Abstract
Purpose
It is essential to evaluate the risk of occult lymph node (LN) disease in early-stage non-small cell lung cancer (NSCLC), especially because delivering stereotactic ablative radiotherapy (SABR) assumes no occult spread. This study was designed to assist clinicians in roughly quantifying this risk for cN0 NSCLC.
Methods
The National Cancer Data Base was queried for cN0 cM0 lung squamous cell or adenocarcinoma who underwent surgery and LN dissection without neoadjuvant therapy. Statistics included multivariable logistic regression to evaluate factors associated with pN + disease.
Results
109,964 patients were included. For tumors with size ≤1.0, 1.1–2.0, 2.1–3.0, 3.1–4.0, 4.1–5.0, 5.1–6.0, 6.1–7.0, and >7.0 cm, the pN + rate was 4.4, 7.7, 12.9, 18.0, 20.2, 22.5, 24.4, and 26.4%, respectively. When examining patients with more complete LN dissections (defined as removal of at least 10 LNs), the respective values were 6.6, 11.5, 17.6, 25.3, 26.8, 29.7, 30.7, and 31.6%. Moderately-poorly differentiated disease and adenocarcinomas were associated with a higher rate of pN + disease (p < .001 for both). For every cm increase in tumor size, the relative occult nodal risk increased by 10–14% (p < .001). For every elapsed day from initial diagnosis, the relative risk increased by ∼1% (p < .001). Graphs with best-fit lines were created based on tumor size, histology, and differentiation to aid physicians in estimating the pN + risk.
Conclusions
This nationwide study can allow clinicians to roughly estimate the rate of occult LN disease in cN0 NSCLC. These data can also assist in guiding enrollment on randomized trials of SABR ± immunotherapy, individualizing follow-up imaging surveillance, and patient counseling to avoid post-diagnosis delays.
Introduction
Early-stage, clinically node-negative (cN0) non-small cell lung cancer (NSCLC) is a relatively common clinical occurrence. Workup for these patients in the contemporary era includes positron emission tomography – computed tomography (PET-CT) with or without invasive mediastinal nodal staging (e.g., endobronchial ultrasound guided transbronchial needle aspiration (EBUS-TBNA) or mediastinoscopy) [Citation1]. The primary goal of this workup is to ascertain the presence of occult lymph node (LN) disease. This factor is extremely important because the discovery of occult LN involvement vastly alters patient management.
However, the existing methods of detecting occult LN disease in SABR (stereotactic ablative radiation therapy) patients are not completely satisfactory. Although meta-analyses show that the negative predictive value and specificity of PET-CT for staging the mediastinum is around 90%, the positive predictive value and sensitivity is only 62–72% [Citation2–3]. Additionally, there is a 5–30% false negative rate for EBUS-TBNA with a sensitivity around 78% [Citation3]. As a result, the ‘gold standard’ for evaluating nodal disease is mediastinal LN dissection (MLND), though an EBUS is still considered a reasonable option for mediastinal nodal staging.
The standard of care patients with early-stage cN0 NSCLC is to undergo surgical resection and MLND, but SABR is an acceptable alternative if patients are medically inoperable or refuse surgery [Citation1]. Whereas all surgical patients undergo MLND to fully evaluate the LNs, this is not the case for patients receiving SABR (the vast majority of whom are medically inoperable, and whose medical comorbidities may preclude the ability to undergo pathologic nodal evaluation). Thus, before proceeding with SABR and in the absence of pathologic nodal evaluation, it is useful to accurately gauge the risk of occult LN involvement.
One important way to estimate the risk of occult nodal disease in SABR patients is to utilize data from historical surgical series. Many such investigations have characterized the rates of occult LN involvement based on tumor size, which is a strong predictor of LN metastasis. These studies have shown that the rate of occult nodal positivity is 0–2% for tumors ≤1.0 cm, 5–15% for tumors 1.1–2.0 cm, and 13–31% for tumors 2.1–3.0 cm [Citation4–6]. However, these studies have several shortcomings that limit application to current clinical practice. In addition to the small sample sizes, many studies were published decades ago when imaging techniques were less advanced. There are also concerns regarding a lack of reproducibility, because other data have shown occult nodal risk at much higher rates [Citation7].
For oncologists managing clinically node-negative NSCLC patients (especially SABR cases), there is a need to precisely quantify the risk of occult LN disease before offering therapy. In light of the shortcomings of existing surgical series, we sought to use the large, contemporary National Cancer Database (NCDB) to evaluate the rate and predictors of occult LN involvement in this population, so that clinicians can use discrete criteria to quantify the risk of occult LN metastasis in any given cN0 NSCLC patient.
Material and methods
The NCDB is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society, which consists of de-identified information regarding tumor characteristics, patient demographics, and patient survival for approximately 70% of the US population [Citation8]. All pertinent cases are reported regularly from CoC-accredited centers and compiled into an unified dataset, which is then validated. The data used in the study were derived from a de-identified NCDB file (2006–2015). The American College of Surgeons and the CoC have not verified and are neither responsible for the analytic or statistical methodology employed nor the conclusions drawn from these data by the investigators. As all patient information in the NCDB database is de-identified, this study was exempt from institutional review board evaluation.
Prior to designing this study, it was well recognized that the NCDB does not carry information on the type of preoperative workup, including PET-CT or invasive mediastinal staging. As a result, we chose to examine all-comers using as broad of a dataset as possible in order to enhance applicability to both high-volume quaternary care centers where workup may be more extensive as well as smaller rural facilities where workup may be more limited.
Inclusion criteria for this study were patients with primary cT1–4 cN0 cM0 squamous cell or adenocarcinoma of the lung. Because the ‘gold standard’ for evaluating occult LN metastasis is MLND, only surgical patients were analyzed (provided there was a record of at least one LN dissected). Additionally, because another relevant goal of this investigation was to specifically quantify the occult LN risk by tumor size, patients with unrecorded tumor size were excluded. Lastly, because the impetus for this study was for clinicians delivering up-front SABR or surgery, subjects who underwent neoadjuvant therapy were also removed.
In accordance with the variables in NCDB files, information collected on each patient broadly included demographic, clinical, and treatment data. All statistical tests were two-sided, with a threshold of p < .05 for statistical significance, and were performed using STATA (version 14, College Station, TX). Multivariable logistic regression analysis was utilized to determine variables associated with discovery of pN + disease on MLND (all variables were included in the multivariable model). Given the heterogeneity and agranularity of NCDB data, along with the myriad confounding factors for survival using such datasets, Kaplan–Meier analysis was intentionally not performed. Lastly, in order to assist clinicians with rough estimation of a given patient’s occult LN risk, using the three variables readily obtained on imaging and biopsy (tumor size, histology, and differentiation status), scatter plots were constructed on Microsoft Excel, with a program-generated best-fit line.
Results
A complete flow diagram of patient selection is provided in Supplementary Figure 1. In total, 109,964 patients met study criteria (). The median tumor size was 2.5 cm (interquartile range (IQR), 1.7–3.6 cm); most tumors were moderately or poorly differentiated and underwent lobectomy. The median number of LNs removed on MLND was 8 (IQR, 5–14).
Table 1. Characteristics of the patient population.
illustrates the proportion of patients with occult LN involvement by tumor size. The overall rate in all patients was 13.2% (14,535 pN + cases in 109,964 patients). Amongst patients with tumor size ≤1.0, 1.1–2.0, 2.1–3.0, 3.1–4.0, 4.1–5.0, 5.1–6.0, 6.1–7.0, and >7.0 cm, the proportion of patients with occult LN disease was 4.4, 7.7, 12.9, 18.0, 20.2, 22.5, 24.4, and 26.4%, respectively.
Table 2. Proportion of patients with occult pathologic nodal disease as stratified by tumor size and number of lymph nodes resected.
We surmised that, in a MLND, the detected pN + rates could be lower if the dissection was more of a ‘sampling’ rather than a ‘dissection’ and did not remove enough LNs, which would not allow for a sense of the ‘true’ risk. As a result, we repeated the quantification based on the number of LNs removed. There was no specific rationale for the trichotomization (1–5, 6–10, and >10 LNs resected) because there is no evidence-based ‘optimal’ harvested nodal count for a MLND to date [Citation1]. In patients with over 10 LNs removed, which could be more indicative of the ‘true’ risk, the respective rates for each tumor size were 6.6, 11.5, 17.6, 25.3, 26.8, 29.7, 30.7, and 31.6%.
Noticing the heterogeneity in surgical procedures () – including the use of pneumonectomy in some cases (which is not standard for cN0 NSCLC) – we then sought to perform a sensitivity analysis by stratifying for the type of surgical procedure. These results are displayed in , showing that patients undergoing pneumonectomy had unexpectedly high rates of positive nodal disease even for smaller sized tumors.
Table 3. Proportion of patients with occult pathologic nodal disease as stratified by tumor size and surgical approach.
Based on this information, we performed multivariable logistic regression analysis for factors associated with discovering occult LN metastasis (). Given the concern for pneumonectomy patients as potential outliers, we repeated the analysis in patients who underwent either lobectomy or sublobar resection (). In addition to demographic factors (age, gender, race, comorbidity score), there were several important variables significantly associated with detecting pN + disease on MLND. These included the number of LNs removed and the surgery type (p < .001 for both). Additionally, histology and grade were strongly associated with occult LN involvement, with moderately-poorly differentiated tumors and adenocarcinomas being associated with a higher rate of LN disease (p < .001 for all). The analysis for tumor size as a continuous variable showed that for every 1 cm increase in tumor size, the risk of occult LN disease increased by 9.9% (all patients) to 14.2% (lobectomy or sublobar resection only) (p < .001). Additionally, every elapsed day from initial diagnosis to MLND was associated with a 0.9–1.0% increased rate of discovering pN + disease (p < .001).
Table 4. Multivariable logistic regression analysis for factors associated with the discovery of pathologic nodal involvement at surgery.
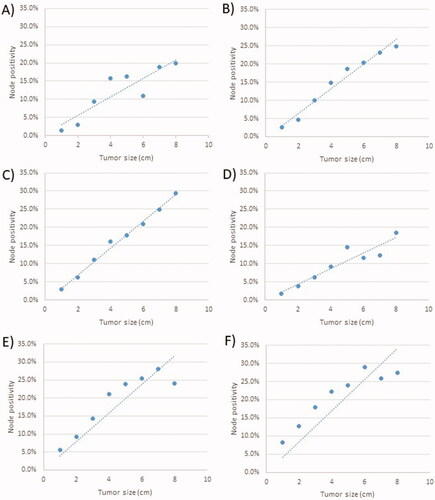
Lastly, in order to allow clinicians to roughly estimate any patient’s occult LN risk using only the information obtained from imaging and biopsy (tumor size, histology, and differentiation status), graphs with best-fit lines were constructed as shown in . Additionally, the proportion of patients with occult nodal positivity by year is shown in Supplementary Figure 2.
Figure 1. Graph of the proportion of patients with occult lymph nodal positivity as a function of tumor size and grade for squamous cell carcinoma with (A) low grade disease; (B) intermediate grade disease and (C) high grade disease; and for patients with adenocarcinoma with (D) low grade disease; (E) intermediate grade disease and (F) high grade disease.
Discussion
Patients with clinically node negative NSCLC harbor a risk of occult pathologic LN involvement, and ascertainment of this risk is essential to management of both SABR and surgical patients. Whereas the latter receive a relatively accurate nodal assessment by virtue of a MLND, SABR is often performed in the absence of pathologic nodal evaluation with the major assumption that there are no occult areas of metastasis. This study of over 100,000 patients treated throughout the United States provides a key tool for clinicians to estimate the rate of occult nodal disease and manage their patients accordingly.
It is important to note that this study is not designed to accurately determine the nodal risk of any patient; it is merely for rough ‘ballpark’ estimation and not fine-tuned assessment. It is clear that an unquantified proportion of patients herein received various combinations of PET-CT staging only, invasive mediastinal staging, both, or neither. This information would have been useful for finer risk assessment, but a major strength of this work is that it can broadly apply to a variety of patients. It is presumed that the use of PET-CT staging and some degree of invasive mediastinal staging in the contemporary era would hint that these numbers are overestimations, but it is also critical to be aware that the lack of both PET-CT and invasive mediastinal staging are not uncommon in rural centers with few resources, even in the contemporary era.
This study also has implications on the adjuvant management of SABR patients (or, theoretically, status post wedge resection or lobectomy with limited LN sampling). A few retrospective studies have suggested that post-SABR chemotherapy may offer outcome benefits in patients with larger tumors expected to have a higher risk of occult nodal and/or distant metastatic disease [Citation9,Citation10]. For this reason, there are several randomized investigations underway that are evaluating the efficacy of post-SABR immunotherapy for high-risk node-negative NSCLC (e.g., NCT03110978, NCT03446547, NCT03833154, NCT03924869, NCT04214262). It is expected that the potential benefit with immunotherapy in those trials may be correlated with tumor size and other such factors, so this study may assist investigators in selecting patients to enroll on those trials.
This study is additionally useful for managing clinicians in order to individualize follow-up schedules after SABR. Patients with a higher risk of pN + disease (as determined by and ) should ideally undergo more aggressive imaging surveillance, especially because early detection of isolated locoregional recurrences can be effectively salvaged [Citation11,Citation12].
The findings of this study largely corroborate previous small historical surgical series [Citation4–7]. The findings of are comparable to those of most prior surgical series [Citation4–6], but suggest that those surgical series may have underestimated the LN risk for tumors ≤1 cm as compared to these data. Those studies did not have the luxury of high sample sizes in order to perform stratification based on several other variables, so these data of over 100,000 patients in the contemporary era provides a finer assessment than historical series. Emerging methods for even more precise assessment, such as radiomics, could show promise in the future [Citation13].
Additionally, the finding that every cm increase in tumor size is associated with a 10–14% increased risk of occult nodal disease is important for patients who have delays from initial diagnosis to definitive therapy. It is not uncommon for these post-diagnosis delays to manifest as 0.5–1.0 cm increases in tumor size from initial imaging to the time of SABR simulation, and patients must be counseled appropriately based on these data. Additionally, this investigation also revealed that each elapsed day from diagnosis was associated with a ∼1% increase in the rate of occult LN involvement, implying that even small delays from diagnosis to therapy should not be taken lightly, and patients should also be counseled accordingly.
Although the NCDB provides an unique platform with which to study this important clinical question, this investigation is not without additional limitations to those discussed above. First, as mentioned above, this is a retrospective study with a heterogeneous population and workup procedures. As such, this work is not designed to give a more limited but specific assessment, but rather a broad picture that is applicable to a variety of settings and circumstances. Importantly, the NCDB is representative of 70% of the US population, but may not be representative of segments of the population who have limited access to care and therefore these results may be not generalizable to the entire population. Second, there was also no standardization of the MLND for these patients, and the NCDB does not tabulate the specific nodal stations dissected. Third, the inclusion of pneumonectomy cases was necessary because a small proportion of cN0 NSCLC would presumably be in locations that would not allow for sparing of the ipsilateral lobar bronchi. Although these cases constituted a very small proportion of patients and could have been outliers, we conducted repeat multivariable analysis removing these cases for this very reason. Fourth, the NCDB does not keep track of several other factors that could be correlated with occult nodal spread, such as tumor location [Citation14], the amount of PET-CT radiotracer uptake [Citation15], adjacent structure involvement [Citation16], the presence of a ground-glass opacity on imaging [Citation17], and lepidic patterns or other histopathologic features [Citation18]. Lastly, the best-fit line in the graphs assumes a linear correlation between tumor size and nodal spread. This is a relatively safe assumption for most tumor sizes, but for very large tumor sizes this correlation could theoretically become more quadratic in nature; thus, the best-fit line could potentially underestimate the risk at higher tumor sizes. However, the data herein seemed to plateau at the largest tumor sizes, implying that the best-fit line could have overestimated the risk.
Conclusions
This study of over 100,000 cT1–4 cN0 cM0 NSCLC patients treated throughout the United States allows clinicians to estimate the rate of occult nodal disease for a given patient. This study demonstrates increased rates of occult LN positivity with both the size of primary tumor as well as the time from diagnosis to initiation of treatment. In particular, each cm increase in tumor size is associated with a 10–14% increase in the occult LN risk, and each elapsed day from diagnosis to therapy is associated with a ∼1% increase in the occult LN risk. These data may assist in guiding enrollment on randomized trials of SABR ± immunotherapy, individualizing follow-up imaging surveillance, and patient counseling to avoid post-diagnosis delays.
Supplemental Material
Download MS Power Point (38.5 KB)Supplemental Material
Download MS Power Point (37.7 KB)Acknowledgment
There was no funding for this study. This study has not been presented or published in part or full form elsewhere.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- National Comprehensive Cancer Network. Non-small cell lung cancer. Version 5; 2021 [cited 2021 June 18]. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- Wu Y, Li P, Zhang H, et al. Diagnostic value of fluorine 18 fluorodeoxyglucose positron emission tomography/computed tomography for the detection of metastases in non-small-cell lung cancer patients. Int J Cancer. 2013;132(2):E37–47.
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e211S–e250s.
- Oda M, Watanabe Y, Shimizu J, et al. Extent of mediastinal node metastasis in clinical stage I non-small-cell lung cancer: the role of systematic nodal dissection. Lung Cancer. 1998;22:23–30.
- Seok Y, Yang HC, Kim TJ, et al. Frequency of lymph node metastasis according to the size of tumors in resected pulmonary adenocarcinoma with a size of 30 mm or smaller. J Thorac Oncol. 2014;9(6):818–824.
- Ding N, Mao Y, Gao S, et al. Predictors of lymph node metastasis and possible selective lymph node dissection in clinical stage IA non-small cell lung cancer. J Thorac Dis. 2018;10(7):4061–4068.
- Ichinose Y, Yano T, Yokoyama H, et al. The correlation between tumor size and lymphatic vessel invasion in resected peripheral stage I non-small-cell lung cancer: a potential risk of limited resection. J Thorac Cardiovasc Surg. 1994;108(4):684–686.
- Bilimoria K, Stewart A, Winchester D, et al. The national cancer data base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683–690.
- Verma V, McMillan MT, Grover S, et al. Stereotactic body radiation therapy and the influence of chemotherapy on overall survival for large (≥5 centimeter) non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2017;97(1):146–154.
- Kann BH, Miccio JA, Stahl JM, et al. Stereotactic body radiotherapy with adjuvant systemic therapy for early-stage non-small cell lung carcinoma: a multi-institutional analysis. Radiother Oncol. 2019;132:188–196.
- Brooks ED, Sun B, Feng L, et al. Association of long-term outcomes and survival with multidisciplinary salvage treatment for local and regional recurrence after stereotactic ablative radiotherapy for early-stage lung cancer. JAMA Netw Open. 2018;1(4):e181390.
- Brooks ED, Verma V, Senan S, et al.; International Association for the Study of Lung Cancer Advanced Radiation Technology Committee. Salvage therapy for locoregional recurrence after stereotactic ablative radiotherapy for Early-Stage NSCLC. J Thorac Oncol. 2020;15(2):176–189.
- Gu Y, She Y, Xie D, et al. A texture analysis–based prediction model for lymph node metastasis in stage IA lung adenocarcinoma. Ann Thorac Surg. 2018;106(1):214–220.
- Ketchedjian A, Daly BDT, Fernando HC, et al. Location as an important predictor of lymph node involvement for pulmonary adenocarcinoma. J Thorac Cardiovasc Surg. 2006;132(3):544–548.
- Higashi K, Ito K, Hiramatsu Y, et al. 18F-FDG uptake by primary tumor as a predictor of intratumoral lymphatic vessel invasion and lymph node involvement in non-small cell lung cancer: analysis of a multicenter study. J Nuc Med. 2005;46:267–273.
- Suzuki K, Nagai K, Yoshida J, et al. Predictors of lymph node and intrapulmonary metastasis in clinical stage IA non–small cell lung carcinoma. Ann Thorac Surg. 2001;72(2):352–356.
- Matsuguma H, Yokoi K, Anraku M, et al. Proportion of ground-glass opacity on high-resolution computed tomography in clinical T1 N0 M0 adenocarcinoma of the lung: a predictor of lymph node metastasis. Surgery. 2002;124:278–284.
- Sun W, Yang X, Liu Y, et al. Primary tumor location is a useful predictor for lymph node metastasis and prognosis in lung adenocarcinoma. Clin Lung Cancer. 2017;18(1):e49–e55.
