Abstract
Background
Prevalence of peripheral neuropathy (PN) has been studied in patients undergoing treatment with taxanes, platinums and vinca alkaloids. The prevalence is unknown in the general oncological cancer population, characterized by advanced age, comorbidities and heterogeneous treatments.
Material and Methods
A cross-sectional survey was administered to all adult patients, attending outpatient services at three Danish departments of oncology. The survey contained the EORTC-CIPN20, the EORTC-QLQ-C30, the GAD7 and PHQ9 questionnaires. A high PN symptom score was defined as a summary score ≥30 points on the CIPN20. P-values were adjusted for multiple testing.
Results
With an overall response rate of 83% (2839 patients), prevalence of PN was 17% overall, varying from 6 to 33% between diagnosis groups.
A high score was more common among females (19 vs. 14%, p = .008), smokers (21 vs. 15%, p = .04), patients living alone (21 vs. 15%, p = .002) and patients using cannabis (29 vs. 15%, p < .001), as well as patients suffering from diabetes (26 vs. 16%, p < .001), cardiac heart disease (27 vs. 16%, p < .001), arthritis (32 vs. 15%, p < .001) or chronic obstructive pulmonary disease (25 vs. 16%, p = .01). High score patients were also older (69ys vs 67ys, p = .048) and more likely experiencing polypharmacy (OR = 3.38 [95% CI, 2.64;4.35]).
Patients with a high CIPN20 symptom score scored worse on all EORTC QLQ-C30 function and symptom scales. The mean adjusted C30 SumScore difference was −18.66 ([95% CI, −20.31; −17.02], p < .001).
Conclusion
Symptoms of PN are experienced widely across cancer groups in the oncology setting. PN symptoms were associated with clinically relevant worse health-related quality of life and with patient-related factors as living alone, various comorbidities, polypharmacy, and cannabis use.
Background
During the last five decades, combination chemotherapy has been used to increase the survival of patients with cancer but many treatment regimens include neurotoxic components [Citation1]. Chemotherapy-induced peripheral neuropathy (CIPN) is the most prevalent type of cancer-related peripheral neuropathy (PN) experienced by up to 37–84% of patients three months after ending treatment [Citation2]. This is a high number, considering the detrimental and lasting effects PN can have on patients’ quality of life (QoL) [Citation3], and the lack of prevention and treatment options of CIPN [Citation4].
CIPN exemplifies the etiological complexity of cancer-related peripheral neuropathies with numerous complex and inter-dependent disease mechanisms unfolding in multiple physiological systems in a specific patient [Citation5]. More than 100 assessment strategies have been proposed [Citation6], which is one of the reasons why rates of CIPN vary significantly depending on cancer patient group, treatment type and study design [Citation6,Citation7].
Prevalence of peripheral neuropathy among the general middle-aged and elderly population has been estimated to be between 4–9% [Citation8]. No study has investigated an overall estimate of PN for an oncological population. The current CIPN estimates are formed on the basis of relatively homogenous datasets from uniform patient trajectories i.e., patients receiving neurotoxic chemotherapy in the adjuvant setting [Citation2] and data has primarily been collected from before the era of immunotherapy and biological agents [Citation2,Citation3], which can also induce PN [Citation9].
Based on the range, diversity and heterogeneity of PN pathophysiology [Citation10], we wanted to explore the overall extent of PN symptoms in a general oncological population.
Material and methods
The study design was a cross-sectional, anonymous survey. All patients aged 18 years or older and attending ambulatory services were eligible for inclusion. Patients could receive active treatment (chemotherapy, immunotherapy, targeted therapies, or radiotherapy) or attend as part of a follow-up visit. Patients without a cancer diagnosis were excluded. The study was conducted at the Departments of Clinical Oncology, Zealand University Hospital and the Department of Oncology, Odense University Hospital, Denmark. Information was reported as recommended by the STROBE guideline (of the EQUATOR network) for cross-sectional studies (see supplementary material).
Questionnaire formation
The questionnaire was comprised of 91 items in total; 17 study-specific questions on sociodemographics, cancer disease, treatment, lifestyle and comorbidity, number of prescription medicines (>5 prescription medicines was defined as polypharmacy), 7 study-specific questions on cannabis use, and the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 (C30), the EORTC-CIPN20 module (CIPN20), the GAD7 and the PHQ9 questionnaires [Citation3,Citation11–13]. An internal multidisciplinary group revised and validated the survey questions and the questionnaire was reviewed by a board of patient and relative representatives.
A pilot study (N = 14) was conducted to evaluate the feasibility of the questionnaire as well as the validity, relevance and wording of the questions, using retrospective think-aloud interviews [Citation14].
Questionnaire conduction
The survey was distributed within standard opening hours usually 8:30 AM to 3:00 PM, for a three-week period at each department. A serial number identified each survey. Surveys were tallied daily. Response rate was calculated by dividing the total surveys distributed with the total amount of completed surveys collected plus patient refusals. Patient refusal were not questioned, but when given freely, patients primarily refused because of time constraints and language barriers. Patients were assisted with completion of the questionnaires in cases of vision problems or paralysis/paresthesia.
Exposure and outcomes
A summary score was calculated based on items 1–18 of the CIPN20 as recent findings have been unable to confirm stable subscale structures of the conventional sensory, motor and autonomic domains of the CIPN20 [15,16]. A total score of 30 points or more on the CIPN20 score was used to divide patients into a high score and low score group [Citation15,Citation16] using the high score as our definition of PN. We allowed for up to two missing items. Missing item values were imputed with the patient average score (preferentially rounded down), resulting in 34 patient score imputations.
The C30 scores, including five function scores, nine symptom scores, a global health score and the C30 Sum Score were calculated based on the EORTC 3rd Edition Manual [Citation17].
Summary scores were calculated for the PHQ9 and GAD7. These summary scores were divided into a categorical variable of two levels using a cutoff point equal to or above 10 points [Citation18,Citation19]. At this cutoff, the likelihood ratio for the presence of a major depressive disorder is 7.1 with a sensitivity and specificity of 88% [Citation18], and Spitzer et al reported in a study that most patients (89%) with general anxiety disorder (GAD) had GAD-7 scores of 10 or greater, whereas most patients (82%) without GAD had scores less than 10 [Citation19]. We allowed for scoring of respondent total score for up to two missing items [Citation20]. Average imputed scores were rounded down. This procedure was completed for 34 patient GAD7 scores, and 106 patient PHQ9 scores.
Statistical analysis
We used a two-sample, unpaired Wilcoxon test to test median difference in age and Pearson’s chi squared test to test factors containing two categorical variables for proportional equality by CIPN20 high scores such as gender (male/female), active smoking (yes/no), active treatment (yes/no), cohabitation status (living with partner/living alone), presence of each comorbidity (yes/no), type of active treatment (yes/no), GAD7 (non-case/case) and PHQ9 (non-case/case). For age groups (<30, 30–49, 50–64, 65–80, >80), BMI categories (<18, 18–25, 25–30, >30), education (mandatory school, upper secondary/vocational education, short higher education, medium-length higher education, long higher education) and number of prescription medicines (0–3, 4–5, >5) we used a binomial general linear model, presenting estimates as odds ratios (OR). Q-Q plots were graphed for each regression, establishing a normal distribution of residuals for univariate linear models. The definition of polypharmacy was set at >5 daily prescription medications.
The C30 subscales differences were tested using logistic regression. Q-Q plots were graphed for each regression, establishing a normal distribution of residuals. Results from multivariate analysis were adjusted for age, gender, BMI, active treatment and cohabitation status. The calculated Variance Inflation Factor (VIF) of involved variables showed minimal collinearity.
All p-values were controlled for the false discovery rate using the method developed by Benjamini & Hochberg [Citation21]. Cohen’s d was calculated for unadjusted and adjusted mean differences defining effect sizes as negligible (<0.25), small (>0.25), medium (>0.50) and large (>0.75) [Citation22].
All statistical operations were done in R-Studio (ver.1.3.1093)
Ethics
The study was done in compliance with the tenets of the Declaration of Helsinki. The local regional Ethical Committee reviewed the study (record no. 18-000080). The invitation letter explained the purpose of the survey, the thematic nature of the questions, and emphasized the anonymity of the respondent. The project leads, sponsoring organization and sources of funding were named.
Results
Between 22 April to 7 June 2019, 3435 patients were invited to participate. Upon completion, 22 questionnaires were found ineligible for analyses as 8 were blank and 14 participants did not list a cancer diagnosis (). A total of 2839 questionnaires were eligible for analysis resulting in a response rate of 83%. Participant characteristics are summarized in . More women (59%) than men (41%) completed the questionnaires. Missing answers were minimal and between 2–4% for most categories, albeit higher for alcohol consumption and cannabis use (30% and 11%, respectively).
Figure 1. Flow chart of participants in cross-sectional study of cancer-related peripheral neuropathy among Danish oncological patients from 22 April to 7 June 2019.
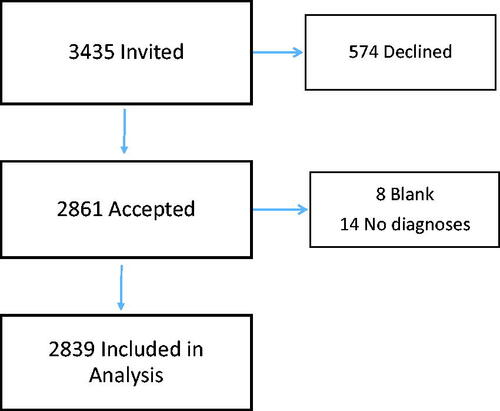
Table 1. Sociodemography, lifestyle, comorbidity and cancer characteristics in a cross-sectional sample of patients in outpatient oncology care.
CIPN20: Prevalence and correlated parameters
Of the 2839 participants, 2533 respondents had evaluable responses of the CIPN20, while 306 had not filled in or partially responded to the CIPN20. The cohort was divided into two groups based on the CIPN20 summary score. A total of 427 participants (17%) scored 30 points or higher (high scorers) and 2107 (83%) scored below (low scorers). The majority of patients received active treatment (67%) and 54% of patients in active treatment received chemotherapy (see ).
summarizes differences between high score and low score groups according to patient related and disease specific characteristics. Several characteristics were significantly associated with a higher proportion of PN high scorers. They were older (median 69 years versus (vs) 67 years, adjusted (adj. p = .048), more often women (19 vs. 14%, adj. p = .01), more lived alone (21 vs. 15%, adj. p = .002), were smokers (21 vs. 15%, adj. p = .04), experienced polypharmacy (OR 3.38, adj. p < .001), and used cannabis (29 vs. 15%, adj. p < .001). There were significantly more participants with a high score among males drinking more than 14 units alcohol per week (22 vs. 11% adj. p = .002) while this was not the case among women (15 vs. 15% p = .877). The proportion of participants in the high score group did not differ on whether patients were actively receiving treatments (17% for both groups, adj. p = .83). There were a higher proportion of high score patients among patients with diabetes (26 vs. 16%, adj. p < .001), cardiac heart disease (CHD) (27 vs. 16%, adj. p < .001), arthritis (32 vs. 15%, adj. p < .001) or chronic obstructive pulmonary disease (COPD) (25 vs. 16%, adj. p = .01) compared to patients without these comorbidities.
Table 2. Differences in demographics in CIPN20 low scorers compared to high scorers (≥30 points) in a cross-sectional sample of patients in outpatient oncology care.
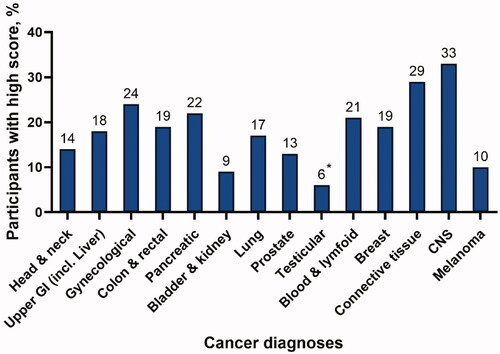
Patients with breast cancer constituted almost 35% of all participants in the high score group, but the prevalence of a high score among patients with breast cancer (19%) was at the same level as for patients with upper gastro-intestinal, colorectal, and lung cancer (17–19%) and less than the prevalence of a high score in patients with gynecological or pancreatic cancer (21–24%). The highest prevalence was found among patients with cancers derived from bone and connective tissue (29%) and CNS tumors (33%) ().
Quality of life
A high PN score was associated with significantly worse mean scores on all C30 subscales when adjusting for age, gender, BMI, active treatment and cohabitation status (). The mean adjusted difference on the C30 SumScore was −18.66 (adj. p < .001), representing a large effect size (Cohen’s d = 1.26) on overall QoL.
Table 3. Health related quality of life for CIPN low scorers vs high scorers among a cross sectional sample of patients in outpatient oncology care.
There were significantly more cases of anxiety (6 vs. 20% adj. p < .001) and depression (8 vs. 30% adj. p < .001) among participants with a PN high score measured with the GAD7 and PHQ9 respectively.
Discussion
To our knowledge, this study is the largest cross-sectional study of PN in a general oncological population to date. The overall prevalence of PN was 17% and thus quite lower than CIPN estimates from prior studies [Citation2,Citation3,Citation23,Citation24], but higher than estimates from general middle aged and elderly non-oncological populations (4–9%) [Citation8]. This is likely due to the heterogeneity of our cohort compared to the more homogenous cohorts in CIPN studies. Our cohort contained patients in follow-up and remission as well as patients in the adjuvant, recurrent and palliative settings, receiving a plethora of other treatments than neurotoxic agents such as platinums, taxanes or vinca alkaloids. This study design resulted in finding a general and somewhat equal distribution of PN symptoms among the most prevalent forms of cancer. This was the main aim and strength of the study, but also the main limitation as this design hampers our ability to assess this pattern in more detail. However, a 2019 position paper on CIPN from the Neurological Complications working group under the auspices of the Multinational Association of Supportive Care in Cancer (MASCC) stated that ‘each class of agents, and perhaps each agent within that class, is likely to have independent risk factors.’ [Citation25]. This conceptualization holds the possibility of a multitude of drug-specific neuropathies developing in any given patient with sufficient alignment of risk factors. This could explain the presence of PN symptoms across such a wide spectrum of cancer patients and their treatments.
We found several patient-related factors associated with PN (score above 30 on the CIPN20). To our knowledge, this is the first study to correlate PN with polypharmacy and cannabis use in an oncology population. Similar to us, prior studies have also found that age [Citation26,Citation27], smoking [Citation2] and alcohol consumption [Citation28] are associated to CIPN while being married has been associated with less risk of CIPN [Citation29]. However, not all studies show an association to these patient-related risk factors [Citation30,Citation31] which may be due to cohort variations, definitions of covariates and limitations in research designs. For instance we found a higher proportion of women in the high score group, while other studies found CIPN equally distributed between genders [Citation24,Citation32], but most research on CIPN has actually been conducted on cohorts of one gender [Citation3,Citation27,Citation31]. Due to the lack of information of specific types of chemotherapy (which may differ by gender) and doses received based on our data we are not able interpret this difference as reflecting differences in use of chemotherapy as has been argued by others [Citation33]. Furthermore, many cross sectional studies of CIPN risk factors rarely report response rates and when they do, they have rates of around 60% [Citation26] while in our population-based study we obtained a response rate of 83%. Although this hopefully increases generalizability of our findings, we know from other health surveys that non-participants are more often men and patients with worse clinical outcomes [Citation34]. Analysis of comorbidities found higher proportions of high scores among patients also sufferings from diabetes, coronary heart disease (CHD), chronic obstructive pulmonary disease (COPD) and arthritis. Diabetes has been shown to increase risk of CIPN [Citation30], but many have not found this in subsequent studies [Citation23,Citation24,Citation27,Citation28]. Arthritis has been identified as possible CIPN risk factor [Citation26,Citation29,Citation32] while the association with CHD and COPD seems a novel finding. However, the research design of the present study precludes any meaningful discussion of the possible pathophysiological link between these disorders and CIPN. Specific estimates for each risk factor should be pursued in studies specifically designed for this, taking into account the impact of multiple confounders of CIPN.
In this study, having a high score on the CIPN20 was associated with clinically meaningful worse scores on all subscales of the C30 even when adjusted for multiple confounders indicating that irrespective of PN etiology these patients have worse health related quality of life (HR-Qol). Previous studies have found smaller differences on all subscales in patients with ovarian cancer (except diarrhea) [Citation26] and patients with breast cancer [Citation3] and CIPN. Pain has been noted as an strong mediator of lower HR-QoL in CIPN [Citation32], however the present study did not investigate the impact of individual C30 symptom scales on HR-QoL. This study did find significantly higher rates of possible cases of depression and anxiety among patients with a high score on the CIPN20. These results are consistent with findings among survivors of colorectal and breast cancer [Citation32,Citation35]
The design of this study entails some limitations. Firstly, there is no clinically validated diagnostic cutoff for PN on the CIPN20 score. Paired data analyses of the CIPN20 summary score and CTCAE scores showed no clear grouping, however mean scores above 30 points were associated with a CTCAE grade 3 for oxaliplatin (n = 3269) and a CTCAE grade 1 for paclitaxel (n = 767) treated patients [Citation15]. Secondly, using items 1–18 on the CIPN20 implies using items with varied specificity. Prior studies found that items concerning the sensory qualities of CIPN are more reliable and better associated with other PN measures than those concerning the autonomous qualities of neuropathy [Citation15,Citation36]. However, it should be noted that this may stem from limitations and usage of said correlative measurement methods, which often measure only PN sensory qualities [Citation37,Citation38]. These two design choices may influence the specificity of the PN estimates in our study. The heterogeneity of the sample and solely patient reported data, resulted in an absence of information on specific chemotherapy, other oncological treatments received and time since diagnosis and treatment. These items were regarded as too influenced by recall bias to provide valid data for analysis in this study. The internal validity of this study could also have been further improved by combining objective measures with subjective measures of PN as is now recommended in studies of CIPN [Citation7,Citation39]. Neuropathy symptoms are not pathognomonic for cancer-related PN and may have arisen from other toxic substances or diseases (alcohol, vitamin deficiencies, diabetic), autoimmunity or genetic disorders [Citation10]. We cannot, based on these data, distinguish neuropathy symptoms and severity of these to be based on chemotherapy, by other exposures, or by a combination hereof.
Conclusion
This study investigated symptoms of PN from the perspective of the clinical heterogeneity found in an out-patient oncology clinic. The findings suggest that PN may be a more universal problem in cancer care compared with findings from previous studies that focus on CIPN and suggest that there is more to be learned about cancer-related PN by studying the phenomenon outside patients in adjuvant treatments. Future studies should focus on PN manifestation stratified by associated comorbidities such as diabetes, CHD or arthritis, or PN symptom development through multiple lines of antineoplastic treatment. The study found novel important patient associated factors for PN such as polypharmacy or cannabis use and symptoms of PN were associated with a large reduction in health related quality of life.
Supplemental Material
Download PDF (18.1 KB)Disclosure statement
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Funding
References
- Staff NP, Grisold A, Grisold W, et al. Chemotherapy-induced peripheral neuropathy: a current review. Ann Neurol. 2017;81(6):772–781.
- Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and Meta-analysis. Pain [Internet]. 2014;155(12):2461–2470.
- Eckhoff L, Knoop A, Jensen MB, et al. Persistence of docetaxel-induced neuropathy and impact on quality of life among breast cancer survivors. Eur J Cancer [Internet]. 2015;51(3):292–300.
- Loprinzi CL, Lacchetti C, Bleeker J, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. J Clin Oncol. 2020;38(28):3325–3348.
- Starobova H, Vetter I. Pathophysiology of Chemotherapy-Induced peripheral neuropathy. Front Mol Neurosci. 2017;10:174.
- McCrary JM, Goldstein D, Boyle F, et al.; IN FOCUS Delphi working party. Optimal clinical assessment strategies for chemotherapy-induced peripheral neuropathy (CIPN): a systematic review and delphi survey. Support Care Cancer. 2017;25(11):3485–3493.
- Molassiotis A, Cheng HL, Lopez V, et al. Are we mis-estimating chemotherapy-induced peripheral neuropathy? Analysis of assessment methodologies from a prospective, multinational, longitudinal cohort study of patients receiving neurotoxic chemotherapy. BMC Cancer. 2019;19(1):19.
- Hanewinckel R, Drenthen J, Van Oijen M, et al. Prevalence of polyneuropathy in the general Middle-aged and elderly population. Neurology. 2016;87(18):1892–1898.
- Stone JB, DeAngelis LM. Cancer-treatment-induced neurotoxicity-focus on newer treatments. Nat Rev Clin Oncol. 2016;13(2):92–105.
- Smith DI, Tran HT, Poku J. Hemodynamic considerations in the pathophysiology of peripheral neuropathy. Blood Press - From bench to bed [internet]. InTech. 2018. Available from: http://www.intechopen.com/books/blood-pressure-from-bench-to-bed/hemodynamic-considerations-in-the-pathophysiology-of-peripheral-neuropathy.
- Groenvold M, Klee MC, Sprangers MAG, et al. Validation of the EORTC QLQ-C30 quality of life questionnaire through combined qualitative and quantitative assessment of patient-observer agreement. J Clin Epidemiol. 1997;50(4):441–450.
- Esser P, Hartung TJ, Friedrich M, et al. The generalized anxiety disorder screener (GAD-7) and the anxiety module of the hospital and depression scale (HADS-A) as screening tools for generalized anxiety disorder among cancer patients. Psychooncology. 2018;27(6):1509–1516.
- Pedersen SS, Mathiasen K, Christensen KB, et al. Psychometric analysis of the patient health questionnaire in danish patients with an implantable cardioverter defibrillator (the DEFIB-WOMEN study). J Psychosom Res. 2016;90:105–112.
- Boren T, Ramey J. Thinking aloud: reconciling theory and practice. IEEE Trans Prof Commun. 2000;43(3):261–278.
- Le-Rademacher J, Kanwar R, Seisler D, et al. Patient-reported (EORTC QLQ-CIPN20) versus physician-reported (CTCAE) quantification of oxaliplatin- and paclitaxel/carboplatin-induced peripheral neuropathy in NCCTG/alliance clinical trials. Support Care Cancer. 2017;25(11):3537–3544.
- Kieffer JM, Postma TJ, van de Poll-Franse L, et al.; In Collaboration with the CI-PeriNomS Group. Evaluation of the psychometric properties of the EORTC chemotherapy-induced peripheral neuropathy questionnaire (QLQ-CIPN20). Qual Life Res. 2017;26(11):2999–3010.
- Giesinger JM, Kieffer JM, Fayers PM, et al.; EORTC Quality of Life Group. Replication and validation of higher order models demonstrated that a summary score for the EORTC QLQ-C30 is robust. J Clin Epidemiol. 2016;69:79–88.
- Manea L, Gilbody S, McMillan D. Optimal cut-off score for diagnosing depression with the patient health questionnaire (PHQ-9): a Meta-analysis. CMAJ. 2012;184(3):E191–E196.
- Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097.
- Arrieta J, Aguerrebere M, Raviola G, et al. Validity and utility of the patient health questionnaire (PHQ)-2 and PHQ-9 for screening and diagnosis of depression in rural Chiapas, Mexico: a Cross-Sectional study. J Clin Psychol. 2017;73(9):1076–1090.
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B [Internet]. 1995;57(1):289–300.
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. New York: Lawrence Erlbaum Associates; 1988.
- Greenwald MK, Ruterbusch JJ, Beebe-Dimmer JL, et al. Risk of incident claims for chemotherapy-induced peripheral neuropathy among women with breast cancer in a medicare population. Cancer [Internet]. 2019;125(2):269–277.
- Shah A, Hoffman EM, Mauermann ML, et al. Incidence and disease burden of chemotherapy-induced peripheral neuropathy in a population-based cohort. J Neurol Neurosurg Psychiatry. 2018;89(6):636–641.
- Chan A, Hertz DL, Morales M, et al. Biological predictors of chemotherapy-induced peripheral neuropathy (CIPN): MASCC neurological complications working group overview. Support Care Cancer. 2019;27(10):3729–3737.
- Ezendam NPM, Pijlman B, Bhugwandass C, et al. Chemotherapy-induced peripheral neuropathy and its impact on health-related quality of life among ovarian cancer survivors: results from the population-based PROFILES registry. Gynecol Oncol. 2014;135(3):510–517.
- Bulls HW, Hoogland AI, Kennedy B, et al. A longitudinal examination of associations between age and chemotherapy-induced peripheral neuropathy in patients with gynecologic cancer. Gynecol Oncol [Internet]. 2019;152(2):310–315.
- Molassiotis A, Cheng HL, Leung KT, et al. Risk factors for chemotherapy-induced peripheral neuropathy in patients receiving taxane- and platinum-based chemotherapy. Brain Behav. 2019;9(6):1–10.
- Nyrop KA, Deal AM, Reeder‐Hayes KE, et al. Patient‐reported and clinician‐reported chemotherapy‐induced peripheral neuropathy in patients with early breast cancer: Current clinical practice. Cancer [Internet]. 2019;125(17):2945–2954.
- Hershman DL, Till C, Wright JD, et al. Comorbidities and risk of Chemotherapy-Induced peripheral neuropathy among participants 65 years or older in southwest oncology group clinical trials. J Clin Oncol. 2016;34(25):3014–3022.
- Simon NB, Danso MA, Alberico TA, et al. The prevalence and pattern of chemotherapy-induced peripheral neuropathy among women with breast cancer receiving care in a large community oncology practice. Qual Life Res. 2017;26(10):2763–2772.
- Bonhof CS, Trompetter HR, Vreugdenhil G, et al. Painful and non-painful chemotherapy-induced peripheral neuropathy and quality of life in colorectal cancer survivors: results from the population-based PROFILES registry. Support Care Cancer. 2020;28(12):5933–5941.
- Smith EML, Zanville N, Kanzawa-Lee G, et al. Rasch model-based testing of the european organisation for research and treatment of cancer (EORTC) quality of life Questionnaire-Chemotherapy-Induced Peripheral Neuropathy (QLQ-CIPN20) using Alliance for Clinical Trials in Oncology (Alliance) A151408 study data. Support Care Cancer. 2019;27(7):2599–2608.
- Knudsen AK, Hotopf M, Skogen JC, et al. The health status of nonparticipants in a population-based health study: the Hordaland Health Study. Am J Epidemiol. 2010;172(11):1306–1314.
- Bao T, Basal C, Seluzicki C, et al. Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: prevalence, risk factors, and fall risk. Breast Cancer Res Treat. 2016;159(2):327–333.
- Alberti P, Rossi E, Cornblath DR, et al.; CI-PeriNomS Group. Physician-assessed and patient-reported outcome measures in chemotherapy-induced sensory peripheral neurotoxicity: Two sides of the same coin. Ann Oncol. 2014;25(1):257–264.
- Argyriou AA, Park SB, Islam B, et al.; Toxic Neuropathy Consortium (TNC). Neurophysiological, nerve imaging and other techniques to assess chemotherapy-induced peripheral neurotoxicity in the clinical and research settings. J Neurol Neurosurg Psychiatry. 2019;90(12):1361–1369.
- Coumbe BGT, Groarke JD. Cardiovascular autonomic dysfunction in patients with cancer. Curr Cardiol Rep. 2018;20(8):10–17.
- Gewandter JS, Brell J, Cavaletti G, et al. Trial designs for chemotherapy-induced peripheral neuropathy prevention: ACTTION recommendations. Neurology. 2018;91(9):403–413.