Coronavirus disease (COVID-19) infection has caused morbidity and mortality at an unprecedented scale [Citation1]. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel beta coronavirus responsible for new respiratory infections with mild-to-severe presentations in humans. At the beginning of the pandemic, major attention was directed to investigate the acute effect of COVID-19, as well as to identify potential therapeutic and preventive strategies to control the virus spread. More than a year after the first case was described in Wuhan, China, the scientific community faces the long-term impact of this disease. Indeed, subjects affected by COVID-19 may experience various symptoms, such as fatigue, weakness, dyspnea, physical deconditioning lasting more than one month [Citation2]. This condition, recently defined as post-acute COVID-19 syndrome, is characterized by persistent symptoms and/or delayed or long-term complications beyond 4 weeks from the onset symptoms [Citation2].
Patients with cancer receiving systemic anti-cancer treatments are more susceptible to COVID-19, and across the oncological population, patients with lung cancer are the most vulnerable, resulting in higher morbidity and mortality rates [Citation3]. Recent reports found that the mortality rate in patients with cancer affected by COVID-19 was 22.4% [Citation4], whereas, in thoracic malignancies, such percentage increased to 33% [Citation3]. Moreover, oncological patients with COVID-19, particularly those with lung cancer, may be more predisposed to long-term complications and to the development of post-acute syndrome [Citation2]. A recent review advocates the need to expand the knowledge about interventions to manage post-acute COVID-19 syndrome, highlighting that care for patients with COVID-19 does not conclude at the time of the swab negativity but should also be proposed in the outpatient setting, especially for those patients considered more susceptible [Citation2].
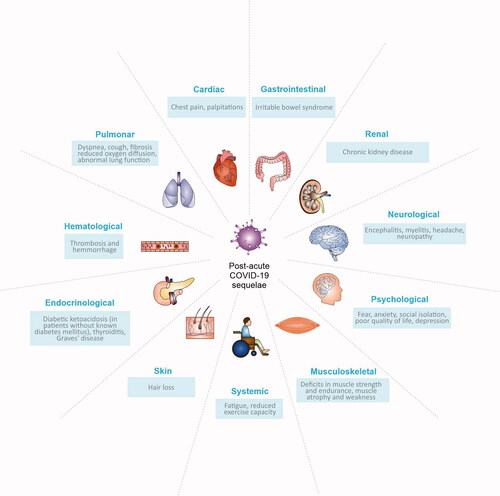
Post-acute COVID-19 sequelae
Although post-acute sequelae are present in a significant number of COVID-19 survivors [Citation5], the only available data on patients with cancer indicates that long-term symptoms may affect up to 15% of patients [Citation6]. Post-acute COVID-19 syndrome can involve different organs, and patients may experience multiple and overlapping sequelae, resulting in a cluster of symptoms (summarized in ) [Citation2]. Given the multiorgan nature and the heterogeneity in the presentation of long-term COVID-19 manifestations, a multidisciplinary approach appears to be the most effective strategy to manage its complexity. Among the possible interventions, exercise is an excellent ally to be included in a multimodal program, able to relieve some virus sequelae, as well as to produce important benefits in patients with cancer and COVID-19.
Exercise strategy in patients with cancer experiencing COVID-19 sequelae
Although no data about the benefits of exercise in patients with cancer suffering from post-acute COVID-19 syndrome are available to date, a strong rationale supports the use of a tailored exercise program to enhance the recovery of long-term COVID-19 sequelae.
First, post-diagnosis physical activity is associated with a reduction in the risk of overall mortality (ranging from 21% to 45%) and cancer-specific mortality (ranging from 26% to 69%), especially in breast, colorectal, and prostate cancer [Citation7]; moreover, cardiorespiratory fitness, muscle strength, and mass are recognized independent prognostic factors in cancer [Citation8]. Furthermore, exercise is largely recognized as an essential therapeutic tool to improve cardiovascular function and diminish the risk of cardiovascular events through an enhancement of central (lung and heart) and peripheral (vasculature and muscle) adaptations. In patients with cancer, exercise significantly increases cardiorespiratory fitness, and consequently, the individual's exercise capacity [Citation9]. Moreover, it may act on the pulmonary system, improving its function, and relieving some associated symptoms like dyspnea. Also, especially in the sub-setting of patients whose lungs are damaged, such as those affected by lung cancer, a structured exercise program can increase respiratory muscle strength and some spirometry values, as forced vital capacity and forced expiratory volume in one second [Citation8]. A targeted exercise, especially strength training or a combined (aerobic + strength) program, was effective in modeling body composition and improving strength in patients with cancer [Citation10,Citation11]. These types of activities are potent body modulators, conferring both morphological (e.g., hypertrophy), and neural (e.g., improvement in intra-, intermuscular coordination and in the nervous system) modifications to skeletal muscle, potentially capable of counteracting some COVID-19 sequelae as muscle weakness and neurological complications. There is evidence that an exercise program may alleviate some symptoms in patients with cancer, such as cancer-related fatigue, chemotherapy-induced peripheral neuropathy, and nausea [Citation12]. Moreover, exercise may act as a psychoactive drug, diminishing the anxiety and depression levels, improving cognitive function, thus becoming a strategy to improve health-related quality of life [Citation12].
Despite the pathogenesis of post-acute COVID-19 syndrome remains to be elucidated, it seems that proinflammatory and immunosuppressive status caused by SARS-CoV-2 may have a role in the onset of late sequelae [Citation2]. Thus, it is possible to speculate that patients with cancer may be at high risk of developing post-acute COVID-19 syndrome because already harboring an immunocompromised and proinflammatory status, which may be further exacerbated by the contagion. Exercise may counteract these mechanisms, enhancing immune surveillance and controlling the inflammation. In this regard, exercise may have an immunostimulant effect, increasing the cytotoxicity activity of natural killers and the lymphocyte level (e.g., CD4+), proliferation, and phagocytic activity in monocytes [Citation8,Citation13]. In fact, during exercise, shear stress and adrenergic signaling may mobilize immune cells to the circulation, favoring immune cell infiltration. More indirectly, muscle contraction, through the releases of immune regulatory cytokines, known as myokines, may promote immune cell proliferation, differentiation, and maturation. However, it is important to remember that the effect of exercise on the immune system largely depends on the "dose" of exercise. This assumption, known as the "Inverted J Hypothesis," suggests that moderate exercise represents the dosage resulting in an enhancement in the immune function [Citation14]. In addition, exercise may control low-grade systemic inflammation, promoting anti-inflammatory cytokines, and diminishing the proinflammatory ones. Particularly, exercise may directly have a modest impact on a series of mediators, such as interleukin (IL)-6, tumor necrosis factor-alpha (TNF-α), IL-8, and IL-2, as suggested in a recent meta-analysis, performed by Meneses-Echavez and colleagues [Citation15]. Moreover, 15-weeks of aerobic exercise has been found to have a beneficial effect on C-reactive protein [Citation16]. Overall, the current evidence seems to support the use of exercise as a potential strategy to manage post-acute COVID-19 syndrome in patients with cancer.
A tailored exercise prescription for patients with cancer affected by post-acute COVID-19 syndrome: a real-life story
Over the years, advancements in exercise science allowed to expand the knowledge about its benefits and the appropriate methods for delivering an individualized exercise program. Exercise in patients with cancer experiencing post-acute COVID-19 syndrome should be ideally tailored according to patients' physical and psychological conditions, considering both the oncological disease and the COVID-19 sequelae. Nevertheless, COVID-19 represents a new condition in which many features remain to be elucidated. There is an urgent need to share the available experience to understand the impact and the feasibility of exercise also in vulnerable patients, as the oncological population, suffering from persistent symptoms after COVID-19.
In this sense, herein we report the case of a 55-year-old never smoker female patient affected by metastatic, EGFR-mutant lung adenocarcinoma, undergoing second-line treatment with osimertinib from December 2019 with a persistent partial response at all disease sites (lung, liver, and bone) (). On 2 December 2020, she presented cough, fever, sore throat, daily headache, fatigue, myalgia, loss of taste/smell, and general weakness, and the Sars-Cov2 molecular test revealed a COVID-19 infection. Whereas fever rapidly resolved, other symptoms progressed over the weeks. Three weeks after her swab, the patient experienced cranial neuropathy associated with dysphagia and focal abnormalities at the right eye. Moreover, she reported symptoms of COVID-19-associate peripheral neuropathy, such as bilateral leg pain, myalgia, weakness, and numbness, affecting her ability to walk. During Sars-Cov2 infection, she continued osimertinib and started prednisone (50 mg/daily for 10 days) to mitigate the COVID-19 effects. Despite the negativity of the nasal and oropharyngeal swab, on 2 January 2021, symptoms of fatigue, headache, physical deconditioning, and neurological impairments continued to worsen in intensity and duration. With the aim to improve deconditioning and COVID-19 sequelae, the patient was enrolled in a home-based exercise intervention program, based on the American College of Sports Medicine guidelines [Citation12], started on 10 January 2021, and lasted 8 weeks. A tailored instruction manual, detailing the program to perform twice a week and a diary to log exercise sessions, were provided. Exercise prescription included: aerobic training, resistance training, and balance task. The program consisted of aerobic activity (walking or cycling), in which the load slowly increased from 10 min to 20 min at a constant intensity of 3–5 on the CR10 Borg Scale of perceived exertion (CR10). Exercises with bodyweight and resistance bands were proposed to increase strength. Each resistance exercise comprised two-three sets of 8–12 repetitions at 3–4 intensity of CR10, which were progressively increased. Balance was stimulated with two exercises, in which static tasks (e.g., standing on one leg position or in a tandem position) were proposed to optimize the somatosensory system. Evaluations included: functional capacity, measured with the "Six minutes walking test" (6MWT) [Citation17], muscular strength, assessed through handgrip and leg press strength tests [Citation18], anthropometric values (body weight, height, hip and waist circumferences) [Citation19]. Moreover, quality of life (QoL) using the European Organization of Research and Treatment in Cancer questionnaire (EORTC QLQ-C30) [Citation20] and the Godin Leisure-Time Exercise Questionnaire [Citation21] about exercise level, were collected.
Figure 2. Timeline of the clinical history and physical intervention (on the left) and results of exercise intervention (on the right). PR: partial response; SBRT: stereotactic body radiotherapy.
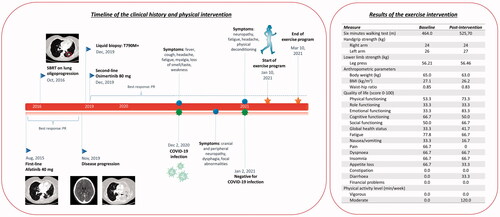
The adherence to the exercise program was excellent (100%), and no adverse events occurred during the training period. Findings from the outcome assessments are summarized in . The amount of light and moderate physical activity levels increased, while muscle strength remained stable. Of note, the patient increased her distance in the 6MWT, revealing a gain of 61.70 m. Gait dysfunction, decreased muscle strength, and physical inactivity are recognized factors correlated with peripheral neuropathy symptoms severity [Citation22,Citation23]. Thus, it could be speculated that a combined exercise training, through enhancement of physical fitness and given its antioxidative capacity, could help in restoring the neurological damage, being a promising strategy to manage the post-infection lower limbs neuropathy. Eight weeks of aerobic, strength, and balance activities were able to improve some QoL domains that the patient reported as post-COVID-19 symptoms, e.g., fatigue, pain, and weakness. Indeed, we found that, after exercise program, fatigue and pain decreased by 11.1 points and 66.7 points, respectively, whereas physical functioning increased by 20.0 points, suggesting that some COVID-19 sequelae ameliorated. Moreover, we may speculate that the exercise program helped in improving a range of QoL subcategories, although the well-known psycho-physical consequences related to the infection and the quarantine period, particularly in oncological patients.
Although under the umbrella of the multidisciplinary approach, multiple healthcare professionals and interventions may be included, we proposed a peculiar and difficult-to-treat situation where exercise demonstrated to be safe and well-tolerated, despite COVID-19 sequelae and prosecution of active oncological therapy. The optimal compliance suggested that the use of telemedicine (as a home-based program) may represent an effective strategy during the pandemic, in which lockdown and social distancing are the most effective and applied measures to contain virus spread.
Conclusion
Patients with cancer affected by SARS-CoV-2 may potentially be at a high risk of developing post-acute COVID-19 syndrome. COVID-19 sequelae, given the frequent multiorgan involvement, could ideally require a multidisciplinary approach, and among them, exercise has a strong rationale supporting its use in patients with cancer. We have reported a case of a metastatic lung cancer patient, tested positive for COVID-19, who reported persistent symptoms, such as fatigue, physical deconditioning, headache, peripheral neuropathy. To treat COVID-19 sequelae, the patient participated in a home-based exercise program, including aerobic exercise, strength training, and balance tasks, to be performed twice a week. Given the promising results, this patient's story suggested that exercise is effective in enhancing the quality of life, accelerating post-infection recovery, and regaining functional capacity, representing an ideal first approach, to be further integrated within a multimodal strategy to manage post-acute COVID-19 syndrome, even in particularly-frail patients' populations as the ontological one.
Disclosure statement
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. M. M. reports personal fees from Pfizer, EUSA Pharma and Astra Zeneca, outside the submitted manuscript. S. P. received honoraria or speakers' fee from Astra-Zeneca, Eli-Lilly, AMGEN, BMS, Boehringer Ingelheim, MSD and Roche, outside the submitted manuscript. All remaining authors have no conflicts of interest to declare.
References
- WHO. Coronavirus Data. (Ed.). 2021. Available from: https://covid19.who.int/
- Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615.
- Garassino MC, Whisenant JG, Huang LC, TERAVOLT Investigators, et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21(7):914–922.
- Zhang H, Han H, He T, et al. Clinical characteristics and outcomes of COVID-19-infected cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2021;113(4):371–380.
- Moreno-Pérez O, Merino E, Leon-Ramirez JM, COVID19-ALC Research Group, et al. Post-acute COVID-19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J Infect. 2021;82(3):378–383.
- Cortellini A, Roldán E, Carmona Garcia MC, et al. Prevalence and impact of COVID-19 sequelae on treatment pathways and survival of cancer patients who recovered from SARS-CoV-2 infection. Ann Oncol. 2021;32:S1130.
- Patel AV, Friedenreich CM, Moore SC, et al. American college of sports medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Med Sci Sports Exerc. 2019;51(11):2391–2402.
- Avancini A, Sartori G, Gkountakos A, et al. Physical activity and exercise in lung cancer care: will promises be fulfilled? Oncologist. 2020;25(3):e555–e569.
- Scott JM, Zabor EC, Schwitzer E, et al. Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: a systematic review and meta-analysis. J Clin Oncol. 2018;36(22):2297–2305.
- Padilha CS, Marinello PC, Galvão DA, et al. Evaluation of resistance training to improve muscular strength and body composition in cancer patients undergoing neoadjuvant and adjuvant therapy: a meta-analysis. J Cancer Surviv. 2017;11(3):339–349.
- Koeppel M, Mathis K, Schmitz KH, et al. Muscle hypertrophy in cancer patients and survivors via strength training. A meta-analysis and meta-regression. Crit Rev Oncol Hematol. 2021;163:103371.
- Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51(11):2375–2390.
- Kruijsen-Jaarsma M, Révész D, Bierings MB, et al. Effects of exercise on immune function in patients with cancer: a systematic review. Exerc Immunol Rev. 2013;19:120–143.
- Woods JA, Davis JM, Smith JA, et al. Exercise and cellular innate immune function. Med Sci Sports Exerc. 1999;31(1):57–66.
- Meneses-Echávez JF, Correa-Bautista JE, González-Jiménez E, et al. The effect of exercise training on mediators of inflammation in breast cancer survivors: a systematic review with meta-analysis. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1009–1017.
- Fairey AS, Courneya KS, Field CJ, et al. Effect of exercise training on C-reactive protein in postmenopausal breast cancer survivors: a randomized controlled trial. Brain Behav Immun. 2005;19(5):381–388.
- Laboratories ACoPSfCPF. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117.
- Innes E. Handgrip strength testing: a review of the literature. Aust Occup Ther J. 1999;46(3):120–140.
- Physical status: the use and interpretation of anthropometry. Report of a WHO expert committee. World Health Organ Tech Rep Ser. 1995;854:1–452.
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376.
- Amireault S, Godin G, Lacombe J, et al. The use of the Godin-Shephard Leisure-Time physical activity questionnaire in oncology research: a systematic review. BMC Med Res Methodol. 2015;15:60.
- McCrary JM, Goldstein D, Wyld D, et al. Mobility in survivors with chemotherapy-induced peripheral neuropathy and utility of the 6-min walk test. J Cancer Surviv. 2019;13(4):495–502.
- van Sloten TT, Savelberg HH, Duimel-Peeters IG, et al. Peripheral neuropathy, decreased muscle strength and obesity are strongly associated with walking in persons with type 2 diabetes without manifest mobility limitations. Diabetes Res Clin Pract. 2011;91(1):32–39.