Abstract
Objective
To evaluate the prevalence of comorbid chronic conditions among Canadian adults with cancer and the impact of socioeconomic background on the distribution of these conditions.
Methods
Canadian Community Health Survey (CCHS) 2017–2018 dataset was accessed and individuals with complete information about cancer history were reviewed. The prevalence of the following 10 chronic conditions was reviewed: asthma, chronic obstructive pulmonary disease, arthritis, hypertension, hypercholesterolemia/hyperlipidemia, heart disease, stroke, diabetes, mood disorder, and anxiety disorder. Stratification of the prevalence was done according to age, sex, and racial subgroups. Multivariable logistic regression analysis was done to evaluate the association between sociodemographic characteristics and having multiple comorbid conditions.
Results
A total of 104,362 participants were included in the current study (including 10,782 participants with a history of cancer; and 93,580 participants without a history of cancer). Among all age, sex, and race strata, participants with a history of cancer were more likely to have multiple chronic conditions (p < 0.05 for all comparisons). The most common three individual comorbid conditions among participants with cancer were arthritis (40.2%), hypertension (36.1%), and hypercholesterolemia (25.2%); while the most common cancer-comorbidity triad among participants with cancer was cancer/arthritis/hypertension (17.7%). In a multivariable logistic regression analysis among participants with cancer, the following sociodemographic factors were associated with having multiple comorbid conditions: older age (OR for age 80+ versus age 18–20 years: 8.32; 95% CI: 5.17–13.39), indigenous racial group (OR: 1.94; 95% CI: 1.43–2.63) and lower income (OR for income ≥80,000 Canadian dollars (CAD) versus income: ≤20,000 CAD: 0.29; 95% CI: 0.23–0.37).
Conclusion
History of cancer is associated with a higher probability of many comorbid conditions. This excess comorbidity burden seems to be unequally shouldered by individuals in the lower socioeconomic stratum as well as minority populations.
Keywords:
Introduction
Cancer is a major cause of morbidity and mortality in Canada with approximately one in two Canadians is estimated to be diagnosed with cancer in their lifetime and one out of four Canadians is expected to die because of cancer [Citation1–3]. Administering effective anticancer treatment(s) can be challenged by many patient-related factors; most notably comorbidities which can limit the ability of the treating physicians to administer potentially life-prolonging and/or curative treatments [Citation4,Citation5]. Thus, identification of the patterns of comorbid chronic conditions among cancer patients, on a population-level, is very important when planning effective anticancer treatment strategies [Citation6]. Moreover, socioeconomic disparities is likely to affect comorbidity patterns among cancer patients and can play an important role in the poor cancer survival reported for some socioeconomic groups [Citation7]. It is thus important to understand the factors at play in driving disparities in comorbidity patterns among cancer patients.
Studying comorbid conditions among cancer patients can be challenged by the fact that most available cancer registries do not provide detailed information about comorbidities [Citation8,Citation9]. Thus, population-based surveys might be a better alternative to provide nationally representative estimates of different comorbid conditions among cancer patients. Canadian Community Health Survey (CCHS) is the largest representative national health survey in Canada with information about cancer history, sociodemographic factors as well as a variety of common comorbid chronic conditions. Thus, it represents an ideal vignette to study this research question in Canada.
Objective
To evaluate the prevalence of comorbid chronic conditions among Canadian adults with cancer and the impact of socioeconomic background on the distribution of these conditions.
Methods
Data sources
CCHS is the largest Canadian national cross-sectional health survey aiming to provide an updated view of the health behaviors, experiences, and attitudes of Canadians. It is conducted on an annual basis through Statistics Canada in collaboration with several federal and provincial health authorities [Citation10]. Approximately, 97% of Canadian residents are represented in this survey; and the following subsets are not represented: (1) some residents of indigenous settlements; (2) members of the Canadian armed forces; (3) residents of some Northern Quebec health regions. This survey follows a multi-stage sampling strategy to ensure adequate representation of all Canadian health regions. It relies on area frame (for residents 18 years and above), and child tax benefit frame (for residents 12–17 years old). CCHS datasets are released on a bi-annual basis, and the current study is based on the CCHS dataset for 2017–2018 (the most recent CCHS release at the time of writing of this manuscript). The response rate for CCHS 2018 is 58.8%
Patients were considered eligible for the current study if they are 18+ years at the time of survey completion and have complete information about the history of a cancer diagnosis. A cancer diagnosis is identified within CCHS through the use of two questions: do you have cancer? and have you ever been diagnosed with cancer (lifetime)? For the sake of the current study, a participant with cancer would be any participant who answered yes to any of these two questions. Cancer status, type, and treatments are not reported within CCHS.
As per article 2.2 of the tri-council policy statement (TCPS2), ethical approval was not required for this study (as this study was based on an open license, anonymized dataset) [Citation11].
Data collection
The following data were collected from each included participant where available: age at survey completion, sex, race (white, indigenous, or nonwhite, non-indigenous), smoking history, body mass index, income in Canadian dollars (CAD), marital status, perceived health, perceived mental health, and province/territory of residence. Age was reported within the CCHS as a categorical variable (in 5 years groups) not as a continuous variable. The following 10 chronic conditions were reported in the CCHS 2017–2018 dataset and thus were included in the current analysis: asthma, chronic obstructive pulmonary disease (COPD), arthritis (e.g., osteoarthritis, rheumatoid arthritis, gout), hypertension, hypercholesterolemia/hyperlipidemia, heart disease, stroke, diabetes, mood disorder (including depression, bipolar disorder, mania, and dysthymia), and anxiety disorder (phobia, obsessive–compulsive disorder, and panic disorder). The questions about comorbidities were meant to represent conditions diagnosed by healthcare providers. Thus, perceived mental health as a variable is different from comorbid mood or anxiety disorder. All the above information was based on self-report by included participants.
Statistical analysis
The two main hypotheses of the current study are: (1) individuals with a history of cancer are more likely to have comorbid chronic conditions; (2) there is a variability in the patterns of chronic conditions among individuals with cancer according to their baseline sociodemographic characteristics.
To account for the complex sampling strategy of the CCHS, sampling weights of the CCHS were incorporated in all of the current study analyses. Differences in baseline demographics between participants with or without a history of cancer were initially described through descriptive statistics with Chi-squared testing. The prevalence of each of the 10 chronic conditions was then evaluated among participants with or without cancer. The prevalence of multiple chronic conditions (> 2 conditions) was then calculated among different strata of the study cohort according to age, sex, and race among participants with or without cancer.
The prevalence of cancer-comorbidity triads (i.e., cancer in addition to two specific chronic conditions in a single participant) was then evaluated among participants with cancer. Multivariable logistic regression analyses were then conducted to analyze the association between history of cancer and history of the 10 comorbid conditions. Each of these analyses was adjusted for age, sex, and racial background. The prevalence of specific chronic conditions among specific age, sex, and race substrata of participants with cancer was then evaluated. Another multivariable logistic regression analysis was then added to assess the association between sociodemographic characteristics (age, sex, race, and income) and having multiple comorbid conditions (>2 conditions of the studied 10 chronic conditions) among participants with cancer. Association between perceived health and perceived mental health and different comorbidities were then examined through Chi-squared testing.
All the above statistical analyses were conducted through STATA software (version 14.0, STATA Corps, College Station, TX, USA).
Results
Participants’ characteristics
Participant selection flow chart is summarized in . A total of 104,362 participants were included in the current study (including 10,782 participants with a history of cancer; and 93,580 participants without a history of cancer). Comparing both groups of participants together, participants with a history of cancer were likely to be older (p < 0.001), females (p < 0.001), married (p < 0.001), white (p < 0.001), have poorer self-perceived health (p < 0.001), obese (p < 0.001), have lower income (p < 0.001), and ever smokers (p < 0.001) ().
Figure 1. Percentage of Canadian adults with >2 conditions of the studied 10 chronic conditions: (a) all participants; (b) participants with a history of cancer.
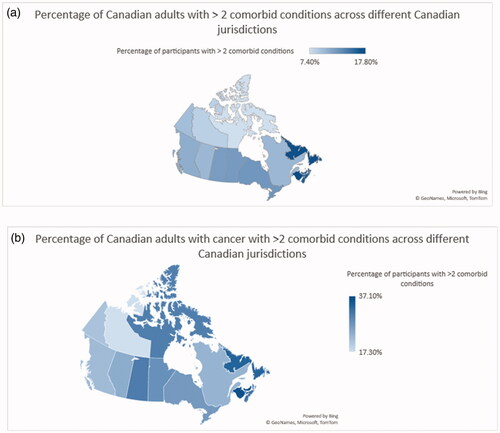
Table 1 Baseline demographics according to history of cancer.
Prevalence of chronic conditions among participants with or without cancer
Comparing both groups together, participants with a history of cancer were likely to have asthma (p = 0.02), COPD (p < 0.001), arthritis (p < 0.001), hypertension (p < 0.001), hypercholesterolemia (p < 0.001), heart disease (p < 0.001), stroke (p < 0.001), diabetes (p < 0.001), and a mood disorder (p < 0.001). The prevalence of these chronic conditions among participants with or without a history of cancer was further detailed in Supplementary Table 1. The most common three comorbid conditions among participants with cancer were arthritis (a prevalence of 40.2%), hypertension (a prevalence of 36.1%), and hypercholesterolemia (a prevalence of 25.2%).
The prevalence of multiple chronic conditions (i.e., > 2 conditions of the studied 10 chronic conditions) was further examined among specific strata of the cohort (according to age, sex, and race) among participants with or without cancer. Among all age, sex, and race strata, participants with a history of cancer were more likely to have multiple chronic conditions (as detailed in Supplementary Table 2) (p < 0.05 for all comparisons).
The percentage of Canadian adults with more than two conditions of the studied 10 chronic conditions was then examined within different Canadian provinces and territories (for all participants as well as for participants with a history of cancer). For all participants, New Brunswick has the highest prevalence of multiple chronic conditions at 17.8% while Northwest Territories has the lowest prevalence at 8.5% (). For participants with a history of cancer, Prince Edward Island has the highest prevalence of multiple chronic conditions at 37.1% while Northwest Territories has the lowest prevalence at 17.3% ().
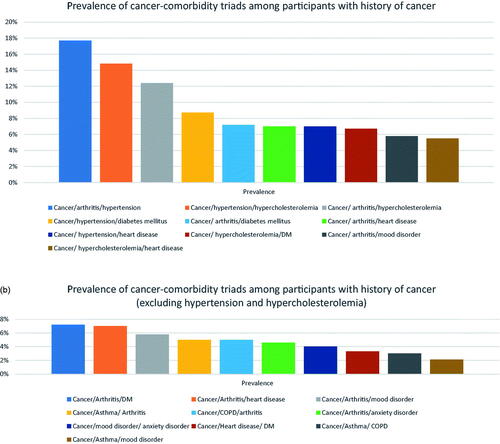
The most common cancer-comorbidity triads among participants with cancer include cancer/arthritis/hypertension (a prevalence of 17.7%), cancer/hypertension/hypercholesterolemia (a prevalence of 14.8%), and cancer/arthritis/hypercholesterolemia (a prevalence of 12.4%) (). The most common cancer-comorbidity triads (after excluding hypertension and hypercholesterolemia) were cancer/arthritis/diabetes mellitus (a prevalence of 7.2%), cancer/arthritis/heart disease (a prevalence of 7%), and cancer/arthritis/mood disorder (5.8%) ().
Figure 2. (a) Prevalence of cancer-comorbidity triads among participants with a history of cancer; (b) prevalence of cancer-comorbidity triads among participants with a history of cancer (after excluding hypertension and hypercholesterolemia).
Multivariable logistic regression analyses were then conducted to analyze the association between history of cancer and diagnosis with different comorbid conditions. History of cancer was associated with a higher probability of concurrent diagnosis of all evaluated comorbid conditions except for stroke ().
Table 2. Multivariable logistic regression analysis for the association of cancer history with each of the studied comorbidities.
Prevalence of different chronic conditions among sub-strata of participants with cancer
The prevalence of different chronic conditions according to age subgroups is detailed in Supplementary Figure 2(a). Among participants with cancer of age 18–40 years (young adults), the most common comorbidities include mood disorder (17.1%), anxiety disorder (16.9%), and asthma (11.1%), while among very old participants with cancer (80+ years), the most common comorbidities include arthritis (54.3%), hypertension (49.4%), and hypercholesterolemia (29.9%).
The prevalence of different chronic conditions among men versus women is detailed in Supplementary Figure 2(b). For both men and women, the most common comorbidities include arthritis (men: 35.5% versus women: 43.8%), hypertension (men: 39.9% versus women: 33.1%) and hypercholesterolemia (men: 29.7% versus women: 21.7%).
The prevalence of different chronic conditions among different racial groups is detailed in Supplementary Figure 2(c). For the three racial groups, the most common comorbid conditions were arthritis (white: 40.5%, indigenous: 49%, nonwhite, non-indigenous: 34%), hypertension (white: 35.7%, indigenous: 36.4%, nonwhite, non-indigenous: 38.8%), and hypercholesterolemia (white: 24.8%, indigenous: 23%, nonwhite, non-indigenous: 28.9%).
In a multivariable logistic regression analysis among participants with cancer, the following sociodemographic factors were associated with having multiple comorbid conditions: older age (OR for age 80+ versus age 18–20 years: 8.32; 95% CI: 5.17–13.39), indigenous racial group (OR: 1.94; 95% CI: 1.43–2.63) and lower income (OR for income ≥80,000 CAD versus income: ≤20,000 CAD: 0.29; 95% CI: 0.23–0.37) ().
Table 3. Multivariable logistic regression analysis for factors associated with multiple comorbid conditions (>2) among patients with cancer.
Correlation between perceived health and self-reported comorbidities among participants with cancer
Poor perceived health was associated with a higher prevalence of all the studied eight physical comorbidities (p < 0.001) for all comparisons; likewise, poor perceived mental health was associated with a higher prevalence of the two studied psychiatric comorbidities (p < 0.001) for all comparisons (Supplementary Tables 3 and 4).
Discussion
The current study reviewed the prevalence of comorbid chronic conditions among Canadian adults with cancer. It suggested that history of cancer is associated with a higher probability of many individual comorbid conditions as well as of multiple co-existing comorbid conditions. That said, this excess comorbidity burden seems to be unequally shouldered by lower socioeconomic strata of the society as well as those within minority groups. Further efforts are needed to address structural roots of health inequality among cancer patients in Canada.
The higher comorbidity burden among participants with cancer is consistent with several previously published studies in different jurisdictions [Citation12,Citation13]. The reasons for this higher comorbidity burden might include common etiological factors between cancer and comorbid condition(s) (e.g., smoking, lung cancer, and COPD), effects of cancer treatment on different body organs (e.g., late effects of cancer treatment among adolescents and young adults with cancer) [Citation14,Citation15], or the simple fact that cancer patients are older, thus, they are expected to have more comorbidities (although, in the current study, individuals with history of cancer were more likely to have comorbid conditions even after adjusting for age category within the multivariable logistic regression).
It is notable that white the non-cancer cohort have equal distribution of men and women; participants with cancer are more likely to be females. A possible reason for this observation is that men are more likely to be affected by more fatal cancers (e.g., lung or pancreatic cancers) [Citation16]. Thus, they are less likely to be available as cancer survivors to be included in such a population-based cancer survey. Geographic differences in the prevalence of comorbidity among cancer as well as non-cancer participants can be viewed in light of the average age within each Canadian jurisdiction. For example, New Brunswick (the jurisdiction with the highest prevalence of multiple comorbid conditions) has the second oldest population in the country; likewise, Northwest Territories (the jurisdiction with the least prevalence of multiple comorbid conditions) has the second youngest population in the country [Citation17].
The discrepancy in the pattern of comorbid conditions between younger and older Canadian adults with cancer is noticeable. While somatic comorbid conditions represent the biggest challenge for older adults with cancer, psychiatric comorbid conditions are particularly challenging for younger adults. Previous studies have suggested that depression is under-diagnosed in the Canadian context among individuals with a history of cancer [Citation18,Citation19]. This has also been linked to a higher risk of suicidal ideation among those patients. These results highlight the particular challenges faced by younger adults with cancer and the need to have dedicated teams with expertise in comprehensive approaches for the management of those patients. It is notable also that mood and anxiety disorders were more likely reported among individuals with indigenous racial background versus other racial groups. Possible reasons might include a relatively younger age but also the previously reported higher prevalence of psychiatric disorders among this subgroup of the Canadian population 8) [Citation20,Citation21]. It has to be noted, however, that all comorbid conditions (including depression and anxiety) are self-reported by participants. Thus, these results need to be approached with caution.
Socioeconomic context represents an important determinant of health in the general population. This has been shown from population-based data in Canada as well as in other parts of the world [Citation22]. The association between lower socioeconomic status and multiple comorbid conditions specifically among cancer patients adds to the growing body of research regarding socioeconomic disparities in cancer care in Canada as well as in other parts of the world [Citation23,Citation24]. This can help also explain prior studies suggesting lower survival among cancer patients who identify as visible minority and those at the lower socioeconomic category of the society [Citation25,Citation26]. Although healthcare in Canada is publicly funded, these results call for addressing deeper causes of healthcare inequality in Canada beyond simple healthcare costs.
The current study has some limitations that need to be acknowledged in their context; first, information about cancer diagnosis, comorbid conditions, and other sociodemographic features is based on self-report by participants. While prior landmark studies from the United States have used the same strategy (in the context of US National Health Interview Survey) [Citation27], this approach is still associated with a theoretical probability of under-, over-, or misreporting information for a variety of reasons. Second, and similar to other population-based surveys, relevant granular data are unavailable (including type, stage, and treatment of cancer, time since cancer diagnosis or treatment, and details of the specific comorbidity). Thus, it is not possible to establish a more elaborate relationship between a specific cancer diagnosis and/or treatment and the subsequent development of a certain comorbidity. While it is possible that many of these comorbid conditions have predated cancer diagnosis (and possibly increased the risk of some of these cancers, e.g., metabolic syndrome and breast/prostate cancers) [Citation28], it is equally possible that some of these comorbid conditions developed (or exacerbated) because of some cancer treatments (e.g., anthracycline-induced cardiotoxicity; androgen-deprivation therapy-induced hypercholesterolemia) [Citation29]. Third, as this is a cross-sectional survey, no long-term follow-up morbidity or mortality data are available. Thus, it is not possible to acknowledge a specific mortality or morbidity impact of these comorbidities on those participants. Fourth, although the 10 reported comorbidities are of interest to the general population as well as to individuals with history of cancer, some relevant comorbidities are not reported within CCHS (specifically, liver and kidney disease). This might have affected the veracity of the analyses within the current study. These limitations generally indicate that these results should inform association rather than causation. Further work is needed to analyze the interaction between history of cancer and diagnosis of other comorbid conditions in Canada. These limitations need to be weighed against the strengths of the current study, most notably, the representative sample size and the known rigorous methodology and data collection approaches of CCHS.
The results of the current study also highlight some of the challenges associated with interpreting interventional clinical trial data in oncology. While patients enrolled in these clinical trials are usually a highly selected cohort of young individuals with very limited comorbidity, the reality is that a considerable proportion of cancer patients are elderly with multiple comorbid conditions [Citation30]. We believe that regulatory bodies (e.g., Health Canada) have a role in advising clinical trialists on the need for their studies to represent real-world cancer patient populations or else approvals for the respective therapeutic interventions would practically apply to a very limited subset of real-world patients. There are also data from real-world settings suggesting that patients treated within clinical trial settings outperform those treated within real-world settings mostly because of younger age and limited comorbidity of clinical trial participants [Citation31].
A possible future research question might be the impact of socioeconomic variables on how participants self-rated their own health. We have not discussed this topic in the manuscript in detail as it is beyond the scope of our manuscript, but we believe that this would deserve a dedicated analysis, with a potentially larger dataset (as the current dataset contained small number of participants who self-rated their health as poor).
In conclusion, history of cancer is associated with a higher probability of many individual comorbid conditions as well as of multiple co-existing comorbid conditions. That said, this higher probability of comorbid conditions seems to be unequally shouldered by certain strata of the society; with an observed association between lower socioeconomic status, minority status, and having multiple comorbid conditions among individuals with cancer. Further work is needed to address structural and systemic causes leading to health inequity among cancer patients. There is a need to have more inclusive designs of cancer clinical trials incorporating patients with multiple comorbidities.
Supplemental Material
Download MS Word (25.6 KB)Supplemental Material
Download MS Word (28.4 KB)Supplemental Material
Download MS Word (16 KB)Acknowledgment
This work is based on CCHS which is a Canadian national population-based survey.
Disclosure statement
No potential relevant conflict of interest was reported by the authors.
References
- Ay C, Beyer-Westendorf J, Pabinger I. Treatment of cancer-associated1 venous thromboembolism in the age of direct oral anticoagulants. Ann Oncol. 2019;30(6):897–907.
- Xie L, Semenciw R, Mery L. Cancer incidence in Canada: trends and projections (1983–2032). Health Promot Chronic Dis Prev Can. 2015;35 (Suppl 1):2–186.
- Poirier AE, Ruan Y, Walter SD, et al. The future burden of cancer in Canada: long-term cancer incidence projections 2013–2042. Cancer Epidemiol. 2019;59:199–207.
- Arnold M, Rutherford MJ, Bardot A, et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 2019;20(11):1493–1505.
- Bowker SL, Pohar SL, Johnson JA. A cross-sectional study of health-related quality of life deficits in individuals with comorbid diabetes and cancer. Health Qual Life Outcomes. 2006;4(1):17.
- Patel R, Liu WK, Patel HR, et al. Are health-care policies restricting further progress in cancer survival outcomes? Lancet Oncol. 2019;20(12):e657.
- Abdel-Rahman O. Impact of socioeconomic status on presentation, treatment and outcomes of patients with pancreatic cancer. J Comp Eff Res. 2020;9(17):1233–1241.
- Austin SR, Wong YN, Uzzo RG, et al. Why summary comorbidity measures such as the Charlson Comorbidity Index and Elixhauser Score work. Med Care. 2015;53(9):e65–e72.
- Mehta HB, Sura SD, Adhikari D, et al. Adapting the Elixhauser comorbidity index for cancer patients. Cancer. 2018;124(9):2018–2025.
- Canadian Community Health Survey – Annual component (CCHS). [cited 2020 Mar 12]. Available from: https://www.statcan.gc.ca/eng/survey/household/3226.
- Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans – TCPS 2. 2018 [cited 2020 Sep 23]. Available from: https://ethics.gc.ca/eng/policy-politique_tcps2-eptc2_2018.html.
- Sarfati D, Koczwara B, Jackson C. The impact of comorbidity on cancer and its treatment. CA Cancer J Clin. 2016;66(4):337–350.
- Williams GR, Deal AM, Lund JL, et al. Patient-reported comorbidity and survival in older adults with cancer. Oncologist. 2018;23(4):433–439.
- Hahn EE, Gould MK, Munoz-Plaza CE, et al. Understanding comorbidity profiles and their effect on treatment and survival in patients with colorectal cancer. J Natl Compr Canc Netw. 2018;16(1):23–34.
- Ettinger DS, Aisner DL, Wood DE, et al. NCCN guidelines insights: non-small cell lung cancer, version 5.2018. J Natl Compr Canc Netw. 2018;16(7):807–821.
- Brenner DR, Weir HK, Demers AA, Canadian Cancer Statistics Advisory Committee, et al. Projected estimates of cancer in Canada in 2020. CMAJ. 2020;192(9):E199–E205.
- Annual Demographic Estimates: Canada, Provinces and Territories. 2018 [cited 2021 Jan 3]. Available from: https://www150.statcan.gc.ca/n1/pub/91-215-x/2018002/sec2-eng.htm.
- Abdel-Rahman O, Salas AS, Watanabe SM, et al. Burden of depression among Canadian adults with cancer; results from a national survey. Expert Rev Pharmacoecon Outcomes Res. 2020;21:1–6.
- Abdel-Rahman O, Koski S, Mulder K. Real-world patterns of chemotherapy administration and attrition among patients with metastatic colorectal cancer. Int J Colorectal Dis. 2021;36(3):493–499.
- Nelson SE, Wilson K. The mental health of indigenous peoples in Canada: a critical review of research. Soc Sci Med. 2017;176:93–112.
- Hajizadeh M, Hu M, Bombay A, et al. Socioeconomic inequalities in health among indigenous peoples living off-reserve in Canada: trends and determinants. Health Policy. 2018;122(8):854–865.
- Key Health Inequalities in Canada: A National Portrait – Executive Summary. [cited 2021 Mar 18]. Available from: https://www.canada.ca/en/public-health/services/publications/science-research-data/key-health-inequalities-canada-national-portrait-executive-summary.html.
- Abdel-Rahman O. Patterns and trends of cancer screening in Canada: results from a contemporary national survey. J Natl Compr Canc Netw. 2021;19(1):68–76.
- Abdel-Rahman O. Patient-reported cognitive and functional impairments among older Canadians with cancer: a population-based study. J Pain Symptom Manage. 2021;61(2):279–286.
- Nishri ED, Sheppard AJ, Withrow DR, et al. Cancer survival among first nations people of Ontario, Canada (1968–2007). Int J Cancer. 2015;136(3):639–645.
- McGahan CE, Linn K, Guno P, et al. Cancer in first nations people living in British Columbia, Canada: an analysis of incidence and survival from 1993 to 2010. Cancer Causes Control. 2017;28(10):1105–1116.
- Ward BW, Schiller JS. Prevalence of multiple chronic conditions among US adults: estimates from the national health interview survey. Prev Chronic Dis. 2010;10:E65.
- Abdel-Rahman O. Impact of diabetes on the outcomes of patients with castration-resistant prostate cancer treated with docetaxel: a pooled analysis of three phase III studies. Clin Genitourin Cancer. 2019;17(1):e104–e112.
- Ahmed SH, Moussa Sherif DE, Fouad Y, et al. Principles of a risk evaluation and mitigation strategy (REMS) for breast cancer patients receiving potentially cardiotoxic adjuvant treatments. Expert Opin Drug Saf. 2016;15(7):911–923.
- Andersson Y, Bergkvist L, Frisell J, et al. Do clinical trials truly mirror their target population? An external validity analysis of national register versus trial data from the Swedish prospective SENOMIC trial on sentinel node micrometastases in breast cancer. Breast Cancer Res Treat. 2019;177(2):469–475.
- Heng DY, Choueiri TK, Rini BI, et al. Outcomes of patients with metastatic renal cell carcinoma that do not meet eligibility criteria for clinical trials. Ann Oncol. 2014;25(1):149–154.
