference task paradigm to enhance automatic and controlled processes in ADHD. Res Dev Disabil. 2020;97:103542.
- Darnell BC, Valentiner DP. Performance on the multisource interference task moderates the relationship between trauma exposure and posttraumatic stress symptoms. ClinPsycholSci. 2020;8(2):351–358.
- Jung M, Jonides J, Berman MG, et al. Construct validity of the multi-source interference task to examine attention in heart failure. Nurs Res. 2018;67(6):465–472.
- Hearps S, Seal M, Anderson V, et al. The relationship between cognitive and neuroimaging outcomes in children treated for acute lymphoblastic leukemia with chemotherapy only: a systematic review. Pediatr Blood Cancer. 2017;64(2):225–233.
- Pesonen M, Hämäläinen H, Krause CM. Brain oscillatory 4–30 Hz responses during a visual n-back memory task with varying memory load. Brain Res. 2007;1138:171–177.
