Abstract
Background
The incidence of pregnancy-associated cancers has been increasing for decades. (18F)-FDG Positron Emission Tomography (PET)/Computed Tomography (CT) imaging has become a golden standard in the staging of many malignant diseases. The aims of the current study were to evaluate the feasibility, safety and impact of (18F)-FDG PET/CT performed during pregnancy.
Material and methods
A retrospective analysis from the prospective database of the Cancer Associé à La Grossesse (CALG) network (Tenon Hospital, France) including patients who underwent (18F)-FDG PET/CT during their pregnancy between 2015 and 2020.
Results
Of the 536 patients for whom advice from the CALG network was requested during the study period, 359 were diagnosed with cancer during pregnancy. Study population was composed of 63 (17.5%) patients who underwent (18F)-FDG PET/CT. Most cancers were diagnosed during the second trimester. Seventy-five percent were diagnosed with breast cancer, mostly locally advanced invasive ductal carcinomas. Median term of pregnancy at PET/CT was 24.8 weeks of gestation. Twelve (19%), 24 (38.1%) and 22 (34.9%) patients underwent the exam during the 1st, 2nd and 3rd trimester, respectively. (18F)-FDG PET/CT resulted in stage modification for 38 (60.3%) of the patients (28 with more extensive lymph node involvement and 10 with metastatic disease) with subsequently/accordingly modified first-line medical treatment. Fifty patients gave birth to healthy newborns. Two patients had a medical termination of pregnancy, five had a medical abortion, one neonatal death occurred in a patient with severe preeclampsia (unrelated to (18F)-FDG PET/CT). The data of 46 children were available at 6 months, 29 at 12 months, and 15 at 24 months. No cases of mental retardation, childhood cancer, or malformation were reported within 2 years.
Conclusion
(18F)-FDG PET/CT has a major impact on the management of pregnancy-associated cancers and does not appear to cause fetal side effects suggesting that the exam is feasible during pregnancy as maternal benefits outweigh fetal risks.
Background
Pregnancy-associated cancers are defined as cancers diagnosed during pregnancy or the year following delivery. Their incidence is relatively low (1/1000–1500 pregnancies) but has been increasing for decades as more women delay childbearing [Citation1–4]. The most frequent pregnancy-associated cancers are breast cancer, uterine cervical cancer, lymphoma, ovarian cancer, and leukemia [Citation5,Citation6]. The diagnosis, management, and treatment of cancer in the setting of pregnancy constitute major challenges for both maternal and fetal outcomes.
Although pregnancy-associated cancers are known to display different characteristics, national and international guidelines recommend that management should be as similar as possible to that in non-pregnant women [Citation7,Citation8]. However, while studies have already reported differences in treatment and outcomes in pregnancy-associated cancers, tools to stage the cancers remain unclear in this population [Citation6,Citation9–11].
(18F)-Fluorine-(2)-deoxy-glucose ((18F)-FDG) Positron Emission Tomography/Computed Tomography (PET/CT) imaging is a golden standard in the staging of many malignant diseases, including most of those occurring during pregnancy [Citation12–15]. Although some small case series have estimated that the fetal radiation exposure in pregnant women is far below the threshold dose for both deterministic and stochastic effects, the indication of (18F)-FDG PET/CT remains a matter of debate [Citation15,Citation16].
The aims of the current study were to evaluate the feasibility, safety, and impact of (18F)-FDG PET/CT during pregnancy on oncological and obstetrical outcomes based on the analysis of the French national register of pregnancy-associated cancers from the Cancer Associé à La Grossesse (CALG) network.
Material and methods
Type of study
We conducted a retrospective analysis from the prospective database of the CALG network (Tenon Hospital, Paris, France) collecting cases of pregnancy-associated cancers, i.e., cancers diagnosed during pregnancy or during the first post-partum year. The CALG network was established in 2008 to investigate oncological and obstetrical care, and maternal and neonatal outcomes in women with a pregnancy-associated cancer.
Population
Patients diagnosed with a pregnancy-associated cancer between January 2015 and December 2020 and for whom advice from the CALG network was requested, were eligible for inclusion. Subsequently, only patients who underwent a (18F)-FDG PET/CT during pregnancy were analyzed. Exclusion criteria were patients diagnosed with cancer during the first post-partum year and patients who underwent (18F)-FDG PET/CT after delivery. The Ethics Committee (CEROG) of the Collège National des Gynécologues et Obstétriciens Français (CNGOF) approved the study (CEROG-2019-GYN-603).
Of the 536 patients for whom advice from the CALG network was requested between January 2015 and December 2020, 154 were excluded for the following reasons: 96 requests for advice about pregnancy after cancer, five borderline ovarian tumors, 10 gestational trophoblastic diseases, 23 carcinomas in situ (12 breast carcinomas in situ and 11 uterine cervical in situ adenocarcinomas), and 20 non-pregnancy-associated cancers. Among the final population of 382 patients, 359 were diagnosed with cancer during pregnancy and 63 (17.5%) underwent (18F)-FDG PET/CT.
Data collection
Diagnosis of the cancer was systematically proven by histology. The following data were recorded; epidemiological (age and parity at diagnosis, genetic mutations, familial and personal history of cancer, term of pregnancy at diagnosis), type of cancer and histological details (histology, immunohistology), disease stage using TNM classification for solid cancers, exams performed during pregnancy (as well as the gestational age when (18F)-FDG PET/CT was performed), treatment received during the pregnancy and in the post-partum period, term and mode of delivery, and neonatal and maternal outcomes.
(18F)-FDG PET/CT imaging protocol
Although no consensus exist on the recommended procedure for women with a pregnancy-associated cancer requiring a (18F)-FDG PET/CT, the protocol was an intravenous injection of the radioisotope 18F-FDG (1.5MBq/kg body weight) before being examined by a hybrid PET/CT (classic CT X-ray) camera BIOGRAPH mCT Flow (Siemens Medical).
For (18F)-FDG, the standard protocol dosage is 2.5–5 MBq/kg body weight according to the European Association of Nuclear Medicine and Molecular imaging (EANMMI) guidelines and recommendations [Citation17] that was approved on 22 June 2018 by the U.S. scientific society, the Nuclear Medicine and Molecular imaging (SNMMI). The pregnant woman protocol we used is based on reducing the dosage to 1.5 MBq/kg body weight. Therefore, this pregnant-woman dedicated protocol reduces the radiation dose to the fetus from 40% up to 70% less than the standard protocol.
For the CT X-ray part, following parameters were applied: a dose modulation using the manufacturer program (Care Dose 4 D Siemens Medical) allowing a dose reduction of X-rays determined by the scout view and CT parameters (intensity: 35 mA; voltage: 120 kV; cut: 5 mm; pitch: 0.8).
The Supplementary data summaries the parameters of the standard protocol and our modified pregnant-woman protocol for both modalities, (18F)-FDG PET and X-rays CT, and the estimated reduction in fetal irradiation compared to standard protocol.
A detailed protocol for (18F)-FDG PET/CT in pregnancy was provided when it was performed as an externally procedure.
Statistical analysis
Statistical analysis of the data was performed using RStudio version 4.0.3 (10 October 2020) software (Bell Laboratories, Lucent Technologies, Paris, France). Descriptive statistics are shown as medians and IQRs (interquartile range: 25th–75th).
Results
Epidemiological characteristics of the study population (n = 63 patients)
The population characteristics are shown in . Median age at diagnosis was 33 (IQR: 31–37) years and most cancers were diagnosed during the second trimester (median term 22 WG).
Table 1. Population characteristics.
Six patients (9.5%) had a personal history of cancer: three had a history of breast cancer with recurrence during pregnancy, and two had a history of Hodgkin lymphoma (one of whom secondarily developed breast cancer, and the other a lymphoma recurrence). The last patient had a history of breast phyllode sarcoma and developed a mandibular tumor during pregnancy. Seventeen patients (27%) had a familial history of cancer and a genetic mutation was identified in five (8%), mainly BRCA mutations.
The distribution of the cancers in the study population (n = 63) is described in and . Clinical stages at diagnosis were heterogeneous and depended on the cancer type, for example: cervical uterine cancers were diagnosed at early stage; ovarian cancers were mostly diagnosed at locally advanced stages; and lung cancers were diagnosed at metastatic stage.
Figure 1. Distribution of cancers in study population (A) and stage of disease at diagnosis by cancer type (B) (n = 63).
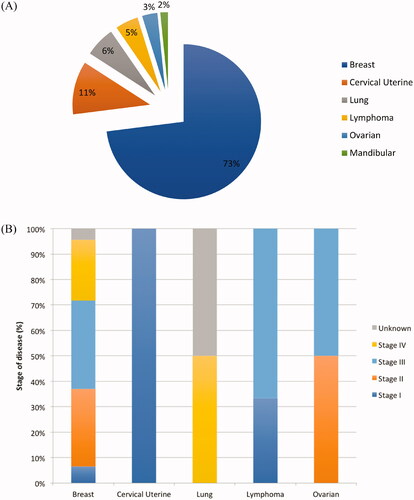
Table 2. Distribution of cancers (n = 63).
Nearly three-quarters of the study population were diagnosed with breast cancer ( and ) which were mainly invasive ductal carcinomas (93.5%) and diagnosed at a locally advanced stage (43.5% T3-TNM and 10.9% T4-TNM). Nearly a quarter (23.9%) of the breast cancer population had metastases at diagnosis.
Table 3. Characteristics of breast cancer occurring during pregnancy.
Cancer management is described in . Median term of pregnancy at (18F)-FDG PET/CT was 24.8 WG (IQR: 15.7–31): 12 patients (19%) underwent the exam during the first trimester, 24 (38.1%) during the second trimester, and 22 (34.9%) during the third trimester. The exam was performed twice in two patients: the first with a T4 invasive ductal carcinoma, at 25 and 31 WG respectively, and the second with a metastatic mutated EGFR + and HER3+ lung cancer, at 26 and 30 WG respectively.
Table 4. (18 F)-FDG PET/CT, treatment, maternal and fetal outcomes.
Impact of (18F)-FDG PET/CT on cancer staging
(18F)-FDG PET/CT modified the initial clinical stage in 38/63 (60.3%) of the patients with subsequently/accordingly modified first-line medical treatment. For 28 (44.4%) patients, (18F)-FDG revealed more extensive lymph node involvement than previously found on clinical staging, and for 10 (15.9%) the exam revealed metastatic disease.
Among these 38 patients, 30 had breast cancer and were subsequently treated by first-line chemotherapy, two had lung cancer harboring mutations related to a first-line medical treatment using tyrosine kinase inhibitors (with the addition of monoclonal antibodies for one), and the remaining six patients had ovarian cancer which was treated by a first-line platinum-based chemotherapy.
Treatment received during pregnancy ()
Of the 63 patients, 46 (73%) received treatment during pregnancy: 42 (66.7%) received an anthracycline-based chemotherapy (doxorubicin or epirubicin + cyclophosphamide) ± taxanes in breast cancer. The three patients with Hodgkin’s lymphoma received vinblastine alone or combined with doxorubicin, bleomycin and dacarbazin.
Targeted therapy was administered to two patients with lung cancer: one received alectinib (tyrosine kinase inhibitor, TKI) and the other a combination of osimertinib (TKI) and trastuzumab (monoclonal antibody).
Surgery was performed in 12 patients (19%), all during the second trimester of pregnancy (median term of 19WG IQR 14–20): one patient underwent laparotomy for ovarian cancer; two patients underwent thoracic surgery for lung cancer; and the remaining nine underwent partial or total mastectomy for breast cancer.
Radiotherapy was delayed until after delivery in all cases.
Maternal outcome
The median follow-up was 287 (extremes 30–1560) days during which seven recurrences were observed. Six of them were subsequent to breast cancer and the last one for cervical uterine cancer.
One patient had had an early medical abortion after the discovery of a metastatic luminal B breast cancer diagnosed at 6WG. The six remaining patients gave birth to healthy newborns.
Among these seven women, four died within 2 years following diagnosis. Three of them were treated for metastatic hormonal HER2-negative breast cancer, the last one for stage IB1 cervical cancer.
Fetal outcomes
Among the 63 patients who underwent (18 F)-FDG PET-CT during their pregnancy, two had a medical pregnancy termination and five a medical abortion. The pregnancy terminations were related to the severity of the disease and the need to start cancer therapy. The first patient with medical pregnancy termination was diagnosed at 21 WG with ovarian cancer, FIGO IIIC. The (18F)- FDG PET-CT and medical pregnancy termination were performed at 24 and 26 WG respectively. The second one was diagnosed with Hodgkin lymphoma at 13 WG. The (18F)- FDG PET-CT and medical pregnancy termination were performed at 13 and 14 WG respectively, in order to start medical treatment. Five patients with locally advanced breast cancer had a medical abortion. The (18F)- FDG PET-CTs and medical abortions were performed between 4–14 and 6–14 WG respectively.
Data about the final outcome of the 56 ongoing pregnancies were available for 51 patients. Among these 51 births, one neonatal death occurred after delivery by emergency cesarean section at 29 WG for severe preeclampsia. This patient was diagnosed with locally advanced T3N1M0 breast cancer at 13 WG. The (18F)-FDG PET/CT was performed at 13 WG and chemotherapy was started at 15 WG.
All the 50 patients delivered healthy newborns with a good neonatal status (normal Apgar score) and no morphological abnormality. The median term at delivery was 37WG (IQR 35–38; extremes 26–39WG) and the median birth weight was 2470 g (IQR 2075–3150; extremes 1040–3520 g).
The data of 46 children were available at 6 months, 29 at 12 months, and 15 at 24 months. No cases of mental retardation, childhood cancer or malformation were reported within the 2 years up to the last update (June 2021).
Discussion
In the present study, (18F)-FDG PET/CT modified the initial clinical stage by detecting more extended lymph node involvement and/or unknown metastases in nearly two thirds of the patients with pregnancy-associated cancer while no fetal adverse effects of PET-CT were found.
The epidemiological and oncological characteristics of our population were similar to those of previous studies on pregnancy-associated cancers including the distribution of cancer types and stage [Citation5,Citation6]. In breast cancers, which are the most common pregnancy-associated cancers, most of the tumors were diagnosed at a locally advanced stage [Citation18,Citation19]. Several studies report nodal involvement in around 53–70% of pregnancy-associated breast cancers [Citation6,Citation19]. Moreover, in our study, almost three-quarters of cancers were diagnosed during the first and second trimesters of the pregnancy imposing treatment before delivery. Pre-therapeutic staging thus determines the oncological approach and contributes to deciding on the best option for both mother and fetus in a shared decision-making manner. It is therefore crucial to evaluate the disease stage by the means of highly accurate imaging tools to assess both lymph node status and distant metastases. In this specific setting, investigations based on ultrasound and chest X-Ray have been shown to be of limited value for evaluating the true initial disease stage [Citation20]. A meta-analysis by Sun et al. found that (18F)-FDG PET/CT was much more accurate than conventional imaging procedures for the assessment of distant metastases in breast cancer [Citation20]: the sensitivities, specificities, and positive and negative likelihood ratios of conventional exams were: 0.57, 0.88, 4.8 and 0.49, respectively, versus 0.99, 0.95, 21.1, and 0.02 for PET-CT [Citation20]. PET/MRI could also be an interesting alternative to (18F)-FDG PET/CT to avoid fetal X-ray radiation exposure [Citation12]. Several studies have compared the diagnostic performance of PET/MRI to PET/CT, MRI and CT, and found that PET/MRI is more accurate than the other imaging procedures for staging [Citation21–23]. Nevertheless, this exam is not available in all centers and also raises the issue of the effects of gadolinium, used as a contrast agent in MRI, which is potentially associated with fetal and newborn risks [Citation24]. In a large Canadian retrospective cohort study, Ray et al. evaluated the long-term safety after exposure to MRI and to gadolinium during pregnancy [Citation24]. Compared with non-exposure, exposure to MRI during the first trimester of pregnancy was not associated with an increased risk of congenital anomalies, neoplasm, vision or hearing loss either in the fetus or in early childhood [Citation24]. However, regardless of the pregnancy term, gadolinium MRI was associated with an increased risk of a broad set of rheumatological, inflammatory, and infiltrative skin conditions and an increased risk of stillbirth or neonatal death [Citation24]. Although the risk of developing nephrogenic systemic fibrosis in utero has been described, a recent review suggests that it is non-significant [Citation25]. Overall, while (18F)-FDG PET/CT would appear to be an appropriate compromise, its innocuity remains a matter of debate [Citation26].
To the best of our knowledge, this study constitutes the largest reported series of women undergoing (18F)-FDG PET/CT during pregnancy. In our cohort, no adverse effects were observed during the pregnancies: no increase in the rate of miscarriage or premature delivery was observed with a median term at delivery of 37 WG. These values are in accordance with those observed in patients with pregnancy-associated cancers not investigated by (18F)-FDG PET/CT with a median term at delivery ranging from 35 to 37 WG [Citation6,Citation27,Citation28]. Except for one case of severe preeclampsia associated with neonatal death, no other pathology associated with pregnancy was noted. Similarly, although a low median birth weight of 2470 g was noted, our results are in accordance with previous studies on pregnancy-associated cancers with a median birthweight ranging from 2415 g to 2770 g [Citation6,Citation27]. However, our results should be analyzed in light of the fact that two-thirds of our population had lymph node involvement and/or distance metastases hence with a potential risk of induction of premature delivery.
To date, only a few small series have estimated fetal radiation exposure associated with (18F)-FDG PET/CT [Citation15,Citation16,Citation29–32]. Fetal exposure to ionizing radiation has long been a matter of concern and the risks are thought to be both from the ionizing radiation and the potential toxicity of the radioisotopes on embryo development and on the fetus. In these case series, (18F)-FDG PET/CT was performed using a lower dose of (18F)-FDG, hyperhydratation, and diuresis increased by furosemide injection and/or the placement of a Foley catheter to decrease the dose received by the fetus. Alternatively, a previous study using PET alone suggested that the radiation dose to the fetus should be as low as possible [Citation15]. However, no data about the accuracy of the PET-only technique are available, which decreases the benefit of the radiological exam while exposing the fetus to radiation. Moreover, the CT component contributes to 8.4–30.8% of the total absorbed dose while the PET component contributes to 70–91.6% [Citation29]. In a study published in 2015, Sawatzke et al. used continuous PET/CT on mice to explore the placental-fetal transport of (18F)-FDG and developed a kinetic model of glucose transport from the placenta to the fetus [Citation33]. They revealed that the avidity of placenta for glucose was greater than any other tissue examined in the maternal or fetal body, and that the fetal uptake of FDG depended on its weight. Thus, the placenta and fetus have a high glucose metabolic system, accounting for a quarter of the total administered FDG dose thus with a potential negative impact [Citation33]. According to the last study by Fregonara et al. in 2017, an administered activity of 200 MBq would expose the fetus to a dose ranging from 5.2 mSv to 1.4 mSv, depending on the term of pregnancy at PET/CT and the position of the fetus [Citation29,Citation32]. Both Takalkar et al. and Zanotti-Fregonara et al. calculated that fetal dose exposure was not only below the threshold dose for deterministic effects during PET/CT, but also below the threshold at which stochastic effects have been documented in humans [Citation34]. In 2017, Gill et al. reviewed one abstract and eight reports of pregnant patients who underwent 18F-FDG PET/CT with dosimetry data [Citation30]. All 18F-FDG fetal doses were below the threshold for deterministic risks from radiation. Expected radiation effects on the fetus, such as mental retardation or malformation could arise above a threshold dose of 100–200 mGy [Citation35]. The effects of radiation depend on the term of pregnancy, and radiation in the first week after conception will result in failure to implant, while organ malformations may arise if the threshold is reached during organogenesis, between 2 and 8 WG [Citation36,Citation37]. Between 8 and 25 WG, the fetal central nervous system (CNS) is especially sensitive to radiation, and exposure above the threshold of 60 mGy could cause mental retardation [Citation38]. In most diagnostic radiological exams, the doses are much lower than the threshold dose for deterministic effects and present no substantial risk of fetal death, malformation, or impairment of mental development [Citation39]. In the current study, no newborn malformations were noted. Moreover, radiation exposure during pregnancy and up to delivery may also cause stochastic effects, independently from the radiation dose received, such as a greater risk of childhood cancer and leukemia. However, none of these effects have been reported in these studies [Citation40,Citation41]. Although no mental alteration was noted in our population, the short follow-up cannot exclude the risk of late mental retardation as, among the 46 children with available follow-up, only one-third had a follow-up of 2 years.
Some limitations of the present study deserve to be underlined. First, although the present series is the largest to date, the small sample size cannot exclude potential underestimation of side effects associated with the use of (18F)-FDG PET/CT during pregnancy. Second, we were not able to have access to a comparison group with PET/MRI. Third, the relatively short follow-up may also underestimate the risk of late complications particularly on childhood development and on oncological risks. Fourth, although the exact protocol for (18F)-FDG PET/CT performed out of Tenon hospital was not systematically recorded, probably using higher dose, no fetal abnormality was reported. Finally, among the 63 patients undergoing (18F)-FDG PET/CT in our study, data were available for only 50 newborns representing a potential bias. This underlines the crucial role of practitioners in requesting advice from the CALG or other reference centers to aliment the database so that patients can be better informed about the risk of 18F-FDG PET/CT during pregnancy.
In conclusion, given the low absorbed doses received by the fetus, radiological exams should not be withheld during pregnancy when they are the golden standard in the general population. This is particularly true if a radiological exam would have an impact on stage assessment and therefore change the oncological management. However, its administration should be optimized in consultation with the nuclear medicine department to reduce fetal exposure. As neonatal outcomes seem to be reassuring after (18F)-FDG PET/CT imaging, the benefits for maternal health seem to outweigh the potential risks of fetal radiation, and (18F)-FDG PET/CT should not be delayed because of the on-going pregnancy.
Supplemental Material
Download MS Word (14.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Smith LH, Danielsen B, Allen ME, et al. Cancer associated with obstetric delivery: results of linkage with the California cancer registry. Am J Obstet Gynecol. 2003;189(4):1128–1135.
- Lee YY, Roberts CL, Dobbins T, et al. Incidence and outcomes of pregnancy-associated cancer in Australia, 1994–2008: a population-based linkage study. BJOG. 2012;119(13):1572–1582.
- Parazzini F, Franchi M, Tavani A, et al. Frequency of pregnancy related cancer: a population based linkage study in Lombardy, Italy. Int J Gynecol Cancer. 2017;27(3):613–619.
- Matthews TJ, Hamilton BE. First births to older women continue to rise. NCHS Data Brief. 2014;(152):1–8.
- de Haan J, Verheecke M, Van Calsteren K, et al. Oncological management and obstetric and neonatal outcomes for women diagnosed with cancer during pregnancy: a 20-year international cohort study of 1170 patients. Lancet Oncol. 2018;19(3):337–346.
- Boudy AS, Zaccarini F, Selleret L, et al. Oncological management of pregnancy-associated cancers: analysis from the French CALG (Cancer Associé à La Grossesse) network. Acta Oncol. 2020;59(9):1043–1050.
- Loibl S, Schmidt A, Gentilini O, et al. Breast cancer diagnosed during pregnancy: adapting recent advances in breast cancer care for pregnant patients. JAMA Oncol. 2015;1(8):1145–1153.
- Peccatori FA, Azim HA Jr, Orecchia R, et al. Cancer, pregnancy and fertility: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi160–vi170.
- Azim HA Jr, Santoro L, Russell-Edu W, et al. Prognosis of pregnancy-associated breast cancer: a meta-analysis of 30 studies. Cancer Treat Rev. 2012;38(7):834–842.
- Litton JK, Warneke CL, Hahn KM, et al. Case control study of women treated with chemotherapy for breast cancer during pregnancy as compared with nonpregnant patients with breast cancer. Oncologist. 2013;18(4):369–376.
- Amant F, von Minckwitz G, Han SN, et al. Prognosis of women with primary breast cancer diagnosed during pregnancy: results from an international collaborative study. J Clin Oncol. 2013;31(20):2532–2539.
- Umutlu L, Antoch G, Herrmann K, et al. PET/MR imaging of the female pelvis. Semin Nucl Med. 2019;49(6):512–520.
- Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32(27):3048–3058.
- Senkus E, Kyriakides S, Ohno S, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 Suppl 5(Suppl 5):v8–v30.
- Takalkar AM, Khandelwal A, Lokitz S, et al. 18F-FDG PET in pregnancy and fetal radiation dose estimates. J Nucl Med. 2011;52(7):1035–1040.
- Zanotti-Fregonara P, Laforest R, Wallis JW. Fetal radiation dose from 18F-FDG in pregnant patients imaged with PET, PET/CT, and PET/MR. J Nucl Med. 2015;56(8):1218–1222.
- Boellaard R, Delgado-Bolton R, Oyen WJ, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42(2):328–354.
- Boudy AS, Naoura I, Selleret L, et al. Propensity score to evaluate prognosis in pregnancy-associated breast cancer: Analysis from a French cancer network. Breast. 2018;40:10–15.
- Amant F, Loibl S, Neven P, et al. Breast cancer in pregnancy. Lancet. 2012;379(9815):570–579.
- Sun Z, Yi YL, Liu Y, et al. Comparison of whole-body PET/PET-CT and conventional imaging procedures for distant metastasis staging in patients with breast cancer: a Meta-analysis. Eur J Gynaecol Oncol. 2015;36(6):672–676.
- Taneja S, Jena A, Goel R, et al. Simultaneous whole-body 18F-FDG PET-MRI in primary staging of breast cancer: a pilot study. Eur J Radiol. 2014;83(12):2231–2239.
- Sawicki LM, Grueneisen J, Schaarschmidt BM, et al. Evaluation of 18F-FDG PET/MRI, 18F-FDG PET/CT, MRI, and CT in whole-body staging of recurrent breast cancer. Eur J Radiol. 2016;85(2):459–465.
- Pujara AC, Kim E, Axelrod D, et al. PET/MRI in breast cancer. J Magn Reson Imaging. 2019;49(2):328–342.
- Ray JG, Vermeulen MJ, Bharatha A, et al. Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA. 2016;316(9):952–961.
- Mervak BM, Altun E, McGinty KA, et al. MRI in pregnancy: Indications and practical considerations. J Magn Reson Imaging. 2019;49(3):621–631.
- Tirada N, Dreizin D, Khati NJ, et al. Imaging pregnant and lactating patients. Radiographics. 2015;35(6):1751–1765.
- Loibl S, Han SN, von Minckwitz G, et al. Treatment of breast cancer during pregnancy: an observational study. Lancet Oncol. 2012;13(9):887–896.
- Boudy AS, Naoura I, Zilberman S, et al. Arguments pour différencier les cancers du sein associés à la grossesse de ceux diagnostiqués dans le post-partum: expérience monocentrique du réseau cancer associé à la grossesse (CALG) [clues to differentiate pregnancy-associated breast cancer from those diagnosed in postpartum period: a monocentric experience of pregnancy-associated cancer network (CALG)]. Bull Cancer. 2017;104(6):574–584.
- Xie T, Zanotti-Fregonara P, Edet-Sanson A, et al. Patient-specific computational model and dosimetry calculations for PET/CT of a patient pregnant with twins. J Nucl Med. 2018;59(9):1451–1458.
- Gill MM, Sia W, Hoskinson M, et al. The use of PET/CT in pregnancy: a case report of malignant parathyroid carcinoma and a review of the literature. Obstet Med. 2018;11(1):45–49.
- Zanotti-Fregonara P, Chastan M, Edet-Sanson A, et al. New fetal dose estimates from 18F-FDG administered during pregnancy: standardization of dose calculations and estimations with voxel-based anthropomorphic phantoms. J Nucl Med. 2016;57(11):1760–1763.
- Xie T, Zaidi H. Fetal and maternal absorbed dose estimates for positron-emitting molecular imaging probes. J Nucl Med. 2014;55(9):1459–1466.
- Sawatzke AB, Norris AW, Spyropoulos F, et al. PET/CT imaging reveals unrivaled placental avidity for glucose compared to other tissues. Placenta. 2015;36(2):115–120.
- Brent RL. Protection of the gametes embryo/fetus from prenatal radiation exposure. Health Phys. 2015;108(2):242–274.
- Kal HB, Struikmans H. Radiotherapy during pregnancy: fact and fiction. Lancet Oncol. 2005;6(5):328–333.
- International Commission on Radiological Protection. Pregnancy and medical radiation. Ann ICRP. 2000;30(1):iii–viii, 1–43.
- Streffer C, Shore R, Konermann G, et al. Biological effects after prenatal irradiation (embryo and fetus). A report of the International Commission on Radiological Protection. Ann ICRP. 2003;33(1–2):5–206.
- Otake M, Schull WJ, Lee S. Threshold for radiation-related severe mental retardation in prenatally exposed A-bomb survivors: a re-analysis. Int J Radiat Biol. 1996;70(6):755–763.
- Zanotti-Fregonara P, Stabin MG. New fetal radiation doses for 18F-FDG based on human data. J Nucl Med. 2017;58(11):1865–1866.
- Doll R, Wakeford R. Risk of childhood cancer from fetal irradiation. Br J Radiol. 1997;70:130–139.
- Brent RL. Saving lives and changing family histories: appropriate counseling of pregnant women and men and women of reproductive age, concerning the risk of diagnostic radiation exposures during and before pregnancy. Am J Obstet Gynecol. 2009;200(1):4–24.
