Abstract
Introduction
The comparative effectiveness of radiofrequency ablation (RFA), radiation therapy (RT), transarterial chemoembolization (TACE) and transarterial radioembolization with Yttrium-90 (Y90) relative to one another for the treatment of hepatocellular carcinoma (HCC) is unclear. The aim of this systematic review and network meta-analysis is to compare RFA to RT to TACE to Y90 in the treatment of HCC.
Methods
Pubmed, Embase and Cochrane CENTRAL were searched up until April 19, 2021. Observational analyses with propensity score matched (PSM) cohort analyses and randomized controlled trials (RCT) reporting on two or more treatments relative to one another with respect to overall survival (OS) and/or progression free survival (PFS) were included. Survival data were extracted from Kaplan-Meier survival curves, and meta-analyzed using a multivariate network meta-analysis.
Results
After exclusions, 24 RCTs or PSM observational studies reporting on 5549 patients were included. While 1-year OS was greater for Y90 than TACE (RR 0.85, 95% CI: 0.72–0.99), all other 1-year OS comparisons across the 4 modalities yielded similar OS, and there were no differences across any modalities in 2-year and 3-year OS. TACE had a modest PFS advantage relative to RFA (RR 0.81, 95% CI: 0.68–0.95) and RT (RR 0.65, 95% CI: 0.51–0.83) at 2 years.
Conclusion
All modalities assessed resulted in similar OS, which explains the current heterogenous practice patterns. This conclusion may assist in decision making based on administrative and patient costs, and implementation of these modalities. Other factors such as toxicity rate specific to individual patients could not be assessed using network meta-analysis and may also play a role in selection of modality. Further studies, ideally using PSM cohort analyses or RCT study design, reporting on OS, PFS, local control, complete response and toxicity are needed prior to drawing definitive conclusions.
Introduction
Hepatocellular carcinoma (HCC) is the second leading cause of cancer-related mortality globally, the fifth most common cancer in men, and seventh most common cancer in women [Citation1]. Several non-surgical local therapies exist, including radiofrequency ablation (RFA), radiation therapy (RT), transarterial chemoembolization (TACE) and transarterial radioembolization with Yttrium-90 (Y90) [Citation2], and no one modality has emerged as the preferred treatment approach across all localized HCC patients.
The comparative efficacy/effectiveness of these treatments relative to one another is unclear [Citation3]. There exists only few randomized controlled trials (RCTs), and these have only compared two treatment modalities at a time relative to one another [Citation4]. Any observations and conclusions are thus drawn from studies with low statistical power and lack of generalizability. Previous systematic reviews and meta-analyses have attempted to pool study data together for a more definitive answer, but they too have been limited in their comparisons of only two treatment modalities [Citation5–8]. They, therefore, have only investigated a selection of treatments. This may be due to limited studies comparing each modality to one another, and thereby making certain comparisons via conventional systematic reviews and meta-analyses infeasible. The known costs and toxicity profile of each of these modalities differ in important ways. Yet, the likelihood that a randomized trial comparing these four different treatment modalities will be performed is unlikely.
To review all treatments relative to one another, a network meta-analysis can be employed employed and thereby multiple modalities can be simultaneously compared. Any limitations that would have prevented prior reviews of one treatment relative to another may be overcome through a network meta-analysis’ ability to conduct indirect comparisons. The aim of this systematic review and network meta-analysis is to compare RFA to RT to TACE to Y90 in the treatment of HCC.
Methods
This systematic review has been registered on PROSPERO (CRD 252180). PubMed, Embase and Cochrane CENTRAL were searched up until April 19, 2021 (Appendix 1). Any systematic reviews identified through screening were subjected to backward reference screening, to identify additional references that were not captured through the search.
Inclusion criteria
Articles were screened by title and abstract (level 1) screening to identify articles comparing at least two treatment modalities to each other in a comparative study (i.e., case-control study, cohort study, or RCT). These articles subsequently underwent full-text (level 2) screening to satisfy the following inclusion criteria: a) treatment intent/primary outcome of local control of HCC; and b) comparative effectiveness studies that comprised either RCTs or observational studies with propensity-score matched (PSM) cohort analyses. These full-text articles were then assessed for quantitative synthesis and included in this review and meta-analysis if they reported on overall survival (OS) and/or progression-free survival (PFS).
Quantitative synthesis
Patient demographics and treatment characteristics were recorded for each study. The recorded sample size was either the total sample size of the RCT, or the sample size of patients included in the PSM cohort analysis. OS and PFS data were extracted from Kaplan-Meier survival curves by visual estimation or in-text descriptions at the 1-year, 2-year and 3-year timepoints.
Meta-Analysis
Summary relative risks and corresponding 95% confidence intervals (CI) were calculated using a multivariate network meta-analysis, with a restricted maximum likelihood model. An inconsistency model was used to assess the underlying consistency assumption of network meta-analyses [Citation9]. P-values less than 0.05 were considered statistically significant. All analyses were conducted using Stata version 16.1 (StataCorp, College Station, TX, USA).
Quality assessment
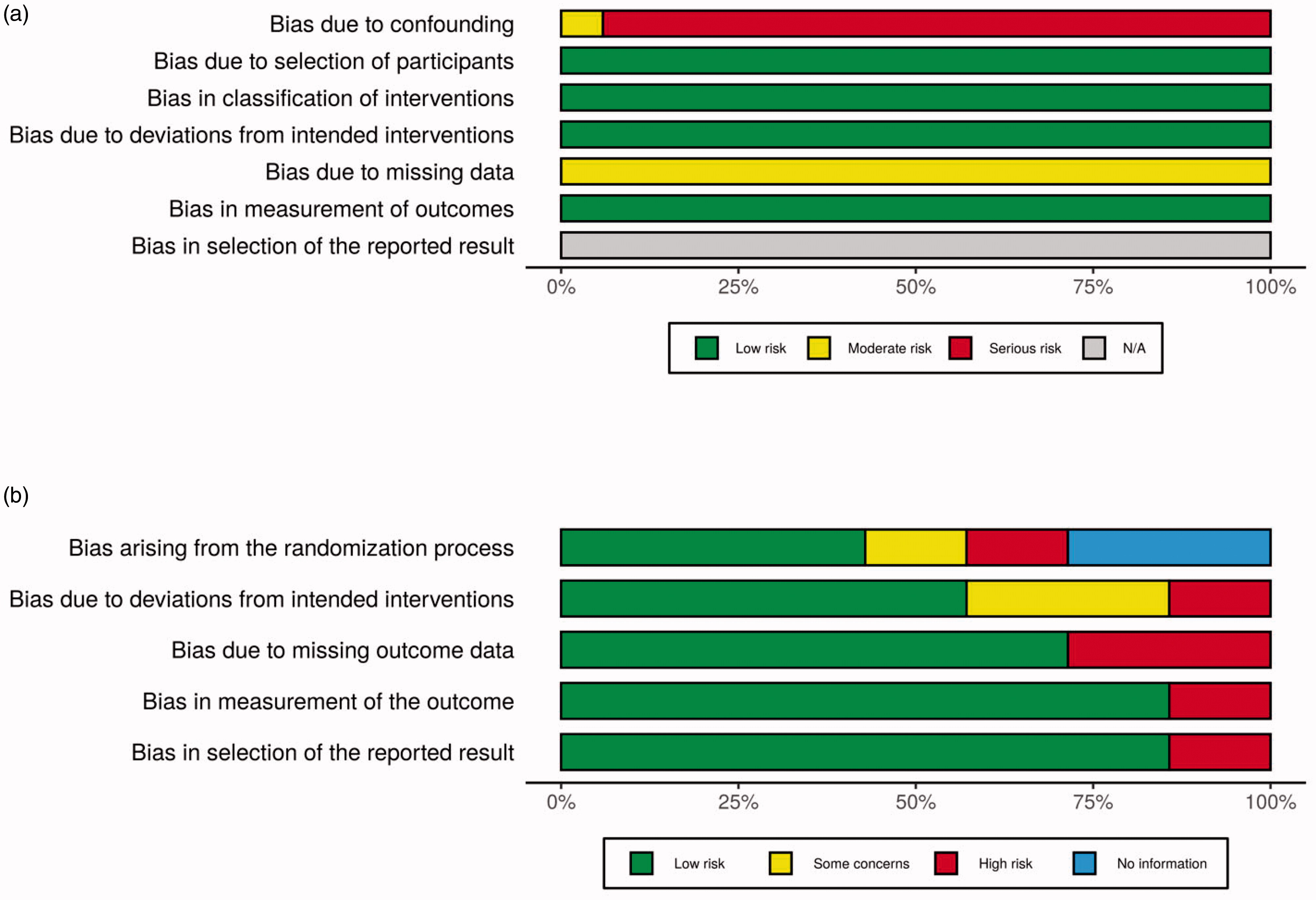
All studies were assessed for risk of bias. RCTs were assessed using the Cochrane Risk of Bias 2 tool [Citation10]. Observational studies were assessed using the ROBINS-I tool [Citation11]. Summary risk of bias in the literature was visualized and is presented by the Risk-of-bias VISualization package [Citation12].
Results
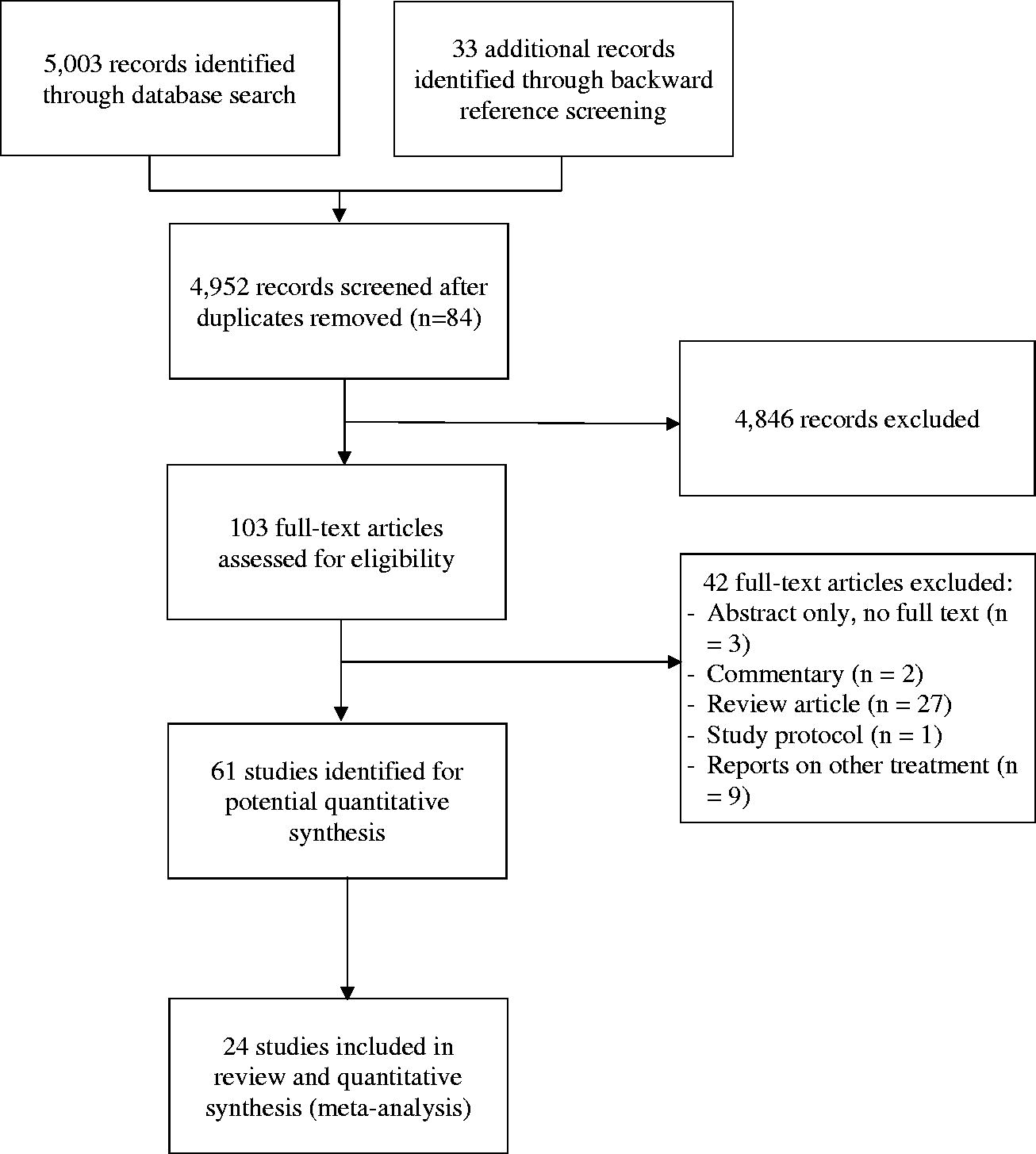
A total of 5003 records were identified through database search, and 33 additional records were identified through backward reference screening of systematic review articles. Of these, 84 were duplicates. The remaining 4952 records underwent level 1 screening to identify 103 full-text articles for level 2 screening. Sixty-one studies were assessed for quantitative synthesis, and 24 [Citation13–36] RCTs or PSM observational studies were included in this review and meta-analysis (Appendix 2).
Study demographics are presented in . Six studies were RCTs, and 18 studies were observational studies with PSM cohort analysis. There were notable concerns for bias due to confounding in observational studies in the unmatched original cohorts, namely that patient demographics by compared treatment modality significantly differed. These concerns did not prevail in their analyses due to the use of propensity-score matching (Appendix 3a). Among RCTs, there was generally a low risk of bias across all assessed criteria (Appendix 3b).
Table 1. Study demographics.
Overall survival (OS)
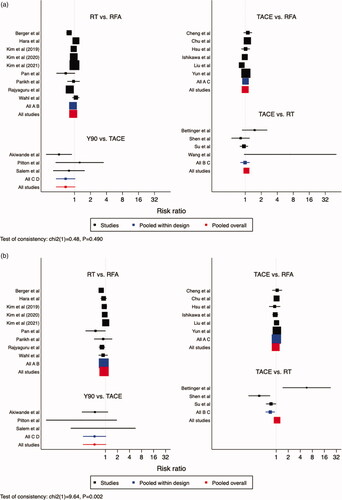
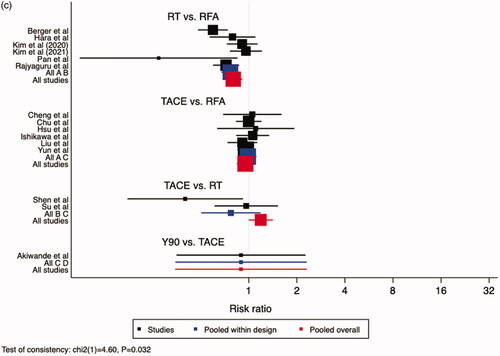
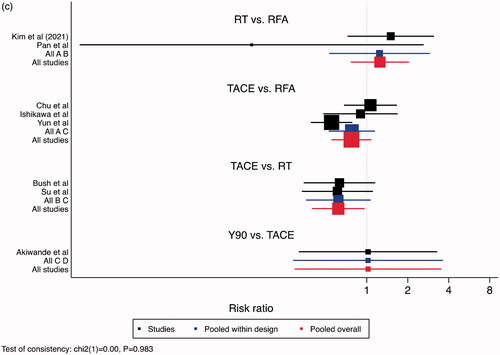
A larger proportion of patients treated with Y90, relative to TACE, survived after one year – RR 0.85, 95% CI: 0.72–0.99. No difference was noted between Y90 and RT (RR 0.90, 95% CI: 0.77–1.05), TACE and RT (RR 1.06, 95% CI: 0.97–1.16), RT and RFA (RR 0.94, 95% CI: 0.88–1.01, and TACE and RFA (RR 1.00, 95% CI: 0.92–1.08) (). No difference was noted between different treatments, with respect to 2-year and 3-year survival, aside from RT over RFA at 3-year timepoint (). RR for RT to RFA was 0.92 (95% CI: 0.86–1.00) for 2-year survival and 0.80 (95% CI: 0.70-0.91) for 3-year survival. When comparing RFA to TACE, 2-year survival had an RR of 0.96 (95% CI: 0.86–1.07) and 3-year survival had an RR of 0.95 (95 CI: 0.79–1.16). RT to TACE had an RR of 0.98 (95 CI: 0.85–1.11) for 2-year survival, and at 3-year survival had an RR of 0.98 (95% CI: 0.75–1.26). When comparing TACE to Y90, 2-year RR was 0.53 (95% CI: 0.27–1.05) and 3-year RR was 0.89 (95% CI: 0.32–2.48).
Figure 1. Comparisons of Overall Survival. (a) 1-Year Overall Survival, (b) 2-Year Overall Survival, (c) 3-Year Overall Survival. Legend: A = RFA, B = RT, C = TACE, D = Y90. The point estimate for “pooled within design” corresponds to the effect estimate based on head-to-head comparison studies. The point estimate for “pooled overall” corresponds to the effect estimate, based on both head-to-head comparison studies and indirect comparisons.
Progression-Free survival (PFS)
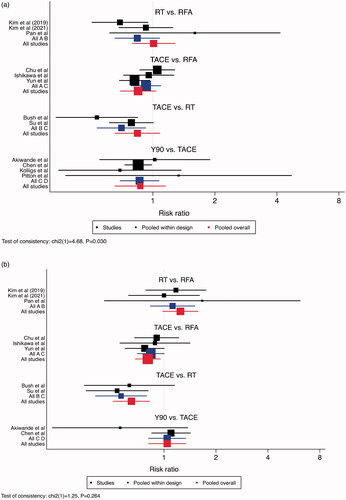
PFS was superior for TACE relative to RFA (RR 0.81, 95% CI: 0.68–0.95) and RT (RR 0.65, 95% CI: 0.51–0.83) at the 2-year endpoint, and to RT at the 3-year endpoint (RR 0.62, 95% CI: 0.40–0.97). Otherwise, all treatment modalities were similar ().
Figure 2. Comparisons of Progression Free Survival. (a) 1-Year Progression Free Survival, (b) 2-Year Progression Free Survival, (c) 3-Year Progression Free Survival. Legend: A = RFA, B = RT, C = TACE, D = Y90. The point estimate for “pooled within design” corresponds to the effect estimate based on head-to-head comparison studies. The point estimate for “pooled overall” corresponds to the effect estimate, based on both head-to-head comparison studies and indirect comparisons.
Discussion
To our knowledge, this is the first systematic review and network meta-analysis for HCC comparing RFA to RT to TACE to Y90. With 5549 patients, this is also one of the largest meta-analysis conducted to date investigating local treatment for HCC patients.
The 1-year OS was superior for patients receiving Y90 relative to TACE. However, Y90 was not found to be superior to RT in 1-year OS. This may be a result of only 1 study reporting on Y90 and RT in a head-to-head comparison. Even with a network meta-analysis, statistical modeling can only increase precision of our summary estimate and cannot overcome the limited head-to-head data. Despite the apparent superiority of Y90 for 1-year OS, a more appropriate conclusion at this time given the paucity of data would be that all treatment modalities yield similar OS. Further trials employing PSM cohort analysis or RCT study design are needed.
Although all treatment modalities had similar impact on OS, TACE had superior PFS. The 2- and 3-year PFS were superior for patients receiving TACE compared to both RFA and RT, and to RT, respectively. TACE may, therefore, be preferred in select patients to achieve longer time without disease progression, and analysis of subsets of patients who may benefit from this modality over other modalities is warranted. It is important to note that TACE, as noted in these studies, refers to either conventional TACE (cTACE) or drug-eluting bead TACE (DEB-TACE). Future studies should assess the comparative effectiveness/efficacy of cTACE to DEB-TACE.
Of further consideration, TACE has been reported to be superior to RT in patients with intermediate stage (Barcelona-Clinic Liver Cancer BCLC stage B) HCC [Citation37]. Our analyses did not separate early stage and intermediate stage HCC patients due to the limited studies comparing these modalities, and thus statistical power would have been even more limited with subgroup analyses. More studies are needed on TACE in the setting of early stage and intermediate stage HCC to assess whether there exists differential effect by disease stage, as different modalities may have different strengths in treating HCC according to stage.
Given the current evidence suggesting equipoise across these commonly utilized modalities, choice of treatment modality should be based on a multitude of factors, including physician and institutional preference and experience, available resources, and individual patient and tumor characteristics.
This systematic review is not without limitations. Intrinsic in systematic review methodology, biases within individual studies are not overcome by pooled analyses of many studies. In an attempt to minimize any such bias and generate robust analyses, our review was intentionally limited only to RCTs and PSM cohort analyses. Study quality was, therefore, generally good, with low risk of bias. Due to the paucity of studies, however, we were unable to meaningfully assess subgroup analyses by different radiation treatment modalities relative to RFA, TACE and Y90; the heterogeneous definition of RT may be the cause of heterogenous conclusions in comparisons to RT and, therefore, nullify any true effect reported of RFA, TACE and Y90 relative to RT. This is notable given the increasing evidence that stereotactic body radiation therapy (SBRT) can lead to improved tumor control and survival compared with conventional radiotherapy techniques [Citation38], that dose-escalated SBRT can further improve outcomes [Citation39], and that proton therapy can reduce toxicities and may improve survival compared with photon therapy [Citation40,Citation41].
Additionally, we similarly were not able to make meaningful comparisons across the four modalities according to different patient subpopulations, and so it is not possible to identify which modality is best for patients with different performance levels, Child–Pugh scores, tumor locations, tumors size, multi-focality of disease, and presence/absence of vascular invasion – all factors known to influence outcomes for HCC. More trials are needed to facilitate subgroup analyses and control for any effect modification by RT modality. Furthermore, our analyses do not report on local control, overall response rates and toxicity endpoints; all important considerations when deciding on appropriate local therapy. Among the studies included in this systematic review, data on the aforementioned outcomes are inconsistent, incomplete, or not reported in the primary published trials. Future studies should strive to report on all outcomes, inclusive of OS, local control, complete response and toxicity, so that more clinically meaningful comparisons between modalities can be made.
In conclusion, the current evidence suggests comparable OS for RFA, RT, TACE, and Y90 relative to each other in the local treatment of HCC; however, the number of studies overall are limited, and further comparative studies are needed prior to drawing definitive conclusions. In particular, additional high-quality and complete data inclusive of OS, response rates, and toxicity are needed. Such studies should use PSM cohort analyses or RCT study design with appropriate patient selection (i.e., BCLC stage stratified) and statistical power in order to inform on best practices of localized therapy for HCC.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47:S2–S6.
- Mahnken AH, Pereira PL, de Baère T. Interventional oncologic approaches to liver metastases. Radiology. 2013;266(2):407–430.
- Pan YX, Fu YZ, Hu DD, et al. Stereotactic body radiotherapy vs. Radiofrequency ablation in the treatment of hepatocellular carcinoma: a Meta-Analysis. Front Oncol. 2020;10:1639.
- Jackson WC, Tao Y, Mendiratta-Lala M, et al. Comparison of stereotactic body radiation therapy and radiofrequency ablation in the treatment of intrahepatic metastases. Int J Radiat Oncol Biol Phys. 2018;100(4):950–958.
- Casadei Gardini A, Tamburini E, Iñarrairaegui M, et al. Radioembolization versus chemoembolization for unresectable hepatocellular carcinoma: a Meta-analysis of randomized trials. Onco Targets Ther. 2018;11:7315–7321.
- Eriguchi T, Takeda A, Tateishi Y, et al. Comparison of stereotactic body radiotherapy and radiofrequency ablation for hepatocellular carcinoma: systematic review and Meta-analysis of propensity score studies. Hepatol Res. 2021;51(7):813–822.
- Ren N, Xue J, Qin S, et al. Comparison of transarterial y90 radioembolization and conventional transarterial chemoembolization in hepatocarcinoma patients: a Meta-analysis. pharmaceutical-sciences. 2020;82(s6):76–81.
- Wang Y, Zeng L, Chen W, et al. Efficacy and safety of radiofrequency ablation and transcatheter arterial chemoembolization for treatment of hepatocellular carcinoma: a Meta-analysis. Hepatol Res. 2016;46(1):58–71.
- White IR, Barrett JK, Jackson D, et al. Consistency and inconsistency in network Meta-analysis: model estimation using multivariate Meta-regression. Res Synth Methods. 2012;3(2):111–125.
- Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
- Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.
- McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and shiny web app for visualizing risk-of-bias assessments. Res Syn Meth. 2021;12(1):55–61.
- Akinwande O, Philips P, Scoggins C, et al. Radioembolization versus chemoembolization (DEBDOX) for the treatment of unresectable hepatocellular carcinoma: a propensity matched study. Anticancer Res. 2016;36:239–246.
- Berger NG, Tanious MN, Hammad AY, et al. External radiation or ablation for solitary hepatocellular carcinoma: a survival analysis of the SEER database. J Surg Oncol. 2017;116(3):307–312.
- Bettinger D, Gkika E, Schultheiss M, et al. Comparison of local tumor control in patients with HCC treated with SBRT or TACE: a propensity score analysis. BMC Cancer. 2018;18(1):807.
- Bush DA, Smith JC, Slater JD, et al. Randomized clinical trial comparing proton beam radiation therapy with transarterial chemoembolization for hepatocellular carcinoma: Results of an interim analysis. Int J Radiat Oncol Biol Phys. 2016;95(1):477–482.
- Cheng BQ, Jia CQ, Liu CT, et al. Chemoembolization combined with radiofrequency ablation for patients with hepatocellular carcinoma larger than 3 cm: a randomized controlled trial. JAMA. 2008;299(14):1669–1677.
- Chu HH, Kim JH, Yoon HK, et al. Chemoembolization combined with radiofrequency ablation for Medium-Sized hepatocellular carcinoma: a Propensity-Score analysis. J Vasc Interv Radiol. 2019;30(10):1533–1543.
- Hara K, Takeda A, Tsurugai Y, et al. Radiotherapy for hepatocellular carcinoma results in comparable survival to radiofrequency ablation: a propensity score analysis. Hepatology. 2019;69:2533–2545.
- Hsu CY, Huang YH, Chiou YY, et al. Comparison of radiofrequency ablation and transarterial chemoembolization for hepatocellular carcinoma within the milan criteria: a propensity score analysis. Liver Transpl. 2011;17(5):556–566.
- Ishikawa K, Chiba T, Ooka Y, et al. Transarterial chemoembolization as a substitute to radiofrequency ablation for treating barcelona clinic liver cancer stage 0/a hepatocellular carcinoma. Oncotarget. 2018;9(30):21560–21568.
- Kim N, Cheng J, Jung I, et al. Stereotactic body radiation therapy vs. radiofrequency ablation in asian patients with hepatocellular carcinoma. J Hepatol. 2020;73(1):121–129.
- Kim N, Kim HJ, Won JY, et al. Retrospective analysis of stereotactic body radiation therapy efficacy over radiofrequency ablation for hepatocellular carcinoma. Radiother Oncol. 2019;131:81–87.
- Kim TH, Koh YH, Kim BH, et al. Proton beam radiotherapy vs. radiofrequency ablation for recurrent hepatocellular carcinoma: a randomized phase III trial. J Hepatol. 2021;74(3):603–612.
- Kolligs FT, Bilbao JI, Jakobs T, et al. Pilot randomized trial of selective internal radiation therapy vs. chemoembolization in unresectable hepatocellular carcinoma. Liver Int. 2015;35(6):1715–1721.
- Liu PH, Lee YH, Hsu CY, et al. Survival advantage of radiofrequency ablation over transarterial chemoembolization for patients with hepatocellular carcinoma and good performance status within the milan criteria. Ann Surg Oncol. 2014;21(12):3835–3843.
- Pan Y-X, Fu Y-Z, Hu D-D, et al. Stereotactic body radiotherapy as a salvage therapy after incomplete radiofrequency ablation for hepatocellular carcinoma: a retrospective propensity score matching study. Cancers. 2019;11(8):1116.
- Parikh ND, Marshall VD, Green M, et al. Effectiveness and cost of radiofrequency ablation and stereotactic body radiotherapy for treatment of early-stage hepatocellular carcinoma: an analysis of SEER-medicare. J Med Imaging Radiat Oncol. 2018;62(5):673–681.
- Pitton MB, Kloeckner R, Ruckes C, et al. Randomized comparison of selective internal radiotherapy (SIRT) versus drug-eluting bead transarterial chemoembolization (DEB-TACE) for the treatment of hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2015;38(2):352–360.
- Rajyaguru DJ, Borgert AJ, Smith AL, et al. Radiofrequency ablation versus stereotactic body radiotherapy for localized hepatocellular carcinoma in nonsurgically managed patients: Analysis of the national cancer database. J Clin Oncol. 2018;36(6):600–608.
- Salem R, Gordon AC, Mouli S, et al. Y90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2016;151(6):1155–1163.e2.
- Shen PC, Chang WC, Lo CH, et al. Comparison of stereotactic body radiation therapy and transarterial chemoembolization for unresectable Medium-Sized hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2019;105(2):307–318.
- Su T-S, Qu S, Chen L, et al. Stereotactic body radiation therapy vs. Transarterial chemoembolization in inoperable barcelona clinic liver cancer stage a hepatocellular carcinoma: a retrospective, Propensity-Matched analysis. Front Oncol. 2020;10:347.
- Wahl DR, Stenmark MH, Tao Y, et al. Outcomes after stereotactic body radiotherapy or radiofrequency ablation for hepatocellular carcinoma. J Clin Oncol. 2016;34(5):452–459.
- Wang G, Shen W, Song M, et al. Results of combined treatment with transcatheter hepatic arterial chemoembolization and whole-liver irradiation with the moving strip technique in unresectable hepatocellular carcinoma. Int J Clin Oncol. 2000;5(6):380–385.
- Yun B-Y, Lee HW, Kim SU, et al. Prognosis of early-stage hepatocellular carcinoma: Comparison between trans-arterial chemoembolization and radiofrequency ablation. Cancers. 2020;12(9):2527–2511.
- Elshaarawy O, Gomaa A, Omar H, et al. Intermediate stage hepatocellular carcinoma: a summary review. J Hepatocell Carcinoma. 2019;6:105–117.
- Rim CH, Kim HJ, Seong J. Clinical feasibility and efficacy of stereotactic body radiotherapy for hepatocellular carcinoma: a systematic review and Meta-analysis of observational studies. Radiother Oncol. 2019;131:135–144.
- Comito T, Clerici E, Tozzi A, et al. Liver metastases and SBRT: a new paradigm? Rep Pract Oncol Radiother. 2015;20(6):464–471.
- Hasan S, Abel S, Verma V, et al. Proton beam therapy versus stereotactic body radiotherapy for hepatocellular carcinoma: practice patterns, outcomes, and the effect of biologically effective dose escalation. J Gastrointest Oncol. 2019;10(5):999–1009.
- Sanford NN, Pursley J, Noe B, et al. Protons versus photons for unresectable hepatocellular carcinoma: Liver decompensation and overall survival. Int J Radiat Oncol Biol Phys. 2019;105(1):64–72.
Appendix 1.
Search strategy
PubMed: 3132 results
(liver neoplasms[mh] OR carcinoma, hepatocellular[mh] OR hepatoma*[tw] OR liver cancer*[tw] OR liver carcinoma*[tw] OR hepatic[tw] OR hepatocellular[tw] OR liver cell carcinoma*[tw] OR HCC[tw] OR intra-hepatic[tw])
AND
(radiofrequency ablation[mh] OR radiation therapy[tw] OR radiotherapy[mh] OR radiotherapy[tw] OR radiotherapy[sh] OR transarterial chemoembolization[tw] OR chemoembolization, therapeutic[mh] OR embolization, therapeutic[mh] OR yttrium-90[tw] OR stereotactic[tw] OR radiosurgery[mh] OR SABR[tw] OR SBRT[tw] OR TACE[tw] OR TAE[tw] OR TRACE[tw] OR irradiat*[tw] OR proton therapy[mh] OR proton therap*[tw] OR tomotherap*[tw])
AND
(randomized[tw] OR randomized[tw] OR randomized controlled trial[pt] OR cohort[tw] OR case-control*[tw] OR controlled clinical trial[pt])
Embase and embase classic: 1761 results
(exp liver tumor/or exp liver cell carcinoma/or hepatoma*.mp. or liver cancer*.mp. or exp liver cancer/or liver carcinoma*.mp. or hepatic.mp. or hepatocellular.mp. or liver cell carcinoma*.mp. or HCC.mp. or intra-hepatic.mp.)
and
(exp radiofrequency ablation/or radiation therapy.mp. or exp radiotherapy/or radiotherapy.mp. or transarterial chemoembolization.mp. or exp chemoembolization/or yttrium-90.mp. or exp yttrium 90/or exp stereotactic radiosurgery/or exp stereotactic body radiation therapy/or stereotactic.mp. or SABR.mp. or SBRT.mp. or TACE.mp. or TAE.mp. or TRACE.mp. or exp irradiation/or irradiat*.mp. or exp proton therapy/or proton therap*.mp. or exp tomotherapy/or tomotherap*.mp.)
and
(exp randomized controlled trial/or randomized.mp. or randomized.mp. or exp cohort analysis/or exp controlled study/or cohort.mp. or exp case control study/or case-control*.mp. or controlled clinical trial.mp. or exp controlled clinical trial/)
Cochrane Central register of controlled trials: 110 results
(exp liver tumor/or exp liver cell carcinoma/or hepatoma*.mp. or liver cancer*.mp. or exp liver cancer/or liver carcinoma*.mp. or hepatic.mp. or hepatocellular.mp. or liver cell carcinoma*.mp. or HCC.mp. or intra-hepatic.mp.)
and
(exp radiofrequency ablation/or radiation therapy.mp. or exp radiotherapy/or radiotherapy.mp. or transarterial chemoembolization.mp. or exp chemoembolization/or yttrium-90.mp. or exp yttrium 90/or exp stereotactic radiosurgery/or exp stereotactic body radiation therapy/or stereotactic.mp. or SABR.mp. or SBRT.mp. or TACE.mp. or TAE.mp. or TRACE.mp. or exp irradiation/or irradiat*.mp. or exp proton therapy/or proton therap*.mp. or exp tomotherapy/or tomotherap*.mp.)
and
(exp randomized controlled trial/or randomized.mp. or randomized.mp. or exp cohort analysis/or exp controlled study/or cohort.mp. or exp case control study/or case-control*.mp. or controlled clinical trial.mp. or exp controlled clinical trial/).
Appendix 2.
PRISMA flow diagram.
Appendix 3a.
Risk of bias of cohort studies 3b. Risk of bias of randomized controlled trials.