Abstract
Aim
Academic and high volume hospitals have better outcome for pancreatic cancer (PC) surgery, but there are no reports on oncological treatment. We aimed to determine the influence of facility types on overall survival (OS) after treatment with chemotherapy for inoperable PC.
Material and methods
2,657 patients were treated in Denmark from 2012 to 2018 and registered in the Danish Pancreatic Cancer Database. Facilities were classified as either secondary oncological units or comprehensive, tertiary referral cancer centers.
Results
The average yearly number of patients seen at the four tertiary facilities was 71, and 31 at the four secondary facilities. Patients at secondary facilities were older, more frequently had severe comorbidity and lived in non-urban municipalities. As compared to combination chemotherapy, monotherapy with gemcitabine was used more often (59%) in secondary facilities than in tertiary (34%). The unadjusted median OS was 7.7 months at tertiary and 6.1 months at secondary facilities. The adjusted hazard ratio (HR) of 1.16 (confidence interval 1.07–1.27) demonstrated an excess risk of death for patients treated at secondary facilities, which disappeared when taking type of chemotherapy used into account. Hence, more use of combination chemotherapy was associated with the observed improved OS of patients treated at tertiary facilities. Declining HR’s per year of first treatment indicated improved outcomes with time, however the difference among facility types remained significant.
Discussion
Equal access to modern combination chemotherapy at all facilities on a national level is essential to ensure equality in treatment results.
Introduction
Pancreatic cancer (PC) is presently the tenth most common cancer worldwide [Citation1], and projected to become the second leading cause of cancer-related death in the US within the next ten years [Citation2]. The prognosis is dismal with a 5-year survival rate of only 3–8% [Citation3]. However, in the last decade large, randomized trials of combination chemotherapy compared with gemcitabine have shown improvements in short-term survival of patients with metastatic PC [Citation4,Citation5], and treatment with gemcitabine has become preferred only for patients unfit for combination chemotherapy [Citation6,Citation7].
For a number of complex surgical oncological procedures (e.g., pancreatectomy and esophagectomy), a higher volume at the hospital or physician level is associated with better outcomes [Citation8–10]. In addition, cancer patients surgically treated at academic versus nonacademic facilities may fare better [Citation10–12]. Similar studies in the medical management of cancers are few and primarily confined to hematological malignancies of high complexity [Citation13–16], however these studies also in general show improved survival at academic and high volume facilities.
The complexity of management in oncology is increasing, e.g., with introduction of new predictive markers, increasingly specific treatment guidelines and staging systems, more treatment options and advanced supportive care [Citation6,Citation17,Citation18]. Access to multidisciplinary teams has been recognized as an important factor for optimizing treatment and outcome [Citation19,Citation20]. Therefore, a relation between degree of facility specialization and outcome may also exist for the medical treatment of non-hematological cancers.
The Danish population of PC patients is well suited for studying treatment facility factors influencing outcome. All costs for patients at all facilities are covered by the public health system and registries are virtually complete. The treatment is regulated through national guidelines [Citation7], there are centralized procedures for drug reimbursement, National Board of Health-determined ‘cancer pathway packages’ (to reduce delay in work-up and time to treatment start [Citation21]), and governmental accreditation of units handling surgery and oncology. Moreover, the treatment of cancer patients is hierarchically organized. Surgical treatment of PC is considered a highly specialized field and is carried out at four certified university centers, whereas medical treatment of PC is categorized as a basic oncological service and is offered at eight oncological facilities. These include four units at larger hospitals with comprehensive diagnostic, surgical and oncological cancer care and other modalities of high specialization (tertiary facilities), and four units at smaller hospitals with standard oncological care (secondary facilities).
The treatment course and outcome of all Danish pancreatic cancer patients have been reported previously [Citation22]. In the current study we aimed to investigate whether the type of oncological facility had an influence on survival of patients with inoperable PC treated with chemotherapy, and whether differences in distribution of treatment and patients’ characteristics influenced outcome.
Material and methods
Data source
Diagnostic and treatment data were retrieved from the Danish Pancreatic Cancer Database (DPCD), a nationwide clinical quality database [Citation23]. The DPCD combines data from The Danish Civil Registration System, The Danish National Patient Registry and The Danish Pathology Registry with supplementary manually recorded data. These registries have high validity and completeness [Citation24]. In the DPCD, all patients in Denmark with a diagnosis of pancreatic or periampullary cancer, except patients with neuroendocrine tumors and patients diagnosed at autopsy, are included. The average yearly incidence in DPCD is almost 950 patients. The completeness of the registry has gradually improved from 76% the first year (2011) to 95–100% in 2018 [Citation23,Citation25].
Patients and variables
From 1 January 2012 to 31 December 2018, we prospectively registered in the DPCD 2,657 patients, who had received chemotherapy as their first treatment. According to national guidelines, all patients that received initial chemotherapy had non-resectable disease or were medically inoperable [Citation7]. Therefore, none of the included patients had prior surgery or chemotherapy for pancreatic cancer. We excluded 2 patients due to emigration. Hence, 2,655 patients were included for final analysis. We classified patients according to the hospital facility at which their first oncological visit was registered. Only 39 patients (1.5%) were registered at more than one oncological facility during their disease course.
From DPCD we retrieved information on gender, age, pretreatment clinical stage (cM0 or cM1), subsequent resection, participation in a standardized cancer pathway, type of 1st line chemotherapy and numbers of further lines of chemotherapy. Type of 1st line chemotherapy was defined by the first 3 series recorded. Chemotherapy was categorized into gemcitabine monotherapy, gemcitabine combination regimens, Folfirinox, and others. Treatment of 60 patients was at one institution erroneously registered as gemcitabine monotherapy wherefore data was manually corrected.
The Charlson Comorbidity Index (CCI) score [Citation26] was calculated from the Danish National Patient Registry from each patient's medical history registered in the decade preceding their PC diagnosis. We defined three levels of comorbidity: low (CCI score 0), moderate (CCI score 1–2) and severe (CCI score >2). Performance status or tobacco use were not recorded.
The cM-stage was cross referenced with the National Pathology Registry. This resulted in a change of stage in a total of 626 patients (24%) to pathological M1 given malignant biopsies from a metastatic lesion at the time of diagnosis.
We also retrieved information on residential municipality at the time of cancer diagnosis. We used the official classification from the Danish Ministry of Environment and Food to classify patients into four types of municipalities: remote area, rural, regional and metropolitan municipality.
Treatment facility characteristics
Facilities were classified as either secondary or tertiary referral centers. In this aspect, the oncological unit at Herlev University Hospital, Copenhagen, and the surgical unit at Rigshospitalet, Copenhagen, were considered a common unit, geographically distributed only 10 km apart. There were 4 tertiary center facilities at larger hospitals with comprehensive diagnostic, surgical and oncological cancer care, all having inhouse pancreatic surgery, multidisciplinary team conferences and other highly specialized modalities. There were 4 secondary facilities at smaller hospitals with standard oncological care and without inhouse pancreatic surgery. One of these facilities first started treating patients in 2017.
Study outcome
The primary outcome of interest was overall survival (OS) measured from the date of the first chemotherapy. We followed each subject until death, emigration, or 15 June 2020, whichever occurred first. Ninety-two patients (3.5%) who had resection after chemotherapy were censored at the time of resection.
Statistical analyses
Categorical values are presented as numbers and percentage. Comparisons were made using Pearson’s Chi square test. Continuous data were compared using Mann–Whitney U test.
For survival analysis unadjusted Kaplan–Meier functions were used to estimate OS, and a log-rank test to compare survival functions. For time specific survival the direct method was used. To illustrate time-trends in survival, the survival of patients treated the last two years was compared with the survival of patients treated the first five years. This cut point was arbitrarily chosen to provide sufficient statistical power.
We used Cox proportional hazards models to calculate hazard ratios (HRs) of PC survival in relation to facility type. A Wald test was used to test the difference in HRs between facilities. For time specific survival the direct method was used. In all analyses, the facility and type of facility were independent variables. In comparing relative risks of individual facilities, the one with the highest patient volume and thus most precisely estimated HR was used as reference. In a first step, estimates of death hazards were investigated by univariate analyses for each of the variables included. In a second step multivariate models were established with facility type as independent variable, adjusted for year of first treatment, age, gender, CCI score and M-stage. Since the use of chemotherapy varied between facilities and over time, a third model including type of chemotherapy was made. All variables were categorical except age. Entering age as a categorical variable, using 25 and 75% percentiles as cut points, produced equal results (data not shown). We checked the proportional hazards assumption by visual inspection of log-minus-log plots and a proportional hazard test. We did not find any significant violations of proportionality.
All tests of statistical significance were 2-sided, and a p-value < .05 was considered statistically significant. We performed analyses using Stata v. 16 (StataCorp LLC, Texas, USA).
Ethical considerations
No ethical board approval is needed in Denmark for this type of study and no consent from patients is needed to use this type of data. The study was approved by The Danish Data Protection Agency (2008-58-0028) and the Danish Patient Safety Authority (3-3013-1678/1/).
Results
Descriptive characteristics
Our cohort included 2,655 patients who were treated at eight oncological facilities in a seven-year period. The yearly median volume across all facilities was 43 patients, median for tertiary facilities 71 patients and 31 patients for secondary facilities.
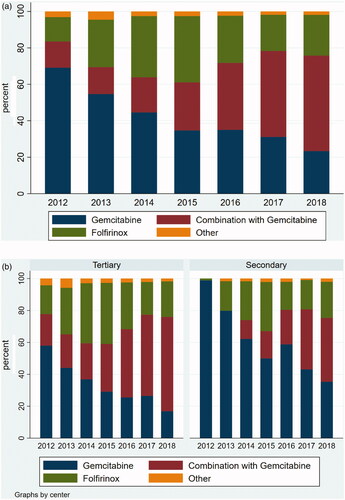
The distribution of variables for the total population and according to facility category is shown in . In tertiary facilities more patients had low CCI score, were resected after chemotherapy, did not participate in the standardized cancer pathway, were treated with further lines of chemotherapy, and lived in metropolitan areas. More patients were among the 25% youngest and less patients among the 25% oldest, however the median ages were almost similar (68.5 versus 69.5 years, p = .006). Monotherapy with gemcitabine was used considerably less often (34%) in tertiary facilities compared with secondary (59%). The average time to treatment start from diagnosis was longer (median 28 days (IQR 16–43 days) versus 25 days (IQR 14–37 days)) at tertiary facilities compared to secondary (p < .001).
Table 1. Characteristics of all patients and of patients distributed according to oncological facility type (numbers and percent).
Evolution in use of chemotherapy regimens
We further explored the distribution of chemotherapy regimens used for first-line treatment according to year of treatment initiation (). Gemcitabine monotherapy was used for 69% of patients in 2012 declining to 23% in 2018, whereas the use of gemcitabine combinations increased from 14 to 52%. The use of Folfirinox increased from 13% the first year to a maximum of 36% in 2015. Secondary facilities used monotherapy gemcitabine for virtually all patients in 2012, whereas this was the case for only 58% at tertiary facilities. The fraction of patients treated with gemcitabine monotherapy generally decreased from 2013 and onwards, although in 2018 there was still a 18%-point difference among the facility types. The type of chemotherapy used for patients with or without distant metastases, distributed according to facility type and year of treatment initiation, showed similar trends (data not shown).
Difference in survival among facilities
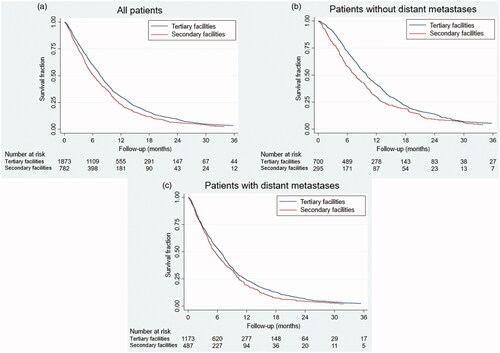
Survival curves for patients distributed according to facility types are shown in . A total of 91 patients were still alive with a median observation time of 27 months. The mOS was longer in patients treated at tertiary (7.7 months) as compared with secondary facilities (6.1 months), corresponding to an absolute difference in mOS of 1.6 months or 26%. IQR’s were non-overlapping. For the subgroup of patients with non-metastatic disease the mOS was 10.0 months at tertiary facilities as compared with 7.7 months at secondary facilities and IQR’s were non-overlapping, whereas patients with metastatic disease treated at tertiary facilities had a mOS of 6.6 months as compared with 5.4 months at secondary facilities (IQR’s 2.8–11.7 months and 2.6–10.4 months, respectively).
Figure 2. (a) The overall survival for patients with pancreatic cancer treated with chemotherapy as first line treatment according to facility type for all patients. The mOS was 7.7 and 6.1 months, respectively (p = .0001). (b) The overall survival for patients with pancreatic cancer treated with chemotherapy as first line treatment according to facility type for patients without distant metastases. The mOS was 10.0 and 7.7 months, respectively (p = .001). (c) The overall survival for patients with pancreatic cancer treated with chemotherapy as first line treatment according to facility type for patients with distant metastases. The mOS was 6.6 and 5.4 months, respectively (p = .02).
The HRs for individual facilities were adjusted for year of first treatment, age, CCI score and M-stage. All secondary facilities, except the one with the highest volume, had significantly increased HRs compared to the reference facility (the largest), with an excess hazard of 27% (CI 7–50%), 47% (CI 12–92%) and 49% (CI 25–77%), respectively. All tertiary facilities had HRs not statistically different from the reference facility, except the one with the lowest volume, which had an excess hazard of 22% (CI 5–41%).
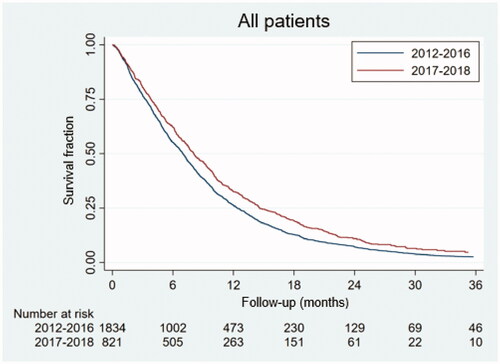
Given the major shift in chemotherapy regimens used over time, the survival analysis was repeated only to include the last two years (2017–2018). This analysis reproduced the finding of a significant difference in OS according to facility type for all patients (p = .01) and for the subgroup of patients with distant metastases (p = .04), but not for patients with non-metastatic disease (p = .10) (Supplementary Figure). As illustrated in , the survival for patients treated in 2017–2018 was improved compared to 2012–2016 with a mOS of 8.1 months (IQR 3.8–14.6 months) compared to 7.0 months (IQR 3.3–12.4 months)). Improvements with time were found both for the subgroup of patients treated at tertiary facilities (mOS 8.5 months compared to 7.3 months, p = .0001), and at secondary facilities (mOS 6.8 months compared to 5.8 months, p = .03).
Figure 3. The overall survival for patients with pancreatic cancer treated with chemotherapy as first line treatment in 2012–2016 and in 2017–2018. The mOS was 8.1 and 7.0 months, respectively (p < .0001).
In a multivariate Cox model with facility type as independent variable () adjustment was made for the year of first treatment, age, gender, CCI score, and M-stage (Model 1). Gender had no independent prognostic significance. The adjusted HR of secondary facilities relative to tertiary was 1.16 (CI 1.07–1.27). The multivariate Cox model with further adjustment for the type of chemotherapy (Model 2) reduced the influence of facility type to 1.06 (CI 0.97–1.15) and the difference was not significant (p = .23). Together the two Cox models, indicate that the different distribution of type of chemotherapy used at secondary and tertiary facilities explains – at least in part – the observed difference in OS among facility types. Furthermore, the HRs generally improved with time, especially in the latter two years. There was no survival difference according to municipalities in either univariate or multivariate analysis (data not shown).
Table 2. Results of multivariate Cox regression analyses of survival with facility as independent variable, excluding (Model 1) and including type of chemotherapy (Model 2).
Discussion
This study demonstrates an association between treatment facility characteristics and outcome for patients treated with chemotherapy for PC. We showed that patients treated at tertiary facilities had 1.6 month (26%) longer mOS compared to those treated at secondary facilities, corresponding to an increased, adjusted death intensity of 16% (CI 7–27%) for patients treated in secondary facilities. The difference in HRs was statistically significant in three of four secondary facilities and death intensity was almost 50% higher at two secondary centers compared to the largest reference center. Significant improvements in survival were observed for both facility types with time, however, the difference in OS results among facility types was maintained.
Our study points to differences in the distribution of patient and treatment variables according to facility type that may influence survival. Patients treated at secondary facilities had more comorbidity and the age distribution was right skewed. However, we found that the effect of facility type on survival was maintained even after adjustment for these factors. We found that patients’ residencies were highly different, but the municipality categories had no influence on survival.
Most striking, the use of multidrug chemotherapy was highly different among facility types. Of these, the Folfirinox regimen was introduced in Denmark at tertiary facilities in 2011 [Citation27], whereas gemcitabine/nab-paclitaxel was used from 2012. Because combination chemotherapy has more side effects and is usually more expensive and logistically complex, the speed of introduction and use of such treatments may reflect quality of care and available resources. In our study we found both a longer delay in the introduction and a sustained reduced use of combination chemotherapy in secondary facilities. Interestingly, Canale et al. observed no difference in use of combination chemotherapy according to geography and found no disparities in survival among 659 patients with advanced PC treated in the British Colombia in Canada [Citation28], whereas in a Danish study of PC in all stages, rural residency compared to urban was associated with a slightly worse outcome. This difference disappeared, however, after adjustment for cancer-directed treatment [Citation29]. Similarly, in the current multivariate analysis with adjustment for type of chemotherapy, the prognostic influence of facility type became insignificant, indicating that the difference in use of combination chemotherapy in first line treatment at secondary and tertiary facilities was a major determinant for differences in outcome.
Other treatment related factors may apply; the dismal prognosis of inoperable PC imply that cure or long-term survival of even a minority of patients may have an influence on mOS of the population. Neoadjuvant therapy was not recommended [Citation7], however, we identified 92 patients that had surgery after chemotherapy [Citation30], casuistically with complete pathological response [Citation31]. Resection was done twice as often in patients treated at tertiary facilities. We chose to censor these patients at the time of surgery, although this may contribute to underestimating the survival difference between facility types. The fraction of patients receiving second or further lines of chemotherapy was slightly higher at tertiary facilities compared to secondary (47 versus 44%), but this was unlikely to have a major influence on the results as the effect on survival is small [Citation32].
Fewer patients were included in the standardized cancer pathway at tertiary centers compared to secondary centers (74 versus 79%). We hypothesize that patients at tertiary facilities may more often be diagnosed incidentally, thus not being registered for a cancer-specific pathway. This may imply a minor lead time bias favoring survival in tertiary facilities. The, on average, 3 day longer delay in treatment start observed at tertiary centers did not result in inferior survival measured from start of chemotherapy. Possible lead time bias prior to any contact of the patient with the health system (and its association with facility type) could not be assessed. The work up procedures and guarantied waiting times in the standardized cancer pathway were, however, unchanged during the inclusion period, and we found no indications of diagnosis at an earlier stage with respect to stage (M-stage) during the latter years of the inclusion period for the whole cohort or according to facility type (data not shown).
In general, the data quality of the Danish national registers is high [Citation24]. However, we found that M-staging was inadequate for 24% of patients, and type of chemotherapy had to be manually entered for 2.3% of patients. Pathological staging data are now automatically incorporated in the DPCD. Our study suggests an inverse correlation between HRs and number of patients treated per year at individual facilities. Studies in larger populations are needed to assess a minimum volume required for optimal results as has been done for several malignant diseases for which treatment has traditionally been regarded as more complex [Citation11,Citation14,Citation16,Citation33].
The strengths of our study are the inclusion of a large cohort of PC patients treated at all oncological departments in the country, the utilization of data in national health registries with standardized coding definitions and information on specific treatment. Nearly all patients in our study were treated at only one reporting facility and all patients were followed up.
The limitations of our study include the lack of some important individual-level prognostic data including performance status, tobacco use and weight loss, so residual confounding from these cannot be excluded. We did not include information on T- and N-stage or anatomical site of primary tumor, which have limited independent prognostic value in advanced disease and were not consistently reported in the database for inoperable patients. Furthermore, we had no available information on potential histopathological, genetic or biochemical prognostic or predictive factors such as tumor grade, HRD mutational status, serum CA 19-9 or white blood cell counts [Citation34]. Although residency was registered we lacked information on other socio-economical factors, which may be facility-dependent [Citation35,Citation36]. Others have shown that individuals living in rural areas with lower average income and level of education are more likely to receive less efficient treatment [Citation29], whereas socio-economic deprivation may not influence treatment or treatment outcome when offered at a high-volume center [Citation37]. We lacked data on the facility uptake-area-specific allocation of patients to surgery, chemotherapy and best supportive care, which may greatly influence survival [Citation22,Citation38,Citation39]. We were unable to assess the recruitment of patients to facilities outside predetermined uptake areas, which might be skewed toward more patients with undisclosed favorable prognostic factors being treated at tertiary facilities. Finally, we did not study the influence of travel distance [Citation28], as this presumably is of limited importance in a small country.
Although patient’s preferences for certain treatments according to, e.g., logistics, quality of life and toxicity should be taken into account, together with efficacy, when discussing the optimal management of patients with PC, our study suggests an opportunity to improve the outcomes on a national level through proper use and fast introduction of evidence-based palliative treatments. Regulatory interventions, such as guarantied maximal waiting times to start on chemotherapy, may improve survival but may also contribute negatively to the selection of less complex care. Equal access to modern treatment at all facilities should be achievable with increased national collaboration and allocation of resources.
We used the STROBE cohort reporting guidelines [Citation40] (included in supplementary material).
Supplemental Material
Download MS Word (140.5 KB)Acknowledgements
All departments treating PC in Denmark contribute with data for the DPCD. The members of both DPCD and DPCG represent surgeons, oncologists, radiologists, and pathologists from the participating institutions. We acknowledge Birgitta Wanda Nielsen for expert technical assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Patients are not completely anonymised and data can therefore not be shared. The data are extracted from the Danish healthcare registries by the Danish Health Data Authority. These data are available to researchers upon application and can only be obtained with permission from the Danish Data Protection Agency, who gave the permission to conduct this study.
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA. CA A Cancer J Clin. 2020;70(1):7–30.
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921.
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075.
- Conroy T, Ychou M, Bouché O, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1842.
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703.
- ESMO Guidelines Committee. Cancer of the pancreas: ESMO clinical practice guidelines. eUpdate; 2019. Available from: https://www.esmo.org/guidelines/gastrointestinal-cancers/pancreatic-cancer.
- Nationale kliniske retningslinjer. Dansk Pancreas Cancer Gruppe; 2020. Avaiable from: http://dpcg.gicancer.dkdefault?pID=22.
- Klompmaker S, van Hilst J, Gerritsen SL, E-AHPBA DP-CAR Study Group, et al. Outcomes after distal pancreatectomy with celiac axis resection for pancreatic cancer: a Pan-European retrospective cohort study. Ann Surg Oncol. 2018;25(5):1440–1447.
- Taylor LJ, Greenberg CC, Lidor AO, et al. Utilization of surgical treatment for local and locoregional esophageal cancer: Analysis of the national cancer data base. Cancer. 2017;123(3):410–419.
- Merritt RE, Abdel-Rasoul M, Fitzgerald M, et al. The academic facility is associated with higher utilization of esophagectomy and improved overall survival for esophageal carcinoma. J Gastrointest Surg. 2021;25(7):1677–1689.
- Hauser A, Dutta SW, Showalter TN, et al. Impact of academic facility type and volume on post-surgical outcomes following diagnosis of glioblastoma. J. Clin. Neurosci. 2018;47:103–110.
- Chu QD, Zhou M, Peddi P, et al. Influence of facility type on survival outcomes after pancreatectomy for pancreatic adenocarcinoma. HPB. 2017;19(12):1046–1057.
- Majhail NS, Mau L-W, Chitphakdithai P, et al. Transplant center characteristics and survival after allogeneic hematopoietic cell transplantation in adults. Bone Marrow Transplant. 2020;55(5):906–917.
- Go RS, Al-Hamadani M, Shah ND, et al. Influence of the treatment facility volume on the survival of patients with non-Hodgkin lymphoma. Cancer. 2016;122(16):2552–2559.
- Master S, Munker R, Shi Z, et al. Insurance status and other non-biological factors predict outcomes in acute myelogenous leukemia: analysis of data from the national cancer database. Anticancer Res. 2016;36(9):4915–4921.
- Go RS, Bartley AC, Crowson CS, et al. 3rd. Association between treatment facility volume and mortality of patients with multiple myeloma. J Clin Oncol. 2017;35(6):598–604.
- Sohal DPS, Kennedy EB, Khorana A, et al. Metastatic pancreatic cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;36(24):2545–2556.
- Ducreux M, Cuhna AS, Caramella C, ESMO Guidelines Committee, et al. Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v56–68.
- Kirkegård J, Aahlin EK, Al-Saiddi M, et al. Multicentre study of multidisciplinary team assessment of pancreatic cancer resectability and treatment allocation. Br J Surg. 2019;106(6):756–764.
- Tamburini N, Maniscalco P, Mazzara S, et al. Multidisciplinary management improves survival at 1 year after surgical treatment for non-small-cell lung cancer: a propensity score-matched study. Eur J Cardiothorac Surg. 2018;53(6):1199–1204.
- Pakkeforløb for kraeft i bugspytkirtlen. Danish Health Authority 1–32; 2016. Avaiable from: https://www.sst.dk/da/sygdom-og-behandling/kraeft/pakkeforloeb//-/media/Udgivelser/2019/Pakkeforloeb-kraeft-2015-2019/Bugspytkirtlen-2016/Pakkeforløb-for-kraeft-i-bugspytkirtlen-–-revision-sep-2016_endelig.ashx.
- Rasmussen LS, Fristrup CW, Jensen BV, et al. Initial treatment and survival in 4163 Danish patients with pancreatic cancer: a nationwide unselected real-world register study. Eur J Cancer. 2020;129:50–59.
- Fristrup C, Detlefsen S, Hansen CP, et al. Danish pancreatic cancer database. Clin Epidemiol. 2016;8:646–648.
- Schmidt M, Schmidt SAJ, Sandegaard JL, et al. The danish national patient registry: a review of content, data quality, and research potential. CLEP. 2015;7:449–490.
- Dansk Pancreas Cancer Database. Sundhed.dk; 2020. Avaiable from: https://www.sundhed.dk/sundhedsfaglig/kvalitet/kliniske-kvalitetsdatabaser/kraeft/kraeft-i-bugspytkirtlen/.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Kraemer PC, Schmidt HH, Ladekarl M. Danish experiences with FOLFIRINOX as first-line therapy in patients with inoperable pancreatic cancer. Dan Med J. 2014;61(4):A4819.
- Canale TD, Cho H, Cheung WY. A population-based analysis of urban-rural disparities in advanced pancreatic cancer management and outcomes. Med Oncol. 2018;35:116.
- Kirkegård J, Ladekarl M, Fristrup CW, et al. Urban versus rural residency and pancreatic cancer survival: a Danish nationwide population-based cohort study. PLoS One. 2018;13(8):e0202486.
- Pfeiffer P, Ladekarl M, Mortensen MB, et al. Chemotherapy for patients with non-resectable pancreatic cancer with additional chemo-radiotherapy for patients with potentially resectable tumours: Final results. J Clin Oncol. 2016;34(15_suppl):e15725–e15725.
- Sharma MB, Carus A, Sunde L, et al. BRCA-associated pancreatico-biliary neoplasms: four cases illustrating the emerging clinical impact of genotyping. Acta Oncol. 2016;55(3):377–381.
- Caparello C, Vivaldi C, Fornaro L, et al. Second-line therapy for advanced pancreatic cancer: evaluation of prognostic factors and review of current literature. Future Oncol. 2016;12(7):901–908.
- Zhu P, Du XL, Zhu J-J, et al. Improved survival of glioblastoma patients treated at academic and high-volume facilities: a hospital-based study from the national cancer database. J. Neurosurg. 2020;132(2):491–502.
- Cai J, Chen H, Lu M, et al. Advances in the epidemiology of pancreatic cancer: trends, risk factors, screening, and prognosis. Cancer Lett. 2021;520:1–11.
- Noel M, Fiscella K. Disparities in pancreatic cancer treatment and outcomes. Health Equity. 2019;3(1):532–540.
- Zhu F, Wang H, Ashamalla H. Racial and socioeconomic disparities in the treatments and outcomes of pancreatic cancer among different treatment facility types. Pancreas. 2020;49(10):1355–1363.
- Powers BD, Fulp W, Dhahri A, et al. The impact of socioeconomic deprivation on clinical outcomes for pancreatic adenocarcinoma at a high-volume cancer center. Ann Surg. 274(6):e564–e573.
- Engberg H, Steding-Jessen M, Øster I, et al. Regional and socio-economic variation in survival after a pancreatic cancer diagnosis in Denmark. Dan Med J. 2020;67:A08190438.
- Shapiro M, Chen Q, Huang Q, et al. Associations of socioeconomic variables with resection, stage, and survival in patients with Early-Stage pancreatic cancer. JAMA Surg. 2016;151(4):338–345.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349.