Abstract
Objectives
This study aims to report on the effectiveness of voice rehabilitation following radiotherapy for laryngeal cancer in a long-term perspective, i.e., up to three years after completion of radiotherapy.
Methods
The study included a total of 74 patients that were randomised into an intervention group (n = 37) or a control group (n = 37). Voice recordings with blinded assessment of voice quality with the GRBAS protocol (Grade, Roughness, Breathiness, Asthenia, Strain) and acoustic analysis was performed at baseline, 12 and 36 months following radiotherapy. Voice rehabilitation was performed in 10 sessions immediately following completion of radiotherapy. Patients also filled out the Swedish Self-Evaluation of Communication Experiences after Laryngeal cancer.
Results
The S-SECEL demonstrated statistically significant improvements in the intervention group when comparing baseline and 36 months, and no changes in the control group. Acoustic measures did not reveal any significant changes. The perceptual analysis demonstrated that when comparing the changes within the groups between baseline and 36 months there were statistically significant differences between the intervention and control group regarding the voice qualities Roughness, Breathiness and Strain. In the control group, 50% demonstrated deterioration in roughness, while in the intervention group only 7% deteriorated during this time. In Breathiness and Strain, 57 and 50%, respectively, improved in the intervention group, while only 32% and 23% improved, respectively, in the control group.
Conclusion
Voice rehabilitation following radiotherapy for laryngeal cancer demonstrate positive effects in patient reported outcomes and perceptual measures of voice quality, and the effects remain up to three years following radiotherapy.
Introduction
With a total of 157 000 new cases annually, laryngeal cancer accounts for 1.1% of all cancer diagnoses [Citation1]. Radiotherapy is the most common treatment and whilst survival rates are high, patients are often left with a sequalae of reduced voice function and quality with roughness as a predominant feature [Citation2], which may be present in up to 95% of patients 10 years following completion of radiotherapy [Citation3].
Fex et al. [Citation4] concluded in 1969 that voice training given concomitant with radiotherapy was effective for their 15 patients irradiated for laryngeal cancer. Despite promising results over 50 years ago, only a handful of studies have to date examined the efficacy of voice rehabilitation in a randomised manner. La Mantia et al. and Angadi compared vocal functional exercises + vocal hygiene advice to vocal hygiene advice alone in their 19 and 12 patients respectively and showed that patients in the intervention group reported better voice scores directly after the voice therapy, as measured by the Voice Handicap Index (VHI) when compared to the control groups [Citation5,Citation6]. Van Gogh et al. [Citation7] also reported improvements in VHI in their 12 patients up to 13 months post-completion of voice rehabilitation.
In response to the small study populations, Karlsson et al. published a randomised study evaluating the effects of voice rehabilitation in 74 patients immediately following radiotherapy completion [Citation8] and with a 12 months follow-up [Citation9]. The studies concluded that patients receiving voice rehabilitation significantly improved their communicative ability, health-related quality of life as well as did not show the deterioration in perceptual quality of roughness observed in the control group and that these positive findings were maintained at 1 year following radiotherapy. Millgård and Tuomi [Citation10] reported on the same cohort at 24 months, demonstrating that perceptual voice quality of the control group was significantly inferior to that of healthy controls, whereas the intervention group only differed from healthy controls in 3 out 5 parameters.
Despite these positive findings, no study exists that investigates the effects of voice rehabilitation on patient-reported outcome combined with perceptual and acoustic measures long-term, i.e., > 1 year post-voice therapy. Hence, this study aims to fill this gap by investigating if voice rehabilitation following irradiated laryngeal cancer is effective 3-years following radiotherapy completion.
Materials and methods
Study participants
All patients diagnosed with laryngeal cancer receiving curatively intended radiotherapy with or without chemotherapy during 2000–2011 (including a two-year interruption) in Gothenburg, Sweden were asked for study participation. Further inclusion criteria were >18 years of age, primary malignancy, adequate cognitive ability and sufficient Swedish language competency to independently fill out questionnaires and partake in the voice rehabilitation.
Study design
This study is a continuation of a randomised control study that has previously been reported according to different time perspectives: up to 6 months [Citation8], 12 months [Citation9] and 24 months [Citation10,Citation11] following radiotherapy.
Following study approval, patients were randomised into either an intervention or a control group. Computerised randomisation was performed by optimal allocation using Pocock’s sequential randomisation method [Citation12] applied to age, tumour site, smoking habits, tumour size and communication function pre-radiotherapy. Dysphonia was used as the main variable when determining sample size based on an 80% power calculation. Patients were followed at baseline (prior to voice rehabilitation and approximately one month following radiotherapy completion), 12 months and 36 months post-radiotherapy. During each visit, patients filled in questionnaires measuring communication function and a voice recording was made, which was then analysed acoustically and perceptually. Comorbidities were measured at baseline according to the Adult Comorbidity Evaluation (ACE-27) [Citation13].
Intervention
The intervention group were given voice rehabilitation by speech-language pathologists in a structured manner. It consisted on 10 sessions totalling 10 weeks and occurred between one and six months post-radiotherapy completion. Each session was specified beforehand, and included breathing, relaxation and phonation exercises. The voice rehabilitation protocol has been described in detail elsewhere [Citation14].
Oncologic treatment
Oncologic treatment was administered as either conventional or hyperfractionated radiotherapy according to the regional treatment guidelines (). The conventional radiotherapy was given once daily in 2–2.4 Grey (Gy) fractions to a total of 62.4–68 Gy. The hyperfractionated radiotherapy consisted of 1.7 Gy fractions given twice daily to a total of 64.6 Gy. Irradiation to the lymph nodes was generally given to patients with T2–T4 tumours (n = 25). Some T3 and T4 tumours also received induction chemotherapy (n = 3).
Table 1. Sociodemographic and clinical data for the study population at baseline and at the 36 months follow-up.
Swedish Self-Evaluation of communication experience after laryngeal cancer (S-SECEL)
The S-SECEL is a 35-item questionnaire adapted to assess communicative function for patients treated for laryngeal cancer. Items are divided into three domains; General, Environmental and Attitudinal as well as a Total score. Items are rated on a four-point Likert scale ranging from never (0) to always (3) and recalls the last 30 days. The cumulative domain scores range from 0–15 for General, 0–42 for Environmental, 0–45 for Attitudinal and 0–102 for the Total domain, where a higher score indicates greater perceived communicative dysfunction. The last question (item 35) ‘Do you speak the same amount now as before your laryngeal cancer’ is answered as Yes/More/Less and is not included in the scoring system [Citation15–17]. Guidelines for interpreting the results using clinically significant differences, and what scores indicates acceptable communicative function have been developed [Citation18]. A total score of ≥20 points indicates the need for voice rehabilitation, and a reduction of 13 points in the Total domain indicates a clinically significant improvement, and an 8 point increase indicates a clinically significant deterioration.
Voice recordings
Voice recordings consisted of the reading of a standard passage and the maximum sustained vowel/a/repeated three times. A headset microphone (Sennheiser MKE 2-p) was set at a distance of 12 cm from the corner of the mouth. Recordings were made at a sampling frequency of 44.1 kHz with a Panasonic Professional Digital Audio Tape (DAT) Recorder SV-3800. Prior to analysis, all recordings were transferred from a DAT to a computer hard drive as an audio file (.wav) using the program Swell Soundfile Editor, version 4.5 (Electronix Hitech).
Acoustic and temporal voice analysis
Acoustic analysis was carried out using Voxalys, a plug-in to the programme Praat. Jitter and shimmer represent irregularities of the waveform from one period to the next, in frequency and amplitude respectively. Harmonics-to-noise ratio (HNR) relates to the relative amount of harmonics and noise in the voice, often caused by turbulence at the vocal fold level due to inadequate vocal fold closure [Citation19]. Jitter, shimmer and HNR were measured from a two-second excerpt in the middle of the second sustained/a/. Maximum phonation time (MPT) reflects the longest time a person can sustain a vowel in one exhalation. It was measured from the sustained vowels, where highest value from three repetitions was used.
Perceptual voice analyses
Two speech-language pathologists performed the perceptual ratings and a third clinician used for consensus rating. All raters attended a half-day’s consensus training. Rating files consisted of two sentences of the reading and the second sustained vowel from each time-point in the study, with anchor samples interspersed at every 20 voice samples. A total of 10 percent of the samples were randomly chosen to be duplicated for intra-rater reliability.
Ratings were blinded to patient status and the GRBAS rating protocol was used [Citation20], consisting of five voice qualities was used: grade, roughness, breathiness, asthenia and strain. Each voice quality is rated on a 4-point scale, where 0 = normal, 1 = mildly impaired, 2 = moderately impaired and 3 = severely impaired.
Statistical analysis
All analyses were performed using IBM SPSS version 25. Mean and standard deviation (SD) of the mean were used for descriptive purposes. Change over time was analysed with Sign test for dichotomous and ordered categorical variables and with Wilcoxon Signed Rank test for continuous variables. For comparison between two groups Fisher’s exact test was used for dichotomous variables, Chi-square test for non-ordered categorical variables, Mantel-Haenszel Chi square test for ordered categorical variables and Mann Whitney U test for continuous variables. All significance tests were two-sided and the significance level set at 0.05. Inter- and intra-rater reliability was calculated using percent exact agreement, percent close agreement and weighted kappa and was interpreted using Landis and Koch guidelines [Citation21].
Ethical considerations
The study was conducted according to the Declaration of Helsinki and was approved by the Regional Ethic Review Board in Gothenburg, Sweden. Written informed consent for participation in the study was obtained from all participants. A part of the study population has been described previously [Citation8,Citation9,Citation14].
Results
Study participants
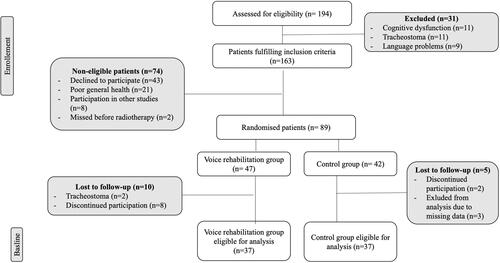
A total of 194 patients were assessed for eligibility of which 31 patients were excluded due to tracheostoma, insufficient cognitive abilities or inadequate language abilities, leaving 163 patients. Of these, 74 were deemed non-eligible, reasons specified in , yielding 89 patients available for randomisation into a voice rehabilitation group (n = 47) and a control group (n = 42). Twelve patients were lost to follow-up during voice rehabilitation and three recordings were missed, resulting in 37 patients in each group available for analysis at baseline.
At 36 months, 22 patients from the intervention group and 15 from the control group were lost to follow-up due to laryngectomy (n = 8), tracheostoma (n = 1), deceased (n = 6) poor general health (n = 4), missed recordings (n = 4) and patient choice/unspecified reason (n = 14).
In , patient characteristics at baseline and at the 36-month follow-up is presented. No statistically significant differences were found between the groups regarding sex, age, tumour localisation, classification or stage, radiotherapy regimen, smoking status or comorbidities at either study occasion.
Swedish Self-Evaluation of Communication Experience after Laryngeal cancer
The intervention group reported significantly worse scores at baseline compared to the control group in domains Environmental, Attitudinal and Total Score (), which improved significantly by the 36-months follow-up. Improvements in the Environmental domain was significantly greater between baseline and 36 months than that in the control group (p = 0.03), whilst those of domains Attitudinal and Total Score nearly reached significant values (p = 0.07). There was no observed statistically significant change in any domain during the study period in the control group.
Table 2. S-SECEL scores presented as mean and standard deviation (SD) at baseline, 12 months and 36 months post-radiotherapy for the intervention and control groups.
In the intervention group, the mean scores in all S-SECEL domains demonstrated values above the threshold indicating the need for voice rehabilitation at baseline. All mean values at 12 and 36 months were below the threshold, except for the General domain at 36 months, which was just above the need for voice rehabilitation. In the control group, at baseline all domains except the Environmental domain indicated the need for vocal rehabilitation, values that remained at 12 months. At 36 months, all domain scores were below the threshold values. Regarding the changes found in the groups, the intervention group demonstrated clinically significant improvements between baseline and 36 months in the Environmental, Attitudinal and Total domain. No clinically significant changes were observed between 12 and 36 months in the intervention group. The control group demonstrated no clinically significant changes during the study period.
Acoustic and temporal analysis
The acoustic and temporal analyses are reported in . At baseline, the intervention group had a significantly higher MPT compared to the control group. For the intervention group, the MPT decreased at the 36 months follow-up (p = 0.04). When comparing the change in MPT from baseline to 36 months between the groups, this reached a statistically significant difference (p = 0.02). Besides this finding, there were no statistically significant differences between or within groups during the study period.
Table 3. Acoustic and temporal measures presented as mean and standard deviation (SD) at baseline, 12 months and 36 months post-radiotherapy for the control and intervention group.
Perceptual data
There was no statistically significant difference between the control and intervention group at baseline in any of the measured qualities of the GRBAS (data not shown). At 12 months post-radiotherapy the control group demonstrated significantly higher degrees of moderate-severe roughness (54% vs 31%). At 36 months, 86% of the intervention group had none-mild roughness compared to 57% in the control group, albeit non-significant. More patients in the intervention group had moderate-severe breathiness compared to the control group (37% vs 17%, p = 0.02) at the 12-month follow-up. No other statistically significant differences were found between the groups regarding the perceptual evaluations at any of the study occasions.
demonstrates the changes in perceptually evaluated voice qualities over time. The results showed that at 36 months post-radiotherapy compared to baseline, 50% of the control group demonstrated deterioration in roughness compared to 7% in the intervention group. At the same time, improvement was demonstrated in 23% of the control group, while only 14% of the intervention group improved (p = 0.03). In the intervention group a majority (79%) remained unchanged regarding roughness during the same period of time. Breathiness also demonstrated statistically significant differences, where at 36 months follow-up 57% of patients in the intervention group improved compared to only 32% in the control group (p = 0.04). Regarding strain, this too demonstrated a statistically significant difference between the groups (p = 0.02) where a total of 50% of the intervention group demonstrated improvement, while only 23% demonstrated improvement in the control group. When comparing the changes between 12 and 36 months, no statistically significant differences were found between the groups.
Table 4. Perceptual evaluation: Changes within the control- and intervention group during the study time-points.
The weighted kappa for intra rater reliability was 0.70, indicating substantial agreement with 71% percent exact agreement and 95% percent close agreement.
Discussion
The positive short-term efficacy of voice rehabilitation following irradiation for laryngeal cancer is well-established, whilst long-term effects are completely lacking. This study is the first to present data from a randomised controlled trial up to three years following rehabilitation after radiotherapy for laryngeal cancer.
The study confirms that the positive effects of voice rehabilitation established in other short-term RCTs (up to 12 months following rehabilitation) [Citation5–7,Citation9,Citation14], remain at three years when utilising patient-reported and perceptual measures. A prior study reporting the results from this cohort short-term, i.e., up to 12 months found significant improvement in 3 out of 4 S-SECEL domains when compared to the control group at 12 months post-radiotherapy, which have now been established to last until 3 years post-radiotherapy. The present study also indicated that the patients in the intervention group, according to the S-SECEL scores, did not indicate the need for voice rehabilitation at 12 months, results which remained at 36 months. There were also clinically significant improvements in the intervention group comparing baseline and 36 months. The control group indicated the need for voice rehabilitation in some domains at 12 months, however, at 36 months, the need was no longer present, even though no clinically significant changes occurred. This may be a result of natural recovery. However, these results indicate that communicative function improves faster following early voice rehabilitation, compared to the possibly natural recovery that may have been the case in the control group. The only contradictory study to the effectiveness of voice rehabilitation is the recently published systematic review published by Taito et al. [Citation22], which concluded that voice rehabilitation might not improve vocal function. This was disputed and deemed misleading information in a Letter to Editor by Karlsson et al. [Citation23], as the three RCTs that constituted the foundation of the review all concluded the opposite, i.e., positive effects of voice training.
Previous studies demonstrated short-term effects regarding roughness, i.e., up to two years following radiotherapy, where moderate and severe grades of roughness increased mainly between 6 and 12 month post-radiotherapy for the control group, but remained stable for the intervention group [Citation9,Citation10]. The present study demonstrated voice rehabilitation to some extent appears to prevent deterioration in roughness up to three years following voice training, as 50% deteriorated in the control group when comparing baseline value to follow-up at 36 months. For the corresponding time-point, only 7% of the patients of the intervention group deteriorated, and 80% remained unchanged. However, improvements were seen in the control group to a greater extent than in the intervention group during the same time point, making conclusions regarding roughness difficult to draw. Most of the changes seem to appear before 12 months, as no further significant changes in perceptual measures appeared when comparing the changes between 12 and 36 months. Millgård and Tuomi highlighted that, at 24 months, patients who had received voice therapy had a voice quality equivalent to healthy controls, whereas the control group still deviated considerably [Citation10]. As radiotherapy causes fibrosis and inflammation affecting the vocal cords [Citation24], it has been hypothesised that rehabilitative exercises can partly prevent fibrotic progression, thereby explaining the lack of deterioration in the intervention group. Voice rehabilitation also teaches laryngeal relaxation, which may counteract the post-radiotherapy tissue tension and hyperfunctional vocal behaviour. This is somewhat reflected in the results regarding strain, where 50% in the intervention group improve regarding strain from baseline to 36 months, compared to 23% in the control group.
Voice therapy appears to initially cause increased voice breathiness, which, in comparison to the control group, improved significantly at the study end-point, compared to baseline. The initial effect may be caused by the elements included in the voice rehabilitation protocol, whereby easy-onset and breathy-voice techniques may generate a greater degree of vocal breathiness [Citation10]. Additionally, Holmberg et al. concluded that an increased breathiness may be reflective of a decrease in phonation hyperfunction and thereby actually indicative of a voice improvement [Citation25].
The present study did not demonstrate any significant difference or changes regarding the acoustic measures jitter, shimmer or harmonics-to-noise ratio. Acoustic variables may not be optimal variables when quantifying vocal character change as no significant differences were found despite changes in PRO and perceptual outcome measures. Both Karlsson et al. and Millgård and Tuomi lacked statistically significant acoustic finding in their studies on voice rehabilitation in laryngeal cancer patients [Citation9,Citation10]. Other studies, such as Van Gogh et al. [Citation7] and La Mantia et al. [Citation6], only found significant improvements in single acoustic measures (jitter and shimmer respectively) following vocal functional exercises. Acoustic measurements are difficult to perform as they are sensitive to mouth-to-microphone distance, analysis software, recording equipment, patient effort and the vowel or speech sample used [Citation26]. Dysphonic voices are also aperiodic, further decreasing the reliability of measurements [Citation27]. Therefore, acoustic variables may not be sensitive or suitable in order to detect changes in severely dysphonic voices in this patient population.
Limitations
This study is limited by the statistically significant difference between the intervention and control groups in S-SECEL data at baseline. However, in the previously published one-year follow-up of this cohort [Citation9], statistical analysis was used to adjust for differing baseline values and significant improvements in the domains Environmental and Total Score still remained. This indicates a larger improvement of communicative function in the intervention group despite the difference in baseline values, and that this difference is likely to remain at 36 months. An additional limitation is the reduced number of participants over time, especially in the intervention group, where only a total of 37 participants (15 in the intervention group, and 22 in the control group) remain at three years which hampers definite conclusions. However, to date, no other study exists regarding the long-term effects of voice rehabilitation in laryngeal cancer patients.
Clinical implications
It has been established that vocal function following radiotherapy is abnormal up to 10-years following oncologic treatment [Citation3]. Voice therapy implemented early, i.e., before 12 months following completion of radiotherapy, appears to have lasting measurable effects on perceptual and self-reported voice outcomes as long as three years after completion. Therefore, it could be of great benefit to implement early voice therapy for laryngeal cancer patients following radiotherapy, using perceptual and self-reported communicative function measures for evaluation before and after treatment.
Conclusions
The positive effects of voice rehabilitation following radiotherapy for laryngeal cancer remain up to three years following therapy, confirmed by the intervention group reporting improved communicative function and improved perceptual qualities of breathiness and strain, and lack of deterioration of roughness compared to the control group. The control group demonstrated some degree of improvement regarding roughness during the same period of time, making conclusions regarding roughness somewhat difficult to interpret, but may be due to natural recovery over time. Acoustic analysis is not recommended to detect change over time or in relation to intervention in this patient group.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86.
- Bibby JR, Cotton SM, Perry A, et al. Voice outcomes after radiotherapy treatment for early glottic cancer: assessment using multidimensional tools. Head Neck. 2008;30(5):600–610.
- Morgan DA, Robinson HF, Marsh L, et al. Vocal quality 10 years after radiotherapy for early glottic cancer. Clin Radiol. 1988;39(3):295–296.
- Fex S, Henriksson B. Phoniatric treatment combined with radiotherapy of laryngeal cancer for the avoidance of radiation damage. Acta Otolaryngol Suppl. 1969;263:128–129.
- Angadi V. Investigating the efficacy of vocal function exercises in improving vocal function in adults irradiated for laryngeal cancers: a three part dissertation; 2016.
- La Mantia I, Cupid F, Andaloro C. Vocal function exercise and vocal hygiene combined treatment approach as a method of improving voice quality in irradiated patients for laryngeal cancers. Acta Med Mediterr. 2018;34:525–529.
- van Gogh CD, Verdonck-de Leeuw IM, Langendijk JA, et al. Long-term efficacy of voice therapy in patients with voice problems after treatment of early glottic cancer. J Voice. 2012;26(3):398–401.
- Karlsson T, Johansson M, Andrell P, et al. Effects of voice rehabilitation on health-related quality of life, communication and voice in laryngeal cancer patients treated with radiotherapy: a randomised controlled trial. Acta Oncol. 2015;54(7):1017–1024.
- Karlsson T, Tuomi L, Andrell P, et al. Effects of voice rehabilitation after radiotherapy for laryngeal cancer: a longitudinal study. Logoped Phoniatr Vocol. 2017;42(4):167–177.
- Millgård M, Tuomi L. Voice quality in laryngeal cancer patients: a randomized controlled study of the effect of voice rehabilitation. J Voice. 2020;34(3):486 e13–486. e22.
- Tuomi L, Karlsson T. Voice quality, function, and quality of life for laryngeal cancer: a prospective longitudinal study up to 24 months following radiotherapy. Ear Nose Throat J. 2020;145561320929941.
- Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–115.
- Piccirillo JF, Spitznagel EL, Vermani N, et al. Comparison of comorbidity indices for patients with head and neck cancer. Med Care. 2004;42(5):482–486.
- Tuomi L, Andréll P, Finizia C. Effects of voice rehabilitation after radiation therapy for laryngeal cancer: a randomized controlled study. Int J Radiat Oncol Biol Phys. 2014;89(5):964–972.
- Tuomi L, Johansson M, Andréll P, et al. Interpretation of the Swedish self evaluation of communication experiences after laryngeal cancer: cutoff levels and minimum clinically important differences. Head Neck. 2016;38(5):689–695.
- Finizia C, Bergman B, Lindstrom J. A cross-sectional validation study of Self-Evaluation of communication experiences after laryngeal cancer–a questionnaire for use in the voice rehabilitation of laryngeal cancer patients. Acta Oncol. 1999;38(5):573–580.
- Johansson M, Ryden A, Finizia C. Self evaluation of communication experiences after laryngeal cancer - a longitudinal questionnaire study in patients with laryngeal cancer [research support, Non-U.S. Bmc Cancer. 2008;8:80.
- Tuomi L, Johansson M, Andrell P, et al. Interpretation of the Swedish Self-Evaluation of communication experiences after laryngeal cancer (S-SECEL): cutoff levels and minimum clinically important differences. Head Neck. 2016;38(5):689–695.
- Ferrand CT. Harmonics-to-Noise ratio: an index of vocal aging. J Voice. 2002;16(4):480–487.
- Hirano M. Clinical examination of voice. London: Springer Limited; 1981.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174.
- Taito M, Taito S, Banno M, et al. Voice rehabilitation for laryngeal cancer after radiotherapy: a systematic review and Meta-analysis. Eur Arch Otorhinolaryngol. 2019;276(6):1573–1583.
- Karlsson T, Johansson M, Andrell P, et al. Letter to editor: Misleading conclusions in the recently published study by taito et al. "voice rehabilitation for laryngeal cancer after radiotherapy: a systematic review and Meta-analysis". Eur Arch Otorhinolaryngol. 2019;276(11):3253–3254.
- Karlsson T, Bergström L, Ward E, et al. A prospective longitudinal study of voice characteristics and health-related quality of life outcomes following laryngeal cancer treatment with radiotherapy. Acta Oncol. 2016;55(6):693–699.
- Holmberg EB, Ihre E, Södersten M. Phonetograms as a tool in the voice clinic: Changes across voice therapy for patients with vocal fatigue. Logopedics Phoniatrics Vocology. 2007;32(3):113–127.
- Oridate N, Homma A, Suzuki S, et al. Voice-related quality of life after treatment of laryngeal cancer. Arch Otolaryngol Head Neck Surg. 2009;135(4):363–368.
- Carding PN, Wilson JA, MacKenzie K, et al. Measuring voice outcomes: state of the science review. J Laryngol Otol. 2009;123(8):823–829.