Abstract
Background
Electrochemotherapy (ECT) harnesses electric pulses to enhance cytotoxic drug delivery into tumors and has entered the armamentarium to treat superficially metastatic melanoma. We performed a systematic review and meta-analysis to assess treatment patterns and patient outcomes.
Methods
PubMed, Medline, Embase, and the Cochrane Library databases were queried for publication from inception to September 2020. Primary outcome measures were overall and complete response rate (ORR and CRR); secondary outcomes included local control rate (LCR) and overall survival (OS).
Results
Twenty-seven studies met the selection criteria for a total of 1161 individuals (mean age 71 years) and 5308 tumors (weighted mean size 14 mm). The majority of patients (n = 1124) underwent bleomycin-ECT. Aggregate ORR was 77.6% (95% confidence interval [CI] 71.0 − 83.2%) and CRR 48% (95% CI 42 − 54%), with no significant difference between the route of bleomycin administration (ORR, 69.2 vs. 81.9% following intravenous or intratumoral bleomycin, p = .37) and tumor size (p = .69). When reported (n = 8 studies), 1- and 2-year LCR ranged from 54 to 89% and 72 to 74%, respectively, and 1-year OS (n = 3 studies) from 67 to 89%.
Conclusions
ECT with either intratumoral or intravenous bleomycin confers a high therapeutic response in cutaneous metastatic melanoma. Moderate evidence supports its low toxicity and durability of local control.
Electrochemotherapy (ECT) is associated with a 77% overall response rate (ORR).
Intravenous and intratumoral bleomycin are equally effective.
There are no relevant toxicity concerns.
One-year local tumor control rate ranges from 54 to 89%.
Current literature has significant variation in reporting.
Highlights
Introduction
The occurrence of skin metastases is a common event in patients with advanced melanoma, with incidence rates ranging from 5 to 10% (in the form of satellites or in-transit deposits) to almost 50% (in the form of distant metastases) [Citation1–4]. As a result, treatment strategies abound, including surgery, radiotherapy, local or locoregional therapies, and, more recently, also systemic treatment with targeted agents and immune checkpoint inhibitors [Citation5,Citation6].
Recent advances in the systemic treatment of melanoma are supported by large randomized trials [Citation7,Citation8]. Conversely, the abundance of local and locoregional therapeutic options makes clinical decision-making challenging owing to the ever-shifting therapy environment and lack of comparative studies. An exception is oncolytic immunotherapy with T-VEC that was approved based on the evidence of a randomized trial, although it was not compared with the current standard of care for stage IV melanoma [Citation9–11].
Still, the path to designing comparative trials requires the standardization of each procedure/approach and the accumulation of solid evidence. Herein, we summarize the current supporting evidence for electrochemotherapy (ECT), a relatively novel treatment modality first described by Mir et al. in the early 1990s and then standardized in 2006 [Citation12–14].
ECT involves the co-delivery of a poorly permeant cytotoxic drug (bleomycin or cisplatin) and short local electric pulses, which induce reversible permeabilization (electroporation) of the cell membrane promoting drug diffusion into cells. The final antitumor effect is the cumulative result produced by a composite mechanism of action, including immediate vasoconstriction, direct cytotoxicity, a delayed vascular disruptive action, and local immune response [Citation15–18].
The procedure was first introduced in Europe, the USA, and Australia to treat skin cancers and cutaneous metastases not amenable to surgical resection [Citation12,Citation13,Citation19–21] and was then standardized in 2006 with the European Standard Operating Procedures of ECT (ESOPE) [Citation22,Citation23]. Since then, the technique has been further refined and optimized, although the basic principle remains unaltered [Citation24]. A 2014 meta-analysis on the efficacy of skin-directed therapies, including 915 patients with 4313 superficial metastases (mostly from melanoma and breast cancer), indicated the comparable efficacy of ECT, radiation, photodynamic, intralesional, and topical therapies [Citation25]. Currently, ECT is applied in the palliation of skin metastases from various tumor histotypes [Citation26] and is included in the European Society of Medical Oncology (ESMO) melanoma guidelines to treat satellite and in-transit disease not amenable to surgical resection [Citation27,Citation28]. Its application in melanoma has been extensively reported, with appreciable benefits in terms of tolerability, local tumor control, and patient quality of life [Citation29–32]. Interestingly, growing evidence supports the feasibility of its application in concert with either targeted therapy or immune checkpoint inhibitors [Citation33–37]. Therefore, we performed a systematic review and meta-analysis to determine the activity of ECT in the treatment of superficially metastatic melanoma, attempt to characterize treatment patterns, and assess patient outcomes.
Material and methods
Search strategy and study selection
A systematic literature search was conducted in PubMed, Embase, Medline, and The Cochrane Library databases for human-only studies written in English and published from inception until September 2020. Search terms were (‘electrochemotherapy or electroporation’ AND (‘melanoma’ OR ‘skin metastases’). Two investigators (FP and RA) independently reviewed all records from the initial search strategy. We considered only the studies including at least four patients with skin metastases from melanoma. Meeting abstracts and case reports were excluded. Only ECT with systemically (intravenous) or locally injected (intratumoral) chemotherapy agents were considered. The studies where ECT was used in association (i.e., in the same procedure) with locoregional chemotherapy were excluded.
Data extraction
AG and MG independently extracted data from the relevant studies. These included patient demographics and tumor characteristics, ECT modalities and eventual additional ECT sessions, previous/following systemic treatment; adopted response criteria, objective overall and complete response rate (ORR and CRR), toxicity, median time to progression (TTP), local control rate (LCR), the median duration of response, and overall survival (OS).
Statistical analysis
The pre-specified outcome measure of interest was tumor response (indicated as either per-tumor or per-patient response) after the first ECT application. The majority of studies analyzed (n = 22, 81%) used either the World Health Organization (WHO) or the response evaluation criteria in solid tumors (RECIST). The primary endpoints were ORR and CRR; if not explicitly stated, ORR equaled CR plus PR. Secondary endpoints were toxicity, TTP or LCR, duration of response, and OS. For all analyses of ORR, CRR, and LCR, pooled event rates were calculated with 95% confidence intervals (CIs). For the meta-analysis, a fixed-effects model or a random-effects model were considered. However, the extent of heterogeneity was significant, so a random-effects model was reported for all analyses. The extent of heterogeneity between studies was performed with the Cochran Q test and I2 test. All probability values were two-tailed with p < .05. Subgroup analysis was performed according to the type (bleomycin or cisplatin), route (intravenous vs. intratumoral) of chemotherapy administration, and tumor size. We then conducted publication bias analyses to control that published studies may present results that differ from those reported in unpublished studies. We examined two publication bias tests for the primary endpoint: Egger’s regression method and Begg and Mazumdar’s rank correlation test [Citation38,Citation39]. In both tests, the absence of publication bias is indicated by non-significant results. Quality assessment was performed in each of the included studies by two independent reviewers (AG and FP) using the Newcastle–Ottawa Scale (NOS) for retrospective cohort studies. A star system of the NOS (range 0–9 stars) was developed for the evaluation [Citation40]. The highest value for quality assessment was nine stars. Statistical analyses were performed using Comprehensive Meta-Analysis version 3 software (Biostat, Englewood, NJ).
Results
Included studies
The initial search yielded 745 records. The study selection process is shown in . We ultimately included 27 retrospective and prospective studies () for a total of 1161 patients (mean 43/study, range 4–283) with a weighted mean age of 71 years). There were 5308 tumors (median 197 per study, range 10–932). Thirteen studies (48%) included only melanoma patients, whereas 14 (52%) also included other histotypes (proportion of melanoma patients, 10–65%). In addition, seven studies did not report the number of treated tumors or the number of tumors in the melanoma subgroup.
Table 1. Study cohort.
Median tumor size was 10 mm (range, 6–23) and weighted mean tumor size was 14 mm. Tumor size (and response) were reported by using different methods (the ‘largest diameter’, [n = 11 studies], the ‘product of the two largest tumor diameters’ [n = 11]; ‘tumor volume’ [n = 3], ‘photo score difference’ [n = 1], or other assessment [n = 1]. Only 14 studies reported a synthetic description of tumor size; among these, median tumor size was 10 mm (range, 6.5–23). The modality of chemotherapy administration was intravenous bleomycin (n = 21 studies, 78%), intratumoral bleomycin (n = 14 studies, 52%), intratumoral cisplatin (n = 4 studies, 15%); intravenous cisplatin, n = 1 study, 4%).
The analyzed publications were from Europe (n = 23 studies), North America (n = 3 studies), and Australia (n = 1 study); one study involved both European and American centers. There were 21 (78%) prospective and 6 (22%) retrospective studies. Among the former, there were seven multicentric studies and three monocentric randomized trials (involving intra-patient – i.e., individual tumor randomization in all cases).
Overall response
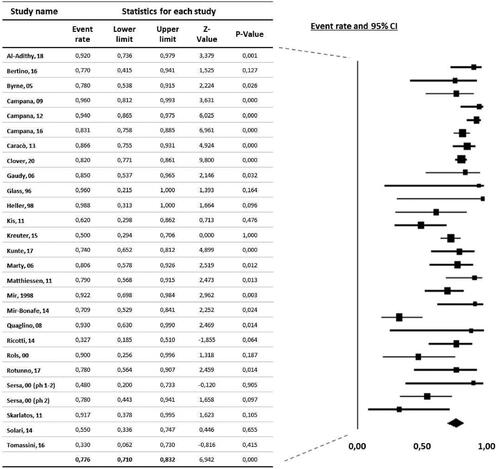
ORR was available from 27 studies. According to the random effect model, the pooled ORR was 77.6% (95% CI 71.0–83.2%) ().
Figure 2. Forest plot diagram of overall response rate in melanoma following electrochemotherapy. Legend: study (first author), year of publication, and overall response rate (ORR) with 95% confidence interval (CI). The vertical line represents the ‘line of no effect’. Black squares indicate ORR, and error bars indicate 95% CI. Square and bar size indicates the proportional weight of the study. The diamond represents the summary effect (pooled results).
Complete response
Data on CR was available in 27 studies. According to the random-effect model, the pooled CR rate was 48% (95% CI 42–54%).
Subgroup analysis
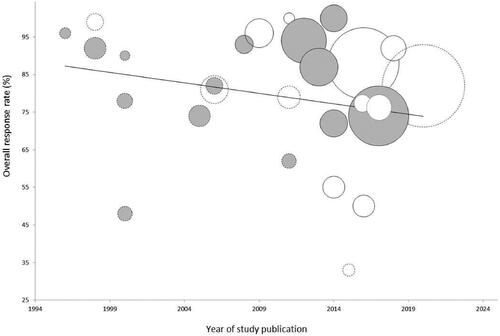
The ORR did not significantly change according to the year of study publication, modality of reporting of tumor response, and size of the study population (). Excluding the two studies on cisplatin-ECT, the pooled ORR was 74.3% (95% CI 64.9–81.9%). Among the studies on bleomycin-ECT, we evaluated response according to the route of chemotherapy administration. The pooled ORR was slightly higher, although not statistically significant, in patients treated with intratumoral (81.9%, 95% CI 64.8–91.7%) compared with intravenous bleomycin (69.2%, 95% CI 53.4–81.4%; p = .37). Finally, the local response did not correlate with the reported median tumor size (p = .69 according to metaregression).
Figure 3. Bubble chart of ECT clinical studies in melanoma according to tumor response and year of publication (1996–2020). Each circle represents a study, whose diameter is proportional to sample size (melanoma patients only). Gray circles represent studies on melanoma; white circles represent studies based on mixed patient populations. Circles with a continuous border represent studies reporting ‘per-patient’ tumor response; circles with dotted lines refer to studies reporting ‘per-tumor’ response. The overall response rate (trend line) did not significantly change over the years (Spearman rs = −0.28, p = .16), or according to the modality of response assessment (point-biserial correlation, r = 0.17, p = .39) and size of the study population (rs = 0.09, p = .66).
Publication bias
Both Begg’s and Egger’s tests were not significant for the ORR analysis (p = .11 and .34, respectively).
Local control and survival
Information on recurrence rates was variable across studies. When reported, 1- and 2-year LCR ranged from 54 to 89% (n = 4 studies) [Citation39–42] and 72 to 87% (n = 4 studies) [Citation27,Citation29,Citation43,Citation44], respectively. Patient survival was also underreported with only three studies providing formal survival analysis; 1-year OS ranged from 67 to 89% [Citation30,Citation40,Citation41].
Retreatment
Seven of 27 (26%) studies included information regarding the percentage of patients receiving additional ECT applications [Citation29,Citation31,Citation32,Citation41–44]. In these reports, the retreatment rate ranged from 18 to 100%, but tumor response was only sporadically indicated [Citation29,Citation31].
Toxicity
The reporting criteria were heterogeneous across publications. The main adverse events included local pain (36–100% of patients) [Citation29,Citation31,Citation32,Citation42,Citation45–47] and various forms of skin toxicity. These included mild erythema and edema (3–100%) and ulceration (0–26%, graded as G3 – according to the CTAE criteria – in 1–18% of cases) [Citation31,Citation32,Citation42,Citation43]. Conversely, there were no significant concerns regarding systemic toxicity, although there was a case of fatal respiratory failure following ECT with intravenous bleomycin in a patient with preexisting lung comorbidity [Citation43].
Patient-reported outcomes
Only a minority of studies – on patients with different histotypes – included quality of life assessment or patient-reported outcomes [Citation30,Citation41,Citation42,Citation48]. Unfortunately, the adopted instruments were fairly consistent, so there are no data available for the melanoma population.
Discussion
The evidence presented offers the most up-to-date synthesis of the efficacy of ECT in the treatment of superficially metastatic melanoma. Skin metastases occur in up to 50% of patients with advanced disease [Citation1–3]. Despite the increasing efficacy of systemic treatment (anti-CTLA-4 and anti-PD1 antibodies, BRAF/MEK inhibitors) and improved patient outcome [Citation49], this condition can cause debilitating symptoms and remains an essential element in determining the quality of life in a subset of patients. Hence, it will become even more critical to provide safe and effective management of skin metastases and secure a satisfactory quality of life. Interestingly, ECT exerts a composite antivascular effect – produced by immediate, short-lived vasoconstriction and a slower disruption of the endothelial cells [Citation50] – which is exploited in the clinic to achieve rapid palliation of hemorrhaging metastases [Citation51].
Activity
Our analysis is based on 27 studies and 1161 patients with more than 5000 superficial metastases treated with ECT. We observed substantial variability in study design, cohort size, and outcome reporting. Notwithstanding this, pooled data confirm the potent and consistent () antitumor activity of ECT, as demonstrated by the 77% ORR after a single course of treatment and nearly half of patients achieving tumor clearance locally. These findings are consistent with the meta-analysis by Mali et al. in patients with skin metastases (36% from melanoma), reporting an ORR of 80.6% (CRR, 56.8%) [Citation52], and with the European ECT registry, indicating an ORR of 82% (CRR 64%) [Citation53]. We cannot exclude that concomitant treatments may have played a role in determining tumor response to ECT and consolidating local control. Generally, this information is either non-available or sparsely reported. As a result, no conclusions can be drawn also regarding the effect of ECT on OS. For this reason, we advocate the reporting of concomitant/adjuvant therapies in future studies, in line with the InspECT recommendations [Citation54]. Nonetheless, the results from a large multi-institutional study, including a formal two-month wash-out from other oncological therapies, are in line with the results of the current meta-analysis [Citation42].
Treatment patterns
There is a clear preference for bleomycin over cisplatin in current ECT practice (). According to the ESOPE, only bleomycin can be administered either locally or systemically [Citation14,Citation24,Citation27]. Additionally, and not surprisingly, it has become the preferred agent thanks to its favorable toxicity profile and low cost. Among patients who underwent bleomycin-ECT, we intratumoral injection was associated with a slightly higher response rate, although not statistically significant, than intravenous infusion (ORR, 81.9 vs. 69.2%, respectively, p = .37). These findings have important implications because they support a patient-targeted approach where drug administration is also informed by the presence of comorbidities and risk for systemic toxicity, rather than tumor burden only. In other words, these results favor the use of intratumoral bleomycin as long as local injection is feasible and provides adequate chemotherapy distribution. This holds true in patients with small-size tumors and whose renal function, respiratory comorbidities, or concomitant risk factors for lung fibrosis contraindicates the use of systemic bleomycin [Citation27]. Although still investigational, calcium electroporation (i.e., the combination of intralesional calcium and electric pulses) is emerging as a safe and effective alternative for these patients [Citation55]. To date, no study has compared the efficacy of the three ECT modalities (i.t. CCDP, i.t. BLM, and i.v. BLM) in melanoma. Previous studies suggest similar efficacy, although comparisons may be biased by small numbers and underlying differences in patient characteristics [Citation22,Citation42]. The results from the cumulative analysis of the InspECT registry (n = 2482 tumors, 932 [37%] from melanoma) indicate a beneficial effect on CRR from i.v. over i.t. BLM starting from a tumor size larger than 2 cm [Citation53].
Interestingly, the ECT procedure is flexible and can be tailored to patient/tumor features. Thus, it can be administered either as a local or locoregional treatment, also depending upon treatment intent [Citation56]. In fact, ECT exerts a local effect when chemotherapy is injected intratumorally; conversely, it acts as a locoregional treatment when chemotherapy is administered systemically, and electrodes are applied over large treatment surfaces.
Retreatment
We were able to assess tumor response only after the first course of ECT. However, in clinical practice, about half of patients undergo repeated sessions to consolidate results or treat new (i.e., out-of-field) lesions [Citation29–32,Citation41,Citation42]. Thus, the actual response rate may be even higher than reported in our analysis. Nonetheless, this outcome is challenging to capture, particularly when the high number of metastases and their heterogeneity coupled with post-treatment inflammation impede reliable tumor assessment. Thus, not surprisingly, only a minority of studies provide data on response following retreatment. Additionally, investigators adopted either per-tumor or per-patient response assessment (, ). To provide meaningful information, we advocate the need to report disease burden in detail, possibly following criteria shared by the melanoma community [Citation57]. As pointed out by the authors of a recent meta-analysis, the lack of outcome reporting following retreatment is a critical issue to address to set standards for the frequency of ECT application and maximize the clinical benefit and the use of resources [Citation58].
Tumor size
Our findings differ from previous reports [Citation29,Citation32,Citation53]. Two meta-analyses observed a significant drop in CRR in lesions larger than 2 or 3 cm. However, these results were based on heterogeneous cohorts including patients with skin cancers and cutaneous metastases from different histotypes [Citation52,Citation59]. We posit the following explanations for the lack of association between tumor size and response in our study. The first is the variation in tumor size measurement and criteria for response assessment (). Second, in the studies where response assessment was performed on target lesions, these might not have been representative of the actual tumor burden, thus introducing a bias toward under/overestimation. Finally, objective measurement of cutaneous metastases may be challenging due to heterogeneous presentation, which ranges from isolated nodules to extensive lymphangitic infiltration or bulky deposits. To be effective, ECT requires simultaneous tumor exposure to chemotherapy and electric fields [Citation60,Citation61]. Hence, while small lesions, even when scattered, can be easily targeted, bulky tumors (i.e., exceeding 2 or 3 cm in thickness) cannot be entirely covered with needle electrodes used in standard ECT [Citation27]. To allow for more reliable correlative studies, we propose to discern between flat and bulky skin metastases and align response assessment with current recommendations on the quality of reporting in ECT clinical studies [Citation54]. Finally, and interestingly, the results of a recent study on variable-geometry ECT (an investigational ECT approach using longer electrode probes able to encompass large target volumes) challenge the supposedly inverse correlation between tumor size and response. In this mono-institutional phase-II study, which enrolled 30 patients with bulky soft tissue tumors (median size 4.7 cm), the CRR was 63% after a single course of treatment [Citation62].
Limitations of current literature
Our review highlights the variation in reporting of current ECT literature and the lack of some relevant outcome metrics. Critically, data on treatment toxicity, response after retreatment, local control, and patient-reported outcomes are under-reported or lacking. As remarked by the authors of a recent review on ECT, this information is essential to properly evaluate palliative treatments [Citation58]. For instance, only a minority of studies include survival statistics on local tumor control. Nonetheless and encouragingly, in these reports, the 2-year in-field control rate – following one or repeated courses – ranges between 74 and 87%, even though the contribution of the following treatments cannot be excluded [Citation29,Citation31]. Finally, in a multicenter study including a formal wash-out from previous/following therapies (n = 376 patients with 1304 tumors, 65% from melanoma), the ORR was 88%, with a CRR of 50% [Citation42].
Toxicity
Skin ulceration seems to be the most relevant concern, although the heterogeneity of reporting across studies prevented more detailed analyses. To better inform multidisciplinary team discussion and patient choices, we encourage investigators to consistently report on this aspect, e.g., adopting the CTCAE and even the PRO-CTCAE criteria [Citation63]. To address this and other methodological issues, the International Network for Sharing Practices on ECT (InspECT) has advanced a proposal to improve the quality of reporting, which is also available as a checklist to investigators [Citation54].
Treatment options for skin metastases
Radical surgical resection should be pursued whenever possible. An ongoing randomized trial investigates the complementary role of surgery in patients with metastatic melanoma who achieve PR/SD with immunotherapy [Citation64]. Conversely, patients with multiple, bulky, or rapidly growing satellite/in-transit metastases not amenable to surgical treatment are still candidates to a range of treatments, including external beam/low dose radiotherapy [Citation65,Citation66], locoregional chemotherapy (either in the form of isolated limb perfusion [ILP] or isolated limb infusion [ILI]), talimogene laherparepvec (T-VEC), Rose Bengal (PV-10), and ECT [Citation28,Citation67]. Outside Europe, other lesional therapies include interleukin-2 (IL-2), diphencyprone (DPCP), with pathologic CR reported in 44 and 20% of patients, respectively [Citation67–70]. These approaches, which vary in the mechanism of action and delivery, provide a valuable therapeutic opportunity in different clinical scenarios. Thus, while locoregional chemotherapy leverages vascular isolation to deliver high-dose chemotherapy to limb-confined disease [Citation71,Citation72], injection therapies (T-VEC, PV-10, ECT, and IL-2) can also be applied in patients with distant metastases. Interestingly, local immunotherapy may exert a beneficial systemic effect, particularly with current anti-PD1 agents [Citation73,Citation74]. However, and importantly, the application of locoregional therapies should be carefully weighed against systemic treatment to maximize the opportunity of long-term benefit from targeted or immunotherapy [Citation75]. Although the major randomized trials have only marginal representation of patients with stage III in-transit and stage IV cutaneous-only melanoma, recent data show sustained response rates to checkpoint blockade and support their early application [Citation76–78]. More recent strategies have been derived from neoadjuvant trials [Citation79,Citation80]. Although investigational, neoadjuvant targeted therapy or double-agent immunotherapy in patients with stage III resectable disease has been associated with 2-year relapse-free survival ranging to 79–96% [Citation81]. These findings open new avenues for novel integrated approaches combining local and systemic treatment [Citation33–37]. In this regard, ECT has been shown to induce a local immune response and, sporadically, an abscopal effect [Citation15–18,Citation82,Citation83].
Integration of ECT with systemic treatment
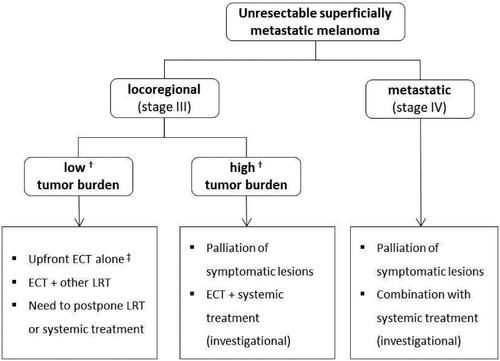
Current ECT indications in melanoma are summarized in . Although the main application is in patients with locoregional disease [Citation28], preliminary findings support the combination of ECT and systemic treatment in the metastatic setting.
Figure 4. Summary of the current indications to ECT in melanoma. ECT: electrochemotherapy; LRT: locoregional therapies. †A consensus on how to define low/high disease burden is lacking. For instance, < 10 lesions and tumor size < 3 cm have been proposed to identify patients with low burden who are candidates to isolated limb infusion; whereas ≤ 20 lesions have been proposed as a threshold of clinical benefit for isolated limb perfusion (Poklepovic AS, Carvajal RD, Prognostic Value of Low Tumor Burden in Patients With Melanoma. Oncology 2018;32(9):e90–e96). Current parameters used in clinical ECT practice include the number, size, distribution and anatomical location of skin metastases and the rate of their appearance. ‡In patients not fit for other LRT, target therapy or immunotherapy and following multidisciplinary evaluation.
A retrospective analysis of 15 patients treated with ipilimumab and ECT reported a 67% local ORR (CRR, 27%) and a 60% systemic ORR, with no toxicity concerns [Citation36]. In addition, lower post-treatment circulating regulatory T cells (Tregs) at week-12 (but not at baseline) was associated with improved response.
A multi-institutional retrospective study evaluated the outcome of 127 patients who received ipilimumab vs. ipilimumab plus local peripheral treatment (in the form of radiation or ECT). Interestingly, the investigators observed a prolonged OS in the combination group (93 vs. 42 weeks HR, 0.46; p = .003) [Citation35] and. the survival benefit was confirmed at multivariate analysis after adjusting for prognostic factors. Likewise, in a recent retrospective matched-cohort study, the HR for systemic PFS and OS was 1.7 and 1.8, respectively, in patients treated with pembrolizumab-ECT or pembrolizumab alone [Citation84], again prompting the conduction of prospective comparative trials.
Given these promising results, future studies should enroll more homogeneous populations (i.e., patients with in-transit only or superficially-only metastatic disease) to better delineate the subgroups most likely to benefit from treatment and establish the most effective protocols. The search for an expert agreement on the use of ECT in melanoma is ongoing [Citation85]. While some procedural aspects (i.e., anesthesia, chemotherapy, and electrodes) are guided by the ESOPE guidelines [Citation14,Citation24], others (i.e., the extension of treatment field, the inclusion of safety margins, and frequency of retreatment) remain controversial [Citation56,Citation85]. The standardization of the procedure represents the way forward to conduct the next generation of ECT studies in combination with immunotherapy.
Study limitations
Some limitations of this analysis are noteworthy to point out. The first relates to the heterogeneity of the included studies, with their inherent risk of selection bias and the lack of patient-level data [Citation86–91]. Thus, we could not perform subgroup analyses according to disease stage. Nonetheless, we provide an up-to-date report and highlight the barriers to more comprehensive systematic overviews. Second, we could assess tumor response only after one ECT cycle, whereas approximately 50% of patients undergo retreatment in clinical practice [Citation30,Citation31]. Lastly, we acknowledge the publication of previous reviews and meta-analyses on ECT, although none of these was focused on melanoma. Two of these reports included a subgroup analysis and reported an ORR of 81%, consistent with our findings (Supplementary Table 1) [Citation52,Citation92].
Conclusions
We report the largest and most comprehensive meta-analysis on the efficacy of ECT in the treatment of metastatic cutaneous melanoma. Altogether, the evidence presented reinforces the notion that this approach yields favorable oncologic outcomes in terms of local response, local tumor control and low toxicity, thus adding to the armamentarium of the oncology team. Nonetheless, the impact on patient quality of life is scarcely reported, and there is variation in ECT clinical practice, emphasizing the need for comprehensive and high-quality reporting in future prospective studies.
Ethics approval
This article does not contain any studies with human or animal subjects.
Author contributions
Conceptualization: Fausto Petrelli; Literature search: Fausto Petrelli, Antonio Ghidini; Data analysis: Fausto Petrelli; Original draft preparation: Luca Giovanni Campana, Andrea Simioni; Review and editing: Luca Giovanni Campana, Andrea Simioni, and Giovanni Ghidini. All authors read and approved the final manuscript.
Supplemental Material
Download MS Word (17.2 KB)Acknowledgments
The authors thank all the authors of the studies we analyzed in this meta-analysis and the participants who made those studies possible. The authors also thank Dr Sara Valpione, CRUK Manchester Institute, for her contribution during various stages of the article preparation.
Disclosure statement
The authors have no relevant financial or non-financial interests to disclose.
Data availability statement
The dataset of this study is available on request from the authors.
Additional information
Funding
References
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2 Pt 1):228–236.
- Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33(2 Pt 1):161–182.
- Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a Meta-analysis of data. South Med J. 2003;96(2):164–167.
- Pawlik TM, Ross MI, Johnson MM, et al. Predictors and natural history of in-transit melanoma after sentinel lymphadenectomy. Ann Surg Oncol. 2005;12(8):587–596.
- Keilholz U, Ascierto PA, Dummer R, et al. ESMO consensus conference recommendations on the management of metastatic melanoma: under the auspices of the ESMO guidelines committee. Ann Oncol. 2020;31(11):1435–1448.
- Coit DG, Thompson JA, Albertini MR, et al. Cutaneous melanoma, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(4):367–402.
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372(1):30–39.
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–2017.
- Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780–2788.
- Andtbacka RHI, Collichio F, Harrington KJ, et al. Final analyses of OPTiM: a randomized phase III trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in unresectable stage III-IV melanoma. J Immunother Cancer. 2019;7:1–11.
- Chesney J, Puzanov I, Collichio F, et al. Patterns of response with talimogene laherparepvec in combination with ipilimumab or ipilimumab alone in metastatic unresectable melanoma. Br J Cancer. 2019;121(5):417–420.
- Mir LM, Orlowski S, Belehradek J, et al. Electrochemotherapy potentiation of antitumour effect of bleomycin by local electric pulses. Eur J Cancer. 1991;27(1):68–72.
- Belehradek M, Domenge C, Luboinski B, et al. Electrochemotherapy, a new antitumor treatment. First clinical phase I-II trial. Cancer. 1993;72(12):3694–3700.
- Mir LM, Gehl J, Sersa G, et al. Standard operating procedures of the electrochemotherapy: instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the CliniporatorTM by means of invasive or non-invasive electrodes. Eur J Cancer Suppl. 2006;4(11):14–25.
- Sersa G, Teissie J, Cemazar M, et al. Electrochemotherapy of tumors as in situ vaccination boosted by immunogene electrotransfer. Cancer Immunol Immunother. 2015;64(10):1315–1327.
- Calvet CY, Mir LM. The promising alliance of anti-cancer electrochemotherapy with immunotherapy. Cancer Metastasis Rev. 2016;35(2):165–177.
- Gerlini G, Di Gennaro P, Borgognoni L. Enhancing anti-melanoma immunity by electrochemotherapy and in vivo dendritic-cell activation. Oncoimmunology. 2012;1(9):1655–1657.
- Di Gennaro P, Gerlini G, Urso C, et al. CD4 + FOXP3+ T regulatory cells decrease and CD3 + CD8+ T cells recruitment in TILs from melanoma metastases after electrochemotherapy. Clin Exp Metastasis. 2016;33(8):787–798.
- Glass LF, Pepine ML, Fenske NA, et al. Bleomycin-mediated electrochemotherapy of metastatic melanoma. Arch Dermatol. 1996;132(11):1353–1357.
- Heller R, Jaroszeski MJ, Reintgen DS, et al. Treatment of cutaneous and subcutaneous tumors with electrochemotherapy using intralesional bleomycin. Cancer. 1998;83(1):148–157.
- Byrne CM, Thompson JF, Johnston H, et al. Treatment of metastatic melanoma using electroporation therapy with bleomycin (electrochemotherapy). Melanoma Res. 2005;15(1):45–51.
- Marty M, Sersa G, Garbay JR, et al. Electrochemotherapy - An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: results of ESOPE (European standard operating procedures of electrochemotherapy) study. Eur J Cancer Suppl. 2006;4(11):3–13.
- Mir LM. Bases and rationale of the electrochemotherapy. Eur J Cancer Suppl. 2006;4(11):38–44.
- Gehl J, Sersa G, Matthiessen LW, et al. Updated standard operating procedures for electrochemotherapy of cutaneous tumours and skin metastases. Acta Oncol. 2018;57(7):874–882.
- Spratt DE, Gordon Spratt EA, Wu S, et al. Efficacy of skin-directed therapy for cutaneous metastases from advanced cancer: a Meta-analysis. J Clin Oncol. 2014;32(28):3144–3155.
- Matthiessen LW, Chalmers RL, Sainsbury DC, et al. Management of cutaneous metastases using electrochemotherapy. Acta Oncol. 2011;50(5):621–629.
- Campana LG, Miklavčič D, Bertino G, et al. Electrochemotherapy of superficial tumors - Current status: basic principles, operating procedures, shared indications, and emerging applications. Semin Oncol. 2019;46(2):173–191.
- Michielin O, van Akkooi A, Lorigan P, et al. ESMO consensus conference recommendations on the management of locoregional melanoma: under the auspices of the ESMO guidelines committee. Ann Oncol. 2020;31(11):1449–1461.
- Quaglino P, Mortera C, Osella-Abate S, et al. Electrochemotherapy with intravenous bleomycin in the local treatment of skin melanoma metastases. Ann Surg Oncol. 2008;15(8):2215–2222.
- Campana LG, Mocellin S, Basso M, et al. Bleomycin-based electrochemotherapy: clinical outcome from a single institution's experience with 52 patients. Ann Surg Oncol. 2009;16(1):191–199.
- Campana LG, Valpione S, Mocellin S, et al. Electrochemotherapy for disseminated superficial metastases from malignant melanoma. Br J Surg. 2012;99(6):821–830.
- Kunte C, Letulé V, Gehl J, et al. Electrochemotherapy in the treatment of metastatic malignant melanoma: a prospective cohort study by InspECT. Br J Dermatol. 2017;176(6):1475–1485.
- Valpione S, Campana LG, Pigozzo I, et al. Consolidation electrochemotherapy with bleomycin in metastatic melanoma during treatment with dabrafenib. Radiol Oncol. 2015;49(1):71–74.
- Heppt MV, Eigentler TK, Kähler KC, et al. Immune checkpoint blockade with concurrent electrochemotherapy in advanced melanoma: a retrospective multicenter analysis. Cancer Immunol Immunother. 2016;65(8):951–959.
- Theurich S, Rothschild SI, Hoffmann M, et al. Local tumor treatment in combination with systemic ipilimumab immunotherapy prolongs overall survival in patients with advanced malignant melanoma. Cancer Immunol Res. 2016;4(9):744–754.
- Mozzillo N, Simeone E, Benedetto L, et al. Assessing a novel immuno-oncology-based combination therapy: ipilimumab plus electrochemotherapy. Oncoimmunology. 2015;4(6):e1008842–8.
- Campana LG, Edhemovic I, Soden D, et al. Electrochemotherapy - emerging applications technical advances, new indications, combined approaches, and multi-institutional collaboration. Eur J Surg Oncol. 2019;45(2):92–102.
- Egger M, Smith GD, Schneider M, et al. Bias in Meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634.
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101.
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. The Ottawa Hospital Research Institute. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Bertino G, Sersa G, De Terlizzi F, et al. European research on electrochemotherapy in head and neck cancer (EURECA) project: results of the treatment of skin cancer. Eur J Cancer. 2016;63:41–52.
- Campana LG, Testori A, Curatolo P, et al. Treatment efficacy with electrochemotherapy: a multi-institutional prospective observational study on 376 patients with superficial tumors. Eur J Surg Oncol. 2016;42(12):1914–1923.
- Mir-Bonafé JM, Vilalta A, Alarcón I, et al. Electrochemotherapy in the treatment of melanoma skin metastases: a report on 31 cases. Actas Dermosifiliogr. 2015;106(4):285–291.
- Caracò C, Mozzillo N, Marone U, et al. Long-lasting response to electrochemotherapy in melanoma patients with cutaneous metastasis. BMC Cancer. 2013;13:564–565.
- Rotunno R, Campana LG, Quaglino P, et al. Electrochemotherapy of unresectable cutaneous tumours with reduced dosages of intravenous bleomycin: analysis of 57 patients from the international network for sharing practices of electrochemotherapy registry. J Eur Acad Dermatol Venereol. 2018;32(7):1147–1154.
- Ricotti F, Giuliodori K, Cataldi I, et al. Electrochemotherapy: an effective local treatment of cutaneous and subcutaneous melanoma metastases. Dermatol Ther. 2014;27(3):148–152.
- Serša G, Štabuc B, Čemažar M, et al. Electrochemotherapy with cisplatin: clinical experience in malignant melanoma patients. Clin Cancer Res. 2000;6:863–867.
- Al-Hadithy N, Dehnel A, George A, et al. Patient reported outcomes in prospective cohort study of electrochemotherapy. Int J Surg. 2018;52:110–119.
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535–1546.
- Jarm T, Cemazar M, Miklavcic D, et al. Antivascular effects of electrochemotherapy: implications in treatment of bleeding metastases. Expert Rev Anticancer Ther. 2010;10(5):729–746.
- Gehl J, Geertsen PF. Efficient palliation of haemorrhaging malignant melanoma skin metastases by electrochemotherapy. Melanoma Res. 2000;10(6):585–589.
- Mali B, Jarm T, Snoj M, et al. Antitumor effectiveness of electrochemotherapy: a systematic review and Meta-analysis. Eur J Surg Oncol. 2013;39(1):4–16.
- Clover AJP, de Terlizzi F, Bertino G, et al. Electrochemotherapy in the treatment of cutaneous malignancy: outcomes and subgroup analysis from the cumulative results from the pan-European international network for sharing practice in electrochemotherapy database for 2482 lesions in 987 patients. Eur J Cancer. 2020;138:30–40.
- Campana LG, Clover AJ, Valpione S, et al. Recommendations for improving the quality of reporting clinical electrochemotherapy studies based on qualitative systematic review. Radiol Oncol. 2016;50(1):1–13.
- Falk H, Matthiessen LW, Wooler G, et al. Calcium electroporation for treatment of cutaneous metastases; a randomized double-blinded phase II study, comparing the effect of calcium electroporation with electrochemotherapy. Acta Oncol. 2018;57(3):311–319.
- Campana LG, Kis E, Bottyán K, et al. Electrochemotherapy for advanced cutaneous angiosarcoma: a European register-based cohort study from the international network for sharing practices of electrochemotherapy (InspECT). Int J Surg. 2019;72:34–42.
- Muilenburg DJ, Beasley GM, Thompson ZJ, et al. Burden of disease predicts response to isolated limb infusion with melphalan and actinomycin D in melanoma. Ann Surg Oncol. 2015;22(2):482–488.
- Morley J, Grocott P, Purssell E, et al. Electrochemotherapy for the palliative management of cutaneous metastases: a systematic review and Meta-analysis. Eur J Surg Oncol. 2019;45(12):2257–2267.
- Mali B, Miklavcic D, Campana LG, et al. Tumor size and effectiveness of electrochemotherapy. Radiol Oncol. 2013;47(1):32–41.
- Miklavcic D, Corovic S, Pucihar G, et al. Importance of tumour coverage by sufficiently high local electric field for effective electrochemotherapy. Eur J Cancer Suppl. 2006;4(11):45–51.
- Miklavcic D, Snoj M, Zupanic A, et al. Towards treatment planning and treatment of deep-seated solid tumors by electrochemotherapy. Biomed Eng Online. 2010;9:10.
- Simioni A, Valpione S, Granziera E, et al. Ablation of soft tissue tumours by long needle variable electrode-geometry electrochemotherapy: final report from a single-arm, single-Centre phase-2 study. Sci Rep. 2020;10(1):2291.
- Atkinson TM, Ryan SJ, Bennett AV, et al. The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): a systematic review. Support Care Cancer. 2016;24(8):3669–3676.
- Holmberg CJ, Katsarelias D, Jespersen H, et al. Surgery of metastatic melanoma after systemic therapy - the SUMMIST trial: study protocol for a randomized controlled trial. Acta Oncol. 2021;60(1):52–55.
- Seegenschmiedt MH, Keilholz L, Altendorf-Hofmann A, et al. Palliative radiotherapy for recurrent and metastatic malignant melanoma: prognostic factors for tumor response and long-term outcome: a 20-year experience. Int J Radiat Oncol Biol Phys. 1999;44(3):607–618.
- Jang HS, Spillane A, Boyle F, et al. Radiotherapy can cause haemostasis in bleeding skin malignancies. Case Rep Med. 2012;2012:168681.
- Read T, Lonne M, Sparks DS, et al. A systematic review and Meta-analysis of locoregional treatments for in-transit melanoma. J Surg Oncol. 2019;119(7):887–896.
- Nadler A, Look Hong NJ, Alavi N, et al. Lesional therapies for in-transit melanoma. J Surg Oncol. 2020;122(6):1050–1056.
- Lo MC, Garioch J, Moncrieff MD. Sequencing in management of in-transit melanoma metastasis: diphencyprone versus isolate limb infusion. J Plast Reconstr Aesthet Surg. 2020;73(7):1263–1267.
- Weitman ES, Zager JS. Regional therapies for locoregionally advanced and unresectable melanoma. Clin Exp Metastasis. 2018;35(5–6):495–502.
- Grünhagen DJ, Kroon HM, Verhoef C. Perfusion and infusion for melanoma in-Transit metastases in the era of effective systemic therapy. Am Soc Clin Oncol Educ Book. 2015;35(35):e528–e534.
- Carr MJ, Sun J, Kroon HM, et al. Oncologic outcomes after isolated limb infusion for advanced melanoma: an international comparison of the procedure and outcomes between the United States and Australia. Ann Surg Oncol. 2020;27(13):5107–5118.
- Ribas A, Dummer R, Puzanov I, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 Immunotherapy. Cell. 2017;170(6):1109–1119.e10.
- Dummer R, Hoeller C, Gruter IP, et al. Combining talimogene laherparepvec with immunotherapies in melanoma and other solid tumors. Cancer Immunol Immunother. 2017;66(6):683–695.
- Valpione S, Carlino MS, Mangana J, et al. Rechallenge with BRAF-directed treatment in metastatic melanoma: a multi-institutional retrospective study. Eur J Cancer. 2018;91:116–124.
- Zaremba A, Philip M, Hassel JC, et al. Clinical characteristics and therapy response in unresectable melanoma patients stage IIIB-IIID with in-transit and satellite metastases. Eur J Cancer. 2021;152:139–154.
- Storey KS, Abdul-Latif M, Kreft S, et al. Checkpoint inhibitor treatment in patients with isolated in-transit melanoma metastases. J Clin Oncol. 2020;38(15):10070–10070.
- Nan Tie E, Lai-Kwon J, Rtshiladze MA, et al. Efficacy of immune checkpoint inhibitors for in-transit melanoma. J Immunother Cancer. 2020;8(1):e000440.
- Blank CU, Rozeman EA, Fanchi LF, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med. 2018;24(11):1655–1661.
- Versluis JM, Long GV, Blank CU. Learning from clinical trials of neoadjuvant checkpoint blockade. Nat Med. 2020;26(4):475–484.
- Menzies AM, Amaria RN, Rozeman EA, et al. Pathological response and survival with neoadjuvant therapy in melanoma: a pooled analysis from the international neoadjuvant melanoma consortium (INMC). Nat Med. 2021;27(2):301–309.
- Falk H, Lambaa S, Johannesen HH, et al. Electrochemotherapy and calcium electroporation inducing a systemic immune response with local and distant remission of tumors in a patient with malignant melanoma – a case report. Acta Oncol. 2017;56(8):1126–1131.
- Calvet CY, Famin D, André FM, et al. Electrochemotherapy with bleomycin induces hallmarks of immunogenic cell death in murine Colon cancer cells. Oncoimmunology. 2014;3(4):e28131–11.
- Campana LG, Peric B, Mascherini M, et al. Combination of pembrolizumab with electrochemotherapy in cutaneous metastases from melanoma: a comparative retrospective study from the InspECT and Slovenian cancer registry. Cancers. 2021;13(17):4289.
- Campana LG, Quaglino P, Bechara F, et al. Electrochemotherapy in melanoma: a European e-Delphi survey to define a consensus on indications, treatment modalities and quality indicators. Eur J Surg Oncol. 2019;45(2):E18.
- Kreuter A, van Eijk T, Lehmann P, et al. Electrochemotherapy in advanced skin tumors and cutaneous metastases - a retrospective multicenter analysis. J Dtsch Dermatol Ges. 2015;13(4):308–315.
- Solari N, Spagnolo F, Ponte E, et al. Electrochemotherapy for the management of cutaneous and subcutaneous metastasis: a series of 39 patients treated with palliative intent. J Surg Oncol. 2014;109(3):270–274.
- Kis E, Oláh J, Ócsai H, et al. Electrochemotherapy of cutaneous metastases of melanoma-a case series study and systematic review of the evidence . Dermatol Surg. 2011;37(6):816–824.
- Gaudy C, Richard MA, Folchetti G, et al. Randomized controlled study of electrochemotherapy in the local treatment of skin metastases of melanoma. J Cutan Med Surg. 2006;10(3):115–121.
- Rols MP, Bachaud JM, Giraud P, et al. Electrochemotherapy of cutaneous metastases in malignant melanoma. Melanoma Res. 2000;10(5):468–474.
- Skarlatos I, Kyrgias G, Mosa E, et al. Electrochemotherapy in cancer patients: first clinical trial in Greece. In Vivo. 2011;25(2):265–274.
- Seyed Jafari SM, Jabbary Lak F, Gazdhar A, et al. Application of electrochemotherapy in the management of primary and metastatic cutaneous malignant tumours: a systematic review and Meta-analysis. Eur J Dermatol. 2018;28(3):287–313.