Abstract
Background
Results from studies addressing age-related patterns of cancer care have found evidence of unjustified differences in management between younger and older patients.
Methods
We examined associations between age and clinical presentation, management and mortality in patients diagnosed with non-small cell lung cancer (NSCLC) between 2002 and 2016. Analyses were adjusted for comorbidity and other factors that may have affected management decisions and outcomes.
Results
The study population encompassed 40,026 patients with NSCLC. Stage at diagnosis did not differ between age groups ≤ 84. The diagnostic intensity was similar in age groups <80 years. In patients with stage IA–IIB disease and PS 0–2, surgery was more common in the youngest age groups and decreased with increasing age, and was rarely performed in those ≥ 85 years. The use of stereotactic body radiotherapy (SBRT) increased with age (≤69 years 5.4%; ≥85 years 35.8%). In patients with stage IIIA disease and PS 0–2, concurrent chemoradiotherapy was more common in younger patients (≤69 years 55.3%; ≥85 years 2.2%). In stage IA–IIIA disease, no major differences in treatment-related mortality was observed. In stage IIIB–IV and PS 0–2, chemotherapy was more common in patients <80 years. However, 58.1% of patients 80–84 years and 30.3% ≥ 85 years received treatment. In stage IA–IIIA, overall and cause-specific survival decreased with increasing age. No age-differences in survival were observed in patients with stage IIIB-IV NSCLC.
Conclusion
Treatments were readily given to older patients with metastatic disease, but to a lesser degree to those with early stage disease. Significant differences in cause specific survival were observed in early, but not late stage disease. Our findings underscore the importance of individualized assessment of health status and life expectancy. Our results indicate that older patients with early stage lung cancer to a higher extent should be considered for curative treatment.
Introduction
Results from studies addressing age-related patterns of cancer care have shown differences in management between younger and older patients [Citation1–4]. While a lower diagnostic and treatment intensity may be justified due to a lack of physiological reserves, comorbidity or short life expectancy, there is a risk that management decisions are routinely made based on high chronological age and preconceptions regarding sensitivity to treatment [Citation5–7]. It is well known that large differences in general health status exist in the oldest age groups with subgroups of older patients in good health who tolerate standard treatments recommended by guidelines [Citation6,Citation8,Citation9]. Selection criteria for clinical studies often result in lower average age in the study population than in the general cancer population [Citation10].
Lung cancer is predominantly diagnosed in the elderly. In Sweden, the median age at diagnosis is 69 years with only 10% of patients being younger than 60 years [Citation11]. Because of increasing life expectancy and population aging, the subgroup of older patients will increase in size. Between 2015 and 2050, the proportion of the world’s population over 60 years will nearly double from 12% to 22% and in the European region the number of people aged 85 years and older is predicted to rise from 19 million to 40 million between 2020 and 2050 [Citation12].
By the use of information in a population-based lung cancer research database, we investigated possible associations between chronological age and clinical presentation, diagnostic intensity, treatment and mortality in patients with non-small cell lung cancer (NSCLC).
Material and methods
Data collection
We conducted a nationwide, register-based cohort study based on data retrieved from Lung Cancer Data Base Sweden (LCBaSe), a research database generated by record linkage between the National Lung Cancer Register (NLCR) and several other high quality Swedish population-based registers. Individual level record linkages were made possible by the use of the national registration number, a personal identifier assigned to all residents in Sweden. Tumors were staged according to the 5th–8th editions of the Union for International Cancer Control (UICC) tumor, node, metastasis (TNM) staging systems in use during the time period under study.
Additional individual level information was obtained from the National Patient Register (NPR), the Swedish Cancer Register (SCR), the social database Longitudinal Integration Database for Health Insurance and Labour Market Studies (LISA) and the Cause of Death Register (CDR).
For estimation of socioeconomic status, data on educational level were retrieved from LISA, a nationwide register including continuously updated information on highest achieved educational level for all residents aged 16 and older [Citation13]. Education was collapsed into three groups based on the total number of years of schooling: low ≤9 years, middle 10–12 years, and high ≥13 years, corresponding to mandatory school, high school, and post-high school (college and university).
Information on comorbidity was based on records of up to eight discharge diagnoses retrieved from the NPR, and from the SCR on malignancies other than lung cancer. Comorbidity burden was estimated by the use of the Charlson Comorbidity Index (CCI) and categorized into three groups; no (CCI 0), mild (CCI 1-2), and severe comorbidity (CCI 3 +) [Citation14].
Smoking history was based on self-reported information at time of diagnosis retrieved from the NLCR and recorded as smoker (current smoker), former smoker (no smoking during the last year) and nonsmoker (never smoked on a regular basis).
Performance status (PS) was assessed and recorded in the NLCR by the treating physician based on the Eastern Cooperative Oncology Group (ECOG)/World Health Organization (WHO) performance scale [Citation15].
Date and cause of death were available in the CDR, a register with high completeness [Citation16] that includes data on main and contributing causes of death.
Since information on date of start of treatment was unavailable, periprocedural mortality was assessed in relation to date of diagnosis. Because of differences between the treatment modalities in duration and expected peaks in mortality, we chose to set different time limits for periprocedural mortality: within 90 days of surgery and 120 days of stereotactic body radiotherapy (SBRT), radiotherapy and concurrent chemoradiotherapy. Since data on SBRT was not reported during the early part of the study period, these analyses were restricted to the years 2012–2016. We also excluded data from previous years on the other treatment modalities to be able to present relevant comparisons in a modern curative setting.
Statistical methods
Demographic and clinical characteristics, diagnostic intensity and treatment modalities of patients diagnosed with non-small cell lung cancer in Sweden 2002–2016 were described by predefined age groups (≤69, 70–74, 75–79, 80–84, and ≥85 years). In a subsequent step, logistic regression models were used to examine whether initial treatment differed by age groups and clinical subgroups (stratified by stage at diagnosis and restricted to patients with PS 0–2), adjusted for sex, educational level, smoking history, stage at diagnosis, CCI, PS, histopathology, region and year of diagnosis, with results presented as adjusted OR. In a final step, cause-specific and overall mortality were compared between age groups. Survival time was calculated as the time interval between the date of lung cancer diagnosis and date of death, or emigration or end of follow-up (31 December 2016).
All tests were two-sided, and a 5% level was considered statistically significant. Statistical analyses were performed using R version 3.5.0.
Research ethics
The study was approved by the Research Ethics Board in Stockholm (2012/1162-31-4; 2016/1137-32; 2017/2026-32; 2017-445-32).
Results
Demographics and clinical characteristics
We identified a total of 52,274 patients diagnosed with lung cancer between 2002 and 2016. Following exclusion of small-cell lung cancer (n = 7137), missing histopathology specimens (n = 2633), unclassified cancer subtypes (n = 1184), carcinoids (n = 986), other rare histopathology (n = 204) and missing data (n = 104), 40,026 patients with non-small cell lung cancer remained for analysis. In the final population under study, 48.6% of patients were 69 years or younger at diagnosis, 19.7% between 70 and 74 years, 16.6% between 75 and 79 years, 10.8% between 80 and 84 years and 4.3% 85 years or older. Demographic and clinical characteristics are presented in .
Table 1. Demographic and clinical characteristics of patients diagnosed with non-small cell lung cancer in Sweden 2002–2016 by age groups.
In all age groups ≥70 years, the majority of patients were men. In the youngest age group ≤69 years, the proportion of women was higher than men. The highest achieved level of education differed markedly between age groups; a low level of education was more common in the oldest cohorts. A history of being never smokers was more common in the oldest age group.
As expected, the comorbidity burden as assessed by CCI was considerably lower in the youngest compared to older age groups. Similarly, the WHO Performance Status score differed markedly by age. Almost a third of patients in age group ≤69 years had a PS score of 0 compared to only to 4.8% in patients 85 years or older. Conversely, a high PS score of 3–4, and, therefore, unfit for therapy, was more common in the oldest cohort.
Adenocarcinomas were more common in younger patients, while the opposite was seen for squamous cell carcinoma. The distribution of large cell and adenosquamous cell carcinomas did not differ between age groups. There were no age-related differences in stage at diagnosis in patients ≤ 84 years of age. The proportion of patients with early stage disease (IA–IIB) was lower in the age group ≥85 years.
Diagnostic intensity and multidisciplinary team assessment
Diagnostic work-up with bronchoscopy, endobronchial ultrasound (EBUS), computed tomography (CT) thorax, ultrasound (US) or CT abdomen, thoracoscopy and positron emission tomography (PET), assessment in a multidisciplinary team setting and testing for epidermal growth factor receptor (EGFR) mutations in adenocarcinomas were evenly performed across all age groups below 80 years, but were less common in patients ≥80 years especially in those ≥ 85 years. Compared to younger patients, a higher proportion of patients ≥ 80 years were diagnosed with thoracocentesis, in the oldest cohort ≥ 85 years one third of patients underwent this procedure ().
Table 2. Diagnostic intensity and multidisciplinary team assessment in patients diagnosed with non-small cell lung cancer in Sweden 2002–2016 by age groups.a
Treatment intensity
Stage IA–IIB
In patients with PS 0–2 and stage IA-IIB disease, surgery was more commonly performed in younger age groups with a marked decrease in patients ≥ 80 years. In contrast, the use of SBRT increased with age ().
Table 3. Treatment modality in patients diagnosed with non-small cell lung cancer in Sweden 2002–2016 with performance status 0–2 by age groups and stage at diagnosis.a
These marked differences remained in multivariable analyses following adjustments for potentially modifying factors including sex, educational level, time period, CCI, smoking history, stage at diagnosis, performance status and histology. Compared to the youngest age group (≤ 69 years), the likelihood for surgery versus SBRT in patients between 70 and 74 years was lower (OR 0.41 (CI 0.33–0.55) and aOR 0.58 (0.45–0.74)), estimates that decreased further with increasing age (75–79 years – OR 0.22 (CI 0.18–0.27); aOR 0.33 (CI 0.26–0.43); 80–84 years – OR 0.07 (CI 0.06–0.09); aOR 0.09 (CI 0.07–0.12); ≥85 years – OR 0.01 (CI 0.01–0.02); aOR 0.01 (CI 0.01–0.02)) (data not shown).
Stage IIIA
In patients with PS 0–2 and stage IIIA disease, combined treatment with concurrent chemoradiotherapy was more commonly given to younger patients. Radiotherapy alone was provided to the same extent regardless of age. Surgery was mainly performed in patients ≤74 years ().
Compared to age groups ≤69 years, the likelihood of receiving curative versus non-curative treatment (concurrent chemoradiotherapy or surgery or SBRT versus chemotherapy or radiotherapy alone or other treatment or no active treatments) was lower for patients 70–74 years (OR 0.53 (CI 0.44–0.64); aOR 0.71 (CI 0.57–0.88)), with estimates decreasing further with increasing age (75–79 years – OR 0.26 (CI 0.21–0.31); aOR 0.38 (CI 0.30–0.47), 80–84 years – OR 0.13 (CI 0.10–0.16); aOR 0.20 (CI 0.15–0.27) and ≥85 years – OR 0.02 (CI 0.01–0.04); aOR 0.03 (CI 0.02–0.07)) (data not shown).
Stage IIIB–IV
In patients with PS 0–2 and advanced stage IIIB–IV disease, chemotherapy and other systemic therapies were more common in patients below 80 years of age. However, treatments were given also in the oldest age groups (80–84 years 58.1%; ≥85 years 30.3%). Radiotherapy was given to the same extent regardless of age. Few patients were eligible for concurrent chemoradiotherapy, but it was used to the same extent in all age groups <80 years ().
Treatment associated mortality
In early stage IA–IIB disease, the all-cause mortality within 90 days following surgery and 120 days following SBRT was slightly higher in age groups ≥ 75 years, but was generally low ().
Table 4. Mortality following start of treatment in IA-IIIA NSCLC patients diagnosed 2012–2016.
In stage IIIA disease, the all-cause mortality within 120 days after diagnosis was higher in the group receiving radiotherapy alone versus concurrent chemoradiotherapy, but without significant differences between age groups ().
Overall and cause-specific mortality
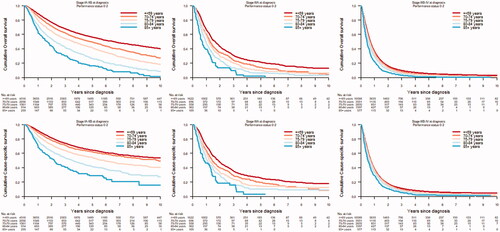
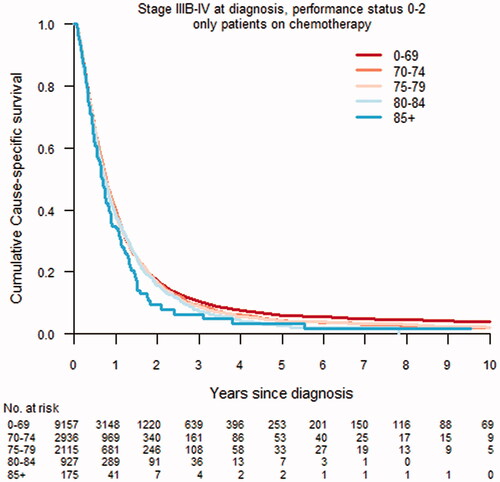
In stage IA–IIB and IIIA disease, overall and cause-specific survival decreased with increasing age (). In advanced stage disease, IIIB–IV, there were no significant age-related differences in overall or cause-specific survival. In separate analyses of patients with PS 0–2 and stage IIIB–IV disease receiving chemotherapy, there were no significant differences in cumulative cause-specific survival ().
Discussion
Summary of results
The challenge of cancer management in older populations has so far received limited attention. The aim of this nationwide study was to examine clinical presentation, management and survival in older patients with NSCLC. An improved understanding of age-related patterns of care and evidence of medically unjustified deviations from guidelines is important to avoid undertreatment of older patients. This is of special importance in light of ongoing population aging and the introduction of lung cancer screening that will further increase the incidence of potentially curable stage IA–IIB cancers.
The differences between age groups in the distribution by sex and histology observed in our study are likely to reflect historical trends in smoking habits. We found no age-related differences in stage at diagnosis in patients below 84 years of age. Diagnostic intensity was similar across age groups, except in patients ≥ 80 years, an age group with a generally higher comorbidity burden and lower PS. As expected, younger patients with early stage disease were to a higher extent accepted for curative treatment compared to older patients. Post-treatment mortality was slightly higher in the older patient groups. In early, but not late stage disease, older age was associated with both lower all-cause and cause specific survival indicating possible underuse of curative treatments. No excess mortality was observed in older patients with stage IIIB–IV disease that received treatment, findings which suggest tolerance to treatment and careful patient selection.
Comparisons to previous studies
Because of strict inclusion and exclusion criteria, clinical studies evaluating lung cancer treatment strategies commonly exclude older patients [Citation17]. As a result clinicians often de-escalate treatment to lower the risk of side effects and complications [Citation18]. Following treatment decisions based on pulmonary function, heart disease, and comorbidity, a previous study on the role of surgery found similar complication and mortality rates in older (≥75 years) and younger patients, indicating that a higher proportion of older patients could be offered surgery [Citation19].
SBRT has been estimated to achieve local control rates approximating 90–95% [Citation20] and there is evidence that use of SBRT in elderly with early stage lung cancer is associated with improved overall survival [Citation21]. To the best of our knowledge, no prospective randomized study has been conducted to compare the effects and safety of surgery and SBRT in the elderly. A retrospective register-based study comparing different treatment modalities in older patients found the lowest periprocedural mortality following SBRT, but the best overall and disease-specific survival after surgery with lobectomy. However, following propensity-matched analysis, SBRT outcomes were not statistically different from those of lobectomy, suggesting that both treatment modalities are possible. In the same study, palliative treatments with conventional radiation or mere observation were associated with poor survival [Citation18]. Based on the results from another study, it was recommended that elderly patients with early stage lung cancer should undergo surgery or SBRT, with SBRT as the treatment of choice in patients with severe comorbidity [Citation22]. The development of minimal invasive surgical techniques may increase older patients’ eligibility for surgery. In our study, patients with early stage disease, aged 80 or older and with a good performance score were to a lesser extent given treatments with a curative intent and had a significantly lower cause-specific survival. Thus, our findings further support the notion of the presence of undertreatment of elderly patients with early stage disease.
The role of concurrent chemoradiotherapy in older patients with locally advanced lung cancer remains debated. A Japanese study including patients 70 years or older was discontinued prematurely because of a clinically significant benefit of concurrent chemoradiotherapy over radiotherapy alone [Citation23]. In a Dutch study, patients 75 years and older with stage III cancer were less frequently treated with concurrent chemoradiotherapy and had poorer general overall survival. Data on cause specific survival were not available, but comparisons within groups receiving treatment showed comparable survival rates [Citation24]. A Danish study found no survival benefit of concurrent chemoradiotherapy in patients ≥70 years [Citation25]. In our study, the proportion of patients 80 years or older with stage IIIA disease and PS 0–2 receiving concurrent chemoradiotherapy was low, indicating a high level of patient selection. No significant differences in mortality were observed in age groups ≥70 years, suggesting that treatment safety and efficacy is possible also in selected older patients.
Several studies have demonstrated similar survival benefits of chemotherapy in older compared to younger patients with metastatic disease [Citation26], but there is evidence that elderly are less often offered systemic treatment [Citation27]. Performance status, a simple scoring system predicting tolerability, has been found to improve patient selection [Citation26]. The use of geriatric assessment tools may have a predictive role for chemotherapy-associated toxicity and can be used to further improve risk stratification [Citation28]. Studies have shown that even the oldest ≥80 years may benefit from treatment [Citation29]. Novel treatment options such as targeted therapies and checkpoint inhibitors may offer a more favorable toxicity and thus enable treatment to a higher degree in older lung cancer patients [Citation30].
Although multidisciplinary team assessments are likely to improve adherence to clinical guidelines, the effect on survival remains debated [Citation31–33]. In our study, a PS 0–2 was found in 67.7% and 56.2% in patients 80–84 years and ≥85 years, respectively, while only 54.6% and 45.6% were discussed in a multidisciplinary team setting. Standardized evaluation of all potentially treatable patients should be a priority in national cancer strategies.
Strengths and limitations
Strengths of our study included the use of information from several high-quality population-based registers encompassing more than 95% of all lung cancer patients diagnosed during the time period under study. The data included individual level information on smoking history, educational level, performance status and comorbidity, reducing the potential confounding influences of co-existing disease. In earlier studies addressing age-related patterns of care, the definition of old patients has often been set to age ≥70 years [Citation8,Citation9,Citation34], and only a few studies to date have investigated lung cancer management in the oldest old [Citation35,Citation36].
Limitations included the absence of information on patient related factors such as social situation, lifestyle, health awareness, and beliefs as well as health care seeking behavior. Information on smoking history was based on self-report and thus subject to misclassification due to misreporting or recall bias. Assessment of comorbidity was based on information on medical conditions requiring medical care. Since no data were available on lung function or exercise capacity, the burden of concomitant disease is likely to have been underestimated. The TNM Classification of Malignant Tumors was revised 2010, but is likely to have affected classification of tumor stage in a similar was across all age groups. Also, no data were available on completion of treatment, toxicity or treatments beyond first line. Furthermore, because of lack of data, we were unable to correctly characterize patients that died shortly after diagnosis or start of treatment. In the oldest age group, the accuracy of cause-specific survival may have been affected by misclassification of cause of death.
Conclusion
We found no age-related differences in stage at diagnosis in patients ≤84 years diagnosed with non-small cell lung cancer. Diagnostic intensity was similar in patients up to age 80, but decreased thereafter. While treatment appears to be readily given also to older patients with metastatic disease, the use of curative treatment with surgery or SBRT in early stage lung cancer was less common in the oldest age groups. Significant differences in cause specific survival were observed in early, but not late stage disease. Taken together, our findings further underscore the importance of correctly assessing health status and life expectancy to avoid unjustified undertreatment of older patients. Our results indicate that older patients with early stage lung cancer to a higher extent should be considered for curative treatment.
Acknowledgments
The project was made possible by the continuous reporting by Swedish clinicians to the National Lung Cancer Register of Sweden and the work by the NLCR steering group: Gunnar Wagenius (chairman), Stefan Bergström, Bengt Bergman, Annelie Behndig, Mikael Johansson, Per Fransson, Kristina Lamberg Lundström, Anna Öjdahl-Bodén, Hanna Carstens, Karl-Gustaf Kölbeck, Andreas Hallqvist, Mona Gilleryd, Anders Vikström, Magnus Kentsson, Lars Ek, and Sven-Börje Ewers.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Bratt O, Folkvaljon Y, Hjälm Eriksson M, et al. Undertreatment of men in their seventies with high-risk nonmetastatic prostate cancer. Eur Urol. 2015;68(1):53–58.
- Vernooij RWM, van Oort I, de Reijke TM, et al. Nationwide treatment patterns and survival of older patients with prostate cancer. J Geriatr Oncol. 2019;10(2):252–258.
- Bouchardy C, Rapiti E, Fioretta G, et al. Undertreatment strongly decreases prognosis of breast cancer in elderly women. J Clin Oncol. 2003;21(19):3580–3587.
- Simmonds P, Best L, George S, et al. Surgery for colorectal cancer in elderly patients: a systematic review. Colorectal cancer collaborative group. Lancet. 2000;356(9234):968–974.
- Bernardi D, Errante D, Tirelli U, et al. Insight into the treatment of cancer in older patients: developments in the last decade. Cancer Treat Rev. 2006;32(4):277–288.
- Everaerts W, Van Rij S, Reeves F, et al. Radical treatment of localised prostate cancer in the elderly. BJU Int. 2015;116(6):847–852.
- Foster JA, Salinas GD, Mansell D, et al. How does older age influence oncologists' cancer management? Oncologist. 2010;15(6):584–592.
- Barta JA, Zinner RG, Unger M. Lung cancer in the older patient. Clin Geriatr Med. 2017;33(4):563–577.
- Blanco R, Maestu I, de la Torre MG, et al. A review of the management of elderly patients with non-small-cell lung cancer. Ann Oncol. 2015;26(3):451–463.
- Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21(7):1383–1389.
- National Lung Cancer Report accounting years 2012–2016 Regional Cancer Center Sweden; 2017. Available from: https://www.cancercentrum.se/globalassets/cancerdiagnoser/lunga-och-lungsack/kvalitetsregister/rapport/nlcr_rapport_tom2016_171120_publicera.pdf. Accessed 6 December 2020.
- WHO Fact sheets on ageing and health. Available from: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health accessed. Accessed 6 December 2020.
- Statistics Sweden. Longitudinal integration database for health insurance and labour market studies (LISA) 1990-onwards. Available from: https://www.scb.se/en/services/guidance-for-researchers-and-universities/vilka-mikrodata-finns/longitudinella-register/longitudinal-integrated-database-for-health-insurance-and-labour-market-studies-lisa/. Accessed 6 December 2020.
- Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682.
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern cooperative oncology group. Am J Clin Oncol. 1982;5(6):649–655.
- Welfare. TSNBoHa. The Swedish Cause of Death Register. Available from: https://www.socialstyrelsen.se/statistik-och-data/register/alla-register/dodsorsaksregistret/. Accessed 6 December 2020.
- Schulkes KJ, Nguyen C, van den Bos F, et al. Selection of patients in ongoing clinical trials on lung cancer. Lung. 2016;194(6):967–974.
- Shirvani SM, Jiang J, Chang JY, et al. Comparative effectiveness of 5 treatment strategies for early-stage non-small cell lung cancer in the elderly. Int J Radiat Oncol Biol Phys. 2012;84(5):1060–1070.
- Baldvinsson K, Oskarsdottir GN, Orrason AW, et al. Resection rate and operability of elderly patients with non-small cell lung cancer: nationwide study from 1991 to 2014. Interact Cardiovasc Thorac Surg. 2017;24(5):733–739.
- Shinde A, Li R, Kim J, et al. Stereotactic body radiation therapy (SBRT) for early-stage lung cancer in the elderly. Semin Oncol. 2018;45(4):210–219.
- Haasbeek CJ, Palma D, Visser O, et al. Early-stage lung cancer in elderly patients: a population-based study of changes in treatment patterns and survival in The Netherlands. Ann Oncol. 2012;23(10):2743–2747.
- Nguyen NP, Godinez J, Shen W, International Geriatric Radiotherapy Group, et al. Is surgery indicated for elderly patients with early stage nonsmall cell lung cancer, in the era of stereotactic body radiotherapy? Medicine (Baltimore). 2016;95(43):e5212.
- Atagi S, Kawahara M, Yokoyama A, Japan Clinical Oncology Group Lung Cancer Study Group, et al. Thoracic radiotherapy with or without daily low-dose carboplatin in elderly patients with non-small-cell lung cancer: a randomised, controlled, phase 3 trial by the Japan Clinical Oncology Group (JCOG0301). Lancet Oncol. 2012;13(7):671–678.
- Driessen EJM, Schulkes KJG, Dingemans AC, et al. Patterns of treatment and survival among older patients with stage III non-small cell lung cancer. Lung Cancer. 2018;116:55–61.
- Hansen O, Schytte T, Nielsen M, et al. Age dependent prognosis in concurrent chemo-radiation of locally advanced NSCLC. Acta Oncol. 2015;54(3):333–339.
- Veluswamy RR, Levy B, Wisnivesky JP. Chemotherapy in elderly patients with nonsmall cell lung cancer. Curr Opin Pulm Med. 2016;22(4):336–343.
- Dawe DE, Pond GR, Ellis PM. Assessment of referral and chemotherapy treatment patterns for elderly patients with non-small-cell lung cancer. Clin Lung Cancer. 2016;17(6):563–572 e2.
- Gajra A, Akbar SA, Din NU. Management of lung cancer in the elderly. Clin Geriatr Med. 2016;32(1):81–95.
- Koyi H, Hillerdal G, Kolbeck KG, et al. Non-small cell lung cancer (NSCLC) in octogenarians in clinical practice. AR. 2016;36(10):5397–5402.
- Kanesvaran R, Roy Chowdhury A, Krishna L. Practice pearls in the management of lung cancer in the elderly. J Geriatr Oncol. 2016;7(5):362–367.
- Boxer MM, Vinod SK, Shafiq J, et al. Do multidisciplinary team meetings make a difference in the management of lung cancer? Cancer. 2011;117(22):5112–5120.
- Pillay B, Wootten AC, Crowe H, et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: a systematic review of the literature. Cancer Treat Rev. 2016;42:56–72.
- Stone E, Rankin N, Kerr S, et al. Does presentation at multidisciplinary team meetings improve lung cancer survival? Findings from a consecutive cohort study. Lung Cancer. 2018;124:199–204.
- Driessen EJ, Aarts MJ, Bootsma GP, et al. Trends in treatment and relative survival among non-small cell lung cancer patients in The Netherlands (1990–2014): disparities between younger and older patients. Lung Cancer. 2017;108:198–204.
- Schulkes KJG, Pouw CAM, Driessen EJM, et al. Lung cancer in the oldest old: a nation-wide study in The Netherlands. Lung. 2017;195(5):627–634.
- DeSantis CE, Miller KD, Dale W, et al. Cancer statistics for adults aged 85 years and older, 2019. CA Cancer J Clin. 2019;190:836–842.