Abstract
Background
Only a few recent phase III trials with targeted therapies or immune checkpoint inhibitors (ICIs) in metastatic clear-cell renal cell carcinoma (m-ccRCC) demonstrated an overall survival (OS) benefit compared to standard of care. We aimed to study the evolution of OS since the start of systemic therapy from 2000 to 2020.
Patients and methods
Retrospective study on all consecutively treated m-ccRCC patients in three Belgian hospitals starting with systemic therapy. The study outcome was OS since the start of systemic therapy. We used a univariable Cox model for OS with year of the start of therapy as a predictor, and a multivariable analysis including known prognostic factors. Linear and non-linear trends of time were tested.
Results
Five hundred patients were included. In a linear model, the HR for OS depending on the year of the start of therapy was 0.95 (95%CI 0.93–0.97; p < 0.0001), estimated for an increase with 1 year in time. In a non-linear model, OS started to improve from 2006 on, when vascular endothelial growth factor receptor tyrosine kinase inhibitors (VEGFR-TKIs) replaced interferon alfa (IFNa) as a standard of care and continued to increase steadily during the following years. On multivariable analysis, the year of the start of therapy remained an independent prognostic factor for OS. Two-year OS after the start of systemic therapy was 23%, 34%, 50% and 59% for patients who started treatment in 2000–2005, 2006–2011, 2012–2017, and 2018–2020, respectively. The five-year OS of the first three groups was 7%, 14% and 24%. The mean number of administered lines of therapy increased over time, with an incidence rate ratio of 1.07 (95%CI 1.05–1.08; p < 0.0001) per year increase for the period 2000–2016.
Conclusion
OS of m-ccRCC patients has been improving significantly over the last 15 years since the introduction of VEGFR-TKIs and ICIs.
Background
Renal cell carcinoma (RCC) accounts for 80% of all kidney cancers [Citation1]. Approximately 20% of the patients present with metastatic RCC (mRCC) at initial diagnosis and 30% of the patients with initially localized disease will sooner or later relapse. Therefore, many RCC patients will eventually need systemic treatment. Clear-cell RCCs (ccRCC) are the most common histologic subtype, representing 80-85% of all RCCs [Citation2–4].
The vascular endothelial growth factor receptor tyrosine kinase inhibitors (VEGFR-TKIs) sunitinib and sorafenib became available from 2006 onwards, followed by the second generation VEGFR-TKIs pazopanib, axitinib, cabozantinib, lenvatinib and tivozanib. The mechanistic target of rapamycin (mTOR)-inhibitors everolimus and temsirolimus were used in routine clinical practice from 2009 on. More recently the advent of immune checkpoint inhibitors (ICIs) targeting PD(L)-1 or CTLA-4 has revolutionized systemic therapy for m-ccRCC. In clinical use, nivolumab became available in monotherapy in 2016 and the combination of ipilimumab/nivolumab from 2019 on. Combinations of VEGFR-TKIs and ICIs have only been introduced very recently [Citation2,Citation5–18].
Although multiple phase III trials with these compounds have shown improved therapeutic efficacy of the experimental arm compared to the previous standard of care in terms of response rate (RR) and progression-free survival (PFS), only a limited number of studies could demonstrate a significant overall survival (OS) benefit (Supplemental Table 1). OS is likely the most important and most objective outcome parameter in clinical research. If drugs are approved based on the positive outcome of trials formed on surrogate endpoints such as RR of PFS, the question arises whether such effects also translate into improved survival. Especially considering the added toxicities of these drugs and the high financial cost [Citation19,Citation20].
Therefore, we aimed to study the evolution of OS of m-ccRCC patients during the last 20-years period (2000–2020) in clinical practice.
Patients and methods
We performed a retrospective cohort study on all m-ccRCC patients treated consecutively in three Belgian hospitals (University Hospitals Leuven, general hospital Imelda in Bonheiden and general hospital Groeninge in Kortrijk). After the approval of the project by the medical ethics committee (S63833), we included all patients who started with systemic therapy (IFNa, mTOR-inhibitors, VEGFR-TKIs or ICIs) between January 2000 and October 2020. We only included ccRCCs, according to the inclusion criteria of the main phase III trials, and because the novel agents are more efficient in ccRCCs compared to other subtypes [Citation21].
The objective of the study was to evaluate the impact of new treatments for m-ccRCC on OS in clinical routine. Therefore, we calculated OS from the start of systemic therapy until death or last contact. As our hypothesis was that the availability and consecutive administration of new efficient therapies improved OS, we recorded the total number of therapy lines that the patients received. We only included IFNa, mTOR-inhibitors, VEGFR-TKIs, ICI and excluded experimental lines of therapy that have never led to positive results or older therapies with poor efficacy such as chemotherapy and thalidomide.
We recorded patient age and gender, Fuhrman grade of the tumor, as well as all previously described factors with a prognostic impact in m-ccRCC: the International Metastatic RCC Database Consortium (IMDC) Risk Score and its determinants, the presence of bone and brain metastasis, and serum albumin and C-reactive protein (CRP) values at the start of first-line therapy [Citation22–25]. We divided the patients into 4 groups depending on the year of the start of first-line therapy: 2000–2005 (the cytokin era, which was in our study predominantly IFNa), 2006–2011 (the first VEGFR-TKIs era), 2012–2017 (during which second-generation VEGFR-TKIs and second-line nivolumab became available) and 2018–2020 (the ipilimumab/nivolumab in first-line era).
Cox proportional hazards regression models were used for time-to-event analyses. Results are presented as hazard ratios (HR) with 95% confidence intervals (CI). We studied univariable Cox models for OS with the year of the start of therapy as a continuous predictor. We used a model fitting a linear trend for the year, assuming a constant effect of time over the entire period. The HR is estimated for an increase with one year in time. Additionally, we used a model fitting a non-linear trend allowing for changes in the effect of time. A multivariable model was applied to test the effect of the year while correcting for IMDC risk groups, CRP levels and the presence of brain or bone metastasis. Serum albumin was not included because of missing values. We checked the impact of the IMDC score on patients treated in first-line with IFNa because this risk classification was developed and originally used during the VEGFR-TKI era.
A Poisson model was used for the analysis of the number of lines of therapy as a count outcome variable. Results are presented as incidence rate ratios (IRR) with 95% CI. The IRR represents the percentage change in outcome variable for a one-year increase of the predictor, an IRR of 1.1 indicating a yearly 10% increase in the number of lines of therapy. A segmented regression approach was applied. Analyses have been performed using SAS software (version 9.4 of the SAS System for Windows) and GraphPad (Prism 9.0.0, San Diego, CA).
As this is a retrospective cohort study, we used the STROBE guidelines for the quality check of this study and draft (see supplemental documents).
Results
Included patients
Five hundred patients were included in this analysis (patient characteristics are reported in ). First-line therapy was IFNa in 92 patients (one in combination with interleukin 2), VEGFR-TKIs in 330 patients (179 patients were started on sunitinib, 135 on pazopanib and 16 on sorafenib), ICIs in 57 patients (3 patients were started on avelumab, 2 on axitinib-pembrolizumab, 4 on nivolumab and 48 on ipilimumab-nivolumab) and mTOR-inhibitors in 21 patients (3 patients were started on everolimus and 18 on temsirolimus). IMDC risk group was good in 17%, intermediate in 54% and poor in 29% of the patients. Database closure and analysis took place in November 2020, there was a median follow-up of 64 months and 384 patients had died. A median of 25 patients was included each year (range 8–34) (Supplemental Table 2).
Table 1. Patient characteristics.
Overall survival depending on year of start of therapy
Median OS (mOS) was 12 months in patients who started first-line therapy between 2000 and 2005, 15 months between 2006 and 2011, 24 months between 2012 and 2017 and not reached in patients who started between 2018 and 2020 (p < 0.0001) (). Estimated two-year OS was 23%, 34%, 50% and 59%, respectively. The five-year OS of the first three groups was 7%, 14% and 24%, respectively (). depicts the improvement of one-, two-, three-, four- and five-year survival probabilities over the study period and the coincidence with the availability of new therapies. During the IFNa era (2000–2005), we saw no improvement, whereas the improvement started to increase from 2006 on, after the introduction of VEGFR-TKIs.
Figure 1. Kaplan–Meier estimates of median overall survival (OS) correlated to start year (panel A) and correlated to first-line therapy (panel B). ICIs: immune checkpoint inhibitors; VEGFR-TKI: vascular endothelial growth factor receptor tyrosine kinase inhibitor; IFN: interferon-alpha.
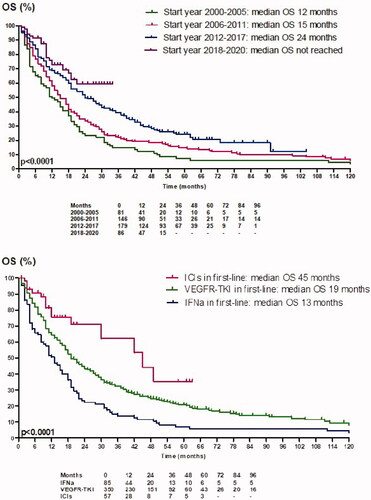
Figure 2. One to five-year survival probabilities depending on start year and availability of treatments (in Belgium). The combination of axitinib/pembrolizumab and axitinib/avelumab became available in 2020.
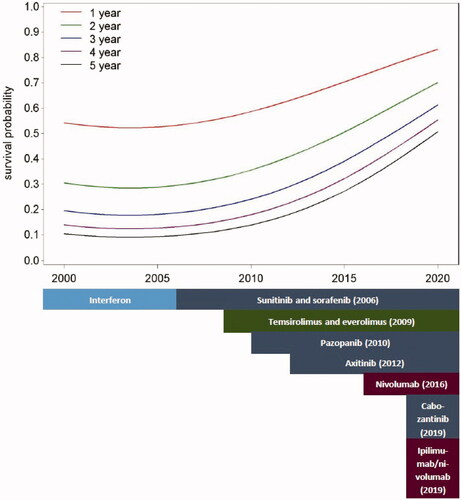
Table 2. One, two, three, four and five-year overall survival depending on starting year and first-line treatment.
In the linear model, OS increased over time (HR per 1 year increase was 0.95, 95%CI 0.93–0.97; p < 0.0001). In the non-linear model, we have found a significant trend with improving HR for better survival at different time points. The HR reported in shows that in earlier years there is no improvement of OS with time, whereas the improvement increased toward the end of the 20 years.
Table 3. Univariable and multivariable analysis.
Multivariable analysis
mOS was 21, 16 and 7 months in patients with IMDC good, intermediate and poor-risk, respectively (p = 0.07). The impact of the year of the start of systemic treatment was independent of all tested prognostic markers. Using a multivariable analysis with a linear approach, the HR was 0.95 for each increase in the year (95%CI 0.93–0.97: p < 0.0001) (). In the non-linear approach, the starting year remained significantly correlated with improved OS, after the initial cytokin era.
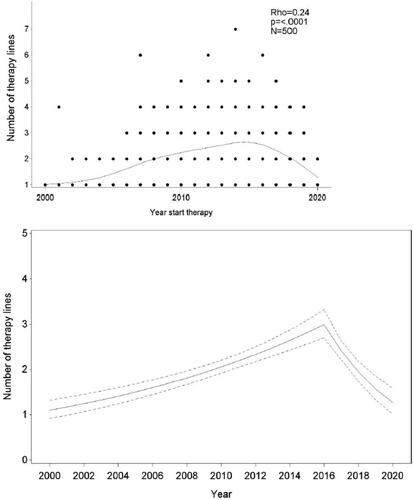
Number of lines of therapy during the period 2000–2020
Of the 500 patients, 227 (45%) only received a single line of therapy, 128 (26%) received two, 81 (16%) three, 53 (11%) four, and 11 (2%) five or more lines of therapy. The mean number of lines of newer, efficient therapies administered to the patients increased over time (). Panel A shows an increasing number of efficient lines of therapy till around 2016, and a decrease afterward. In the next step, a segmented regression approach was applied, dividing the follow-up time in two periods: 2000–2016 and 2017–2020, a cutoff chosen based on model fit. The slope of the number of lines of therapy by year was estimated separately in both periods. IRR was 1.07 (95%CI 1.05–1.08; p < 0.0001) for the period 2000–2016 and 0.81 (95%CI 0.76–0.86; p < 0.0001) for the period 2017–2020. shows the mean number of lines of therapy depending on starting the year with 95%CI.
Type of first-line therapy and overall survival
Finally, we estimated OS differences according to the type of first-line therapy, to illustrate OS improvement in a complimentary way, but without assuming that the global OS might be due to first-line therapy efficacy alone. mOS was 13 months when the first-line treatment was IFNa, 19 months when patients started with VEGFR-TKIs and 45 months when the first-line treatment was based on ICIs (p < 0.0001) (). This difference remained significant even after correction for known risk factors (IMDC risk groups, baseline CRP levels, presence of bone or brain metastasis) (Supplemental Table 3). We did not calculate OS for patients who started in first-line with the mTOR-inhibitor temsirolimus, because temsirolimus is traditionally used only in patients with poor prognosis. Five-year OS was 7%, 21% and 36% in patients treated in first-line with IFNa, VEGFR-TKIs and ICIs, respectively ().
Discussion
After almost 25 years with no improvement in the prognosis of m-ccRCC, several efficient therapies, among which VEGFR-TKIs and ICIs, became available during the last fifteen years [Citation26].
We aimed to assess if the introduction of VEGFR-TKIs and ICIs has led to an OS improvement during the last 20-year period (2000–2020).
Although multiple phase III trials demonstrated the improved therapeutic efficacy of the study arm compared to the previous standard of care in terms of RR and PFS, only a limited number of studies could demonstrate a statistically significant OS benefit (Supplemental Table 1). In first-line therapy, the sorafenib (versus placebo) and the ipilimumab/nivolumab phase III trial (versus sunitinib) were associated with a significant OS benefit. The pivotal phase III trials with pazopanib (versus placebo) or the pivotal phase III trial with cabozantinib (versus sunitinib) did not show a significant OS benefit. For the pivotal trial with sunitinib (versus IFNa), the p-value for OS with sunitinib had borderline significance with a p-value of 0.051. In second- or further line therapy, the pivotal phase III trials with cabozantinib (versus everolimus) and nivolumab (versus everolimus) were associated with a significant OS benefit, but the pivotal phase III trials with everolimus (versus placebo) and axitinib (versus sorafenib) were not.
There are multiple reasons for this lack of OS improvement in most of the published phase III trials. In several studies, cross-over to the experimental agent after the failure of the control arm was allowed. Many patients also gained access to new, presumably more active drugs during the regulatory window between completion of relevant trials and market access. This was well illustrated in the pivotal trial with sunitinib versus IFNa, where patients treated with IFNa in the comparator arm, had a better OS compared to historical IFNa data [Citation27].
Our retrospective analysis of OS depending on the year of the start of first-line therapy shows a significant OS improvement since 2006, even after correction for several well-known prognostic factors such as IMDC risk groups, CRP levels and the presence of brain or bone metastasis. One-year OS was only 49% in the IFNa era, while in the last period (2018–2020) one-year OS has increased to 73%. Five-year OS was only 7% in the IFNa era but is estimated around 40% in patients starting with systemic therapy from 2018 on.
Our primary outcome was OS since the start of systemic therapy in patients with metastatic disease or, less frequently, in patients with locally advanced disease. We did not study OS since the initial diagnosis or since the diagnosis of metastatic disease. In the latter case, OS could have improved due to earlier detection of the disease or the metastatic state. Another possible bias could be the fact that systemic therapy is started earlier in the course of the disease, now that more therapy lines are available. However, the IMDC risk score was evenly distributed over the years and the year of the start of therapy was independent of the IMDC score in the multivariate analysis, so changes in the decision to start therapy should not influence the OS benefit. We can conclude that the improvement in OS is a consequence of the availability of new, efficient drugs such as VEGFR-TKIs and ICI.
To our knowledge, this is the first study reporting data on the impact of systemic therapy on OS in mRCC patients as calculated from the start of systemic therapy in daily clinical practice. In the literature, several population-based studies suggest an OS improvement with the introduction of targeted therapies (VEGFR-TKIs and mTORi). However, these studies were performed before the introduction of ICIs and in three out of these four studies the proportion of patients treated with systemic therapies was low or unreported [Citation28–31].
In a retrospective cohort study including 4.217 Swedish patients diagnosed with mRCC between 2002 and 2005, 2006 and 2008 or 2009 and 2012, OS increased from 10 to 13 and 18 months, respectively. However, only 5.6% of the patients of the 2002–2005 cohort, 32.6% of the patients of the 2006–2008 cohort and 31% of the patients of the 2009–2012 cohort received targeted therapies [Citation28]. In 1.049 Danish mRCC patients referred to four hospitals for first-line treatment between 2006 and 2010, OS increased significantly from 11.5 months in 2006 to 17.2 months in 2010 (p = 0.04) in patients who were treated with systemic therapy (n = 744), whereas survival for untreated patients (n = 305) remained unchanged [Citation29]. In 1.678 Norwegian patients diagnosed with mRCC before (2002–2005) and after (2006–2008 and 2009–2011) introduction of targeted therapies, a significant OS-improvement was observed: mOS was 9, 12 and 14 months, respectively (p < 0.001). Only 41.6% of these patients received at least one targeted therapy, most commonly sunitinib [Citation30]. In 13.670 mRCC patients included in the Surveillance, Epidemiology and End Results (SEER) database (United States), mOS was significantly higher in patients diagnosed in the targeted therapy era (9 months) compared to those diagnosed in the pre-targeted therapy period (7 months). Three-year survival significantly improved from 11.9% in 2000–2005 period to 16.5% in 2006–2010. However, data on treatment regimens were not provided [Citation31]. None of these four studies has taken into account the possible impact of ICIs, as they were performed before ICI approval.
We were able to show that the mean number of lines of therapy administered to the patients increased significantly over time between 2000 and 2016, it may be assumed that the decrease in the last 4 years is due to the fact that many of these patients are still on first-line therapy. In the Danish study [Citation29], a significant increase in second-line treatment (20% versus 40%, p = 0.01) was observed between 2006 and 2010. An increase in therapeutic options will also be associated with a heightened interest of the medical community which possibly leads to more standardized care, more research and better clinical practice which may also impact the survival probabilities of an RCC patient.
Patients in clinical trials are off course selected and known to have a better prognosis than trial ineligible patients. Here we present real-world outcomes where we can show an OS benefit in an unselected population [Citation32].
Recently, new combinations of an ICI and a VEGFR-TKI such as pembrolizumab/axitinib, avelumab/axitinib and nivolumab/cabozantinib became available in first-line, showing important benefit compared to older standards of care [Citation5–7]. In the near future, new drugs (such as the HIF2a inhibitor belzutifan) will probably also be capable to improve outcomes. Therefore, the prognosis of m-ccRCC might improve even more in the coming years.
We conclude that we observed a significant improvement in OS in this real-world cohort of patients with m-ccRCC over the last 15 years, coinciding with the introduction of VEGFR-TKIs and ICIs. Even if a single new therapeutic agent could not demonstrate a significant OS benefit in its pivotal phase III trial, their cumulative impact did result in a clinically relevant OS benefit. Our data are useful when discussing the benefit-risk ratio of systemic therapies with patients and in order to encourage them when facing the metastatic disease.
Supplemental Material
Download MS Word (17.7 KB)Disclosure statement
B. Beuselinck received an honorarium from Merck, MSD, Pfizer, Bristol-Myers-Squibb, Ipsen and Astra-Zeneca. H. Wildiers’s institution received financial compensation for advisory boards and lecture fees from Immutep Pty, MSD, AstraZeneca Ireland, Daiichi, AbbVie, Lilly, PSI CRO AG, KCE, EISAI, AstraZeneca, Roche, Lilly, Congress care, Pfizer, ARIEZ, Sirtex, Roche, Pfizer, TRM Oncology, ORION Corporation, The Planning Shop, Novartis, Biocartes, Puma Biotech. H. Wildiers’s institution received an unrestricted research grant from Roche. Paul M. J. Clement received study budget funds from AstraZeneca; was an advisory board member for AbbVie, AstraZeneca, BMS, Daiichi-Sankyo, Leo Pharma, Merck Serono, MSD and Vifor Pharma. P. Debruyne received an honorarium from Bayer, Merck, MSD, Pfizer, Bristol-Myers-Squibb, Ipsen and Astra-Zeneca and research funding from Pfizer. H. Dumez’s institution received financial compensation for advisory boards and lecture fees from Seattle genetics, MSD, QED, Immunomedics, AstraZeneca, Hoffman-La Roche, Pfizer NV. P. Schöffski reports honoraria from Deciphera, Blueprint Medicines, Exelixis and Boehringer Ingelheim and research funding from PharmaMar, Novartis and Eisai.
References
- Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(5):706–720.
- Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378(9807):1931–1939.
- Verbiest A, Renders I, Caruso S, et al. Clear-cell renal cell carcinoma: molecular characterization of IMDC risk groups and sarcomatoid tumors. Clin Genitourin Cancer. 2019;17(5):e981–e994.
- Alonso-Gordoa T, García-Bermejo ML, Grande E, et al. Targeting tyrosine kinases in renal cell carcinoma: new bullets against old guys. Int J Mol Sci. 2019;20(8):1901.
- Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829–841.
- Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103–1115.
- Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–1127.
- Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–134.
- Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–124.
- Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28(6):1061–1068.
- Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer. 2010;116(18):4256–4265.
- Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1814–1823.
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced Renal-Cell carcinoma. N Engl J Med. 2015; 373(19):1803–1813.
- Choueiri TK, Halabi S, Sanford BL, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the alliance A031203 CABOSUN trial. J Clin Oncol. 2017;35(6):591–597.
- Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–1290.
- Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16(15):1473–1482.
- Motzer RJ, Nosov D, Eisen T, et al. Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: results from a phase III trial. J Clin Oncol. 2013;31(30):3791–3799.
- Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271–2281.
- Nuzzo PV, Pond GR, Abou Alaiwi S, et al. Conditional immune toxicity rate in patients with metastatic renal and urothelial cancer treated with immune checkpoint inhibitors. J Immunother Cancer. 2020;8(1):e000371.
- Su Y, Fu J, Du J, et al. First-line treatments for advanced renal-cell carcinoma with immune checkpoint inhibitors: systematic review, network meta-analysis and cost-effectiveness analysis. Ther Adv Med Oncol. 2020;12:1758835920950199.
- Zhang T, Gong J, Maia MC, et al. Systemic therapy for non-clear cell renal cell carcinoma. Am Soc Clin Oncol Educ Book. 2017;37:337–342.
- Beuselinck B, Oudard S, Rixe O, et al. Negative impact of bone metastasis on outcome in clear-cell renal cell carcinoma treated with sunitinib. Ann Oncol. 2011;22(4):794–800.
- Beuselinck B, Vano YA, Oudard S, et al. Prognostic impact of baseline serum C-reactive protein in patients with metastatic renal cell carcinoma (RCC) treated with sunitinib. BJU Int. 2014;114(1):81–89.
- Roussel E, Kinget L, Verbiest A, et al. C-reactive protein and neutrophil-lymphocyte ratio are prognostic in metastatic clear-cell renal cell carcinoma patients treated with nivolumab. Urol Oncol. 2021;39(4):239.e17–239.e25.
- Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27(34):5794–5799.
- Hsieh JJ, Purdue MP, Signoretti S, et al. Renal cell carcinoma. Nat Rev Dis Primers. 2017;3:17009.
- Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27(22):3584–3590.
- Lindskog M, Wahlgren T, Sandin R, et al. Overall survival in Swedish patients with renal cell carcinoma treated in the period 2002 to 2012: update of the RENCOMP study with subgroup analysis of the synchronous metastatic and elderly populations. Urol Oncol. 2017;35(9):541.e15–541.e22.
- Soerensen AV, Donskov F, Hermann GG, et al. Improved overall survival after implementation of targeted therapy for patients with metastatic renal cell carcinoma: results from the Danish renal cancer group (DARENCA) study-2. Eur J Cancer. 2014;50(3):553–562.
- Beisland C, Johannesen TB, Klepp O, et al. Overall survival in renal cell carcinoma after introduction of targeted therapies: a Norwegian population-based study. OTT. 2017;10:371–385.
- Li P, Wong YN, Armstrong K, et al. Survival among patients with advanced renal cell carcinoma in the pretargeted versus targeted therapy eras. Cancer Med. 2016;5(2):169–181.
- Heng DY, Choueiri TK, Rini BI, et al. Outcomes of patients with metastatic renal cell carcinoma that do not meet eligibility criteria for clinical trials. Ann Oncol. 2014;25(1):149–154.